Abstract
HMGB1, which acts as a DNA chaperone to help maintain nuclear homeostasis, was reported to play a prominent role in cancer progression, angiogenesis, invasion, and metastasis development. Increased expression of HMGB1 has been observed in several tumor entities. However, the molecular mechanisms of HMGB1 in tumorigenesis of bladder cancer have rarely been reported. In the present study, real-time quantitative RT-PCR analysis revealed that the expression of HMGB1 in human bladder urothelial carcinoma (BUC) cells was much higher than that in human normal urethra epithelial cells. In order to investigate the role of HMGB1 in BUC cells, RNA interference and Talen-mediated gene knockout (KO) were used to knockdown and knockout HMGB1, respectively, in BUC cell lines BIU-87 and T24. HMGB1 knockdown/out greatly inhibited proliferation, invasion, and cell cycle G1/S transition of BUC cells. The decrease in cell viability caused by HMGB1 knockdown/out was due to an increase in apoptosis via Bax/Bcl-2, both of which were important molecules involved in the apoptotic pathway. We then investigated the effect of HMGB1 knockdown/out on the sensitivity of BUC cells treated with the anticancer drug cisplatin. Knockdown or knockout of HMGB1 rendered BUC cells more sensitive to cisplatin. The decreased expression of LC3-II and Beclin 1, which resulted in decreased levels of autophagy, could probably explain this phenomenon. Thus, HMGB1 may become a novel promising candidate for the prognosis and therapy for bladder cancer.
Key words: HMGB1, Bladder cancer, Proliferation, Invasion, Apoptosis, Autophagy
INTRODUCTION
Bladder cancer, with more than 385,000 new cases and 150,200 deaths worldwide in 2008, is the second most common type of cancer in the genitourinary tract and the fourth most common cause of cancer in males in Western industrialized countries (1). In China, bladder cancer is also one of the most common genitourinary malignancies, and the incidence of this disease is gradually increasing (2). Urothelial carcinoma of the bladder, the most common histopathologic type of bladder cancer, has a variety of genetic and phenotypic characteristics. Many factors, such as chromosomal anomalies, genetic polymorphisms, genetic and epigenetic alterations, contribute to tumorigenesis and progression of urothelial carcinoma of the bladder. Therefore, identification of key genes and targets in signaling pathways related to tumorigenesis is indispensible for the diagnosis and prevention of bladder cancer (3).
High mobility group box (HMGB) proteins are nonhistone nuclear proteins with many different functions in the cell. HMGB1, HMGB2, and HMGB3 are the members of the HMGB protein family (4). HMGB1 was first isolated and characterized in calf thymus in 1973 and is named for its electrophoretic mobility in polyacrylamide gels. While the expressions of HMGB2 and HMGB3 are limited, HMGB1 expression is common and can be regulated with peripheral factors. In most cells, HMGB1 is located in the nucleus, where it acts as a DNA chaperone to help maintain nuclear homeostasis (5). HMGB1 contains two DNA-binding HMG-box domains (N-terminal A and central B) and an acidic C-terminal tail. Existing studies suggest that HMGB1 may have a prominent role in cancer progression, angiogenesis, invasion, and metastasis development (6).
Increased expression of HMGB1 has been observed in several tumor entities including gastrointestinal stromal tumors, colon tumors, and nasopharyngeal carcinoma (7,8). HMGB1 was also considered to be a useful serological biomarker for early diagnosis, as well as evaluating the tumorigenesis, stage, and prognosis of cancer (9). Recently, it was reported that HMGB1 had high expression in 87 of 164 cases of bladder cancer, of which overexpression was significantly associated with tumor grade and stage (2). However, the clinical significance of HMGB1 in bladder cancer, especially the molecular mechanisms of HMGB1 in tumorigenesis of bladder cancer, has rarely been reported. In the present study, the expression of HMGB1 in bladder urothelial carcinoma (BUC) cells was assessed and compared with human normal urethra epithelial cells by using real-time quantitative RT-PCR. In order to investigate the role of HMGB1 in BUC cells, HMGB1 knockdown and knockout (KO) cell lines were constructed by RNA interference and Talen-mediated gene KO, respectively. Then, the effects of HMGB1 knockdown/out on proliferation, invasion, and cell cycle of BUC cells were evaluated. We also investigated the effect of HMGB1 knockdown/out on the sensitivity of BUC cells treated with the anticancer drug cisplatin, and its probable mechanism was also discussed. This study improves our understanding of the role of HMGB1 in tumorigenesis of bladder cancer.
MATERIALS AND METHODS
Cell Cultures
Human urethra epithelial cell line (SV-HUC-1) and BUC cell lines (EJ, 5637, T24, and BIU-87) were brought from BioHermes Company (China). Cells were maintained in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) at 37°C in humidified air containing 5% CO2 in a monolayer as previously described.
Real-Time RT-PCR
Trizol and RT-PCR Kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). SYBR Green qRCR Mix was purchased from GeneCopoeia (Rockville, MD, USA). Total RNA was extracted from cells with Trizol reagent following the manufacturer’s instructions. Expression of HMGB1 mRNA was detected by real-time RT-PCR using the standard SYBR Green RT-PCR Kit and specific primers synthesized from Sangon Company (Shanghai, China). The specific primer pair for HMGB1 is as follows: sense, 5′-AGAAGTGCTCAGAGAGGTGGA-3′ and antisense, 5′-CCTTTGGGAGGGATATAGGTT-3′. The specific primer pair for β-actin (as an internal control) is as follows: sense, 5′-AGGGGCCGGACTCGTCATACT-3′ and antisense, 5′-GGCGGCACCACCATGTACCCT-3′. Independent experiments were repeated three times, and the relative expression level was analyzed by use of the 2−ΔΔCt method.
RNA Interference and Transfection
The sequence of small interfering RNA (siRNA) targeting HMGB1 was designed by an RNA interference activity-predicting algorithm and synthesized by Eurogentec (Seraing, Belgium). The sequences HMGB1 siRNAs are as follows: 5′-GATCCGCTGAAAAGAGCAAGAAAATTTCAAGAGAATTTTCTTGCTCTTTTCAGCTTTTTGGAAG-3′ (sense); 5′-AGCTCTTCCAAAAAGCTGAAAAGAGCAAGAAAATTCTCTTGAAATTTTCTTGCTCTTTTCAGCG-3′ (antisense). One scrambled siRNA (Scramble II; MWG Biotech) was used as a control. After seeding and adherence for 24 h, BIU-87 and T24 cells were transfected in serum-free OptiMEM with 200 nM of HMGB1 siRNA using DOTAP liposomal transfection reagent according to the manufacturer’s instructions (Roche, Mannheim, Germany). Following transfection (4 h, 37°C), cells were washed with phosphate-buffered saline (PBS) and incubated in serum-containing medium for 72 h. For further analyses, cells were harvested by trypsin treatment (0.05% trypsin/0.02% EDTA, 5 min, 37°C). Detached and adherent cells were pooled and analyzed together. The expression levels of HMGB1 in siRNA-treated cells were examined by Western blotting.
Talen-Mediated Knockout of HMGB1 Gene
To investigate the role of HMGB1 in human BUC cells, a Talen Kit (Sidansai, Shanghai, China) was used to KO the HMGB1 gene in BIU-87 and T24 cells according to the manufacturer’s instructions. Briefly, we constructed plasmid pTalen-HMGB1 (pTalen-NC was used as negative control) and transfected it into BIU-87 and T24 cells, respectively, using Lipofection 2000. The expression levels of HMGB1 in Talen-mediated KO cells were examined by Western blotting using anti-HMGB1 monoclonal antibody (Epitomics, Burlingame, CA, USA).
Western Blotting
Cells were solubilized in cold RIPA lysis buffer. After that, samples were separated with 10% SDS-PAGE and then transferred to PVDF membranes, which were blocked in 10% nonfat dried milk in PBST for 3 h and then incubated overnight with the corresponding first antibody. After incubation with the secondary antibody, immune complexes were detected using the Enhanced Chemiluminescence Kit (GE Healthcare). Quantification was performed using Kodak 1D image analysis software V 3.6 (Fisher Scientific, Schwerte, Germany). Anti-β-actin monoclonal antibody was used as a control in all assays.
Cell Proliferation Assay
HMGB1 siRNA-treated cells, Talen-mediated HMGB1 KO BUC cells, and their corresponding negative control in exponential growth were plated at a final concentration of 5 × 103 cells per well in 96-well plates and incubated at 37°C, 5% CO2 for 0, 24, 48, and 72 h, respectively. To assess cell proliferation, 20 µl of MTT (5 mg/ml) reagent in PBS was added to each well and incubated for another 4 h. Then, the supernatant was removed, and 150 µl of DMSO was added to dissolve the crystal. Within 10 min after adding DMSO, the absorbance was detected at 570 nm with a Microplate Reader (Bio-Rad, USA). Each assay was performed in triplicate wells.
Scratch Assay
The migration of BUC cells was quantitatively assessed by scratch assay. A scratch through the central axis of the plate was gently made using a pipette tip when the cells were transfected for 4 h. Migration of the cells into the scratch was observed at time points of 24 h and 48 h.
Cell Invasion Assay
Invasive ability of siRNA-treated cells and Talen-mediated KO BUC cells was studied in 24-well Transwell chambers (Chemicon, USA), which have a layer of Matrigel. Cells in each group were resuspended in serum-free DMEM at a concentration of 5 × 104 cells/ml, and 500 µl suspension was added into the upper chamber. The bottom chamber was filled with 750 µl DMEM containing 10% FBS. After incubation for 24 h at 37°C, 5% CO2, cotton bud was used to remove the cells that were not through the polycarbonate membrane. Then, the cells transmembraned through the polycarbonate membrane and adhered to the bottom of it were stained with 0.1% crystal violet for 20 min and washed with PBS three times. After that, each well was added with 500 µl 10% ethanol to dissolve the dye on the polycarbonate membrane. Cells were transferred to a 96-well plate to measure the absorbance at 570 nm using a Microplate Reader. Each assay was performed in triplicate wells.
Apoptosis Assay
Double staining for annexin V-FITC and propidium iodide (PI) was performed to detect cell apoptosis as described previously (10). Briefly, after being washed with PBS twice, BUC cells were resuspended in 500 µl binding buffer (10 mM HEPES, 140 mM NaCl, 2 mM MgCl2, 5 mM KCl, and 2.5 mM CaCl2, pH 7.4). Then, 5 µl of FITC-conjugated annexin and 5 µl of PI (Annexin V-FITC Apoptosis detection kit; Immmunostep) were added according to the manufacturer’s protocol. After 15-min incubation in the dark at room temperature, another 400 µl of binding buffer was added, and the samples were immediately analyzed by flow cytometry. Data analysis was carried out using CellQuest software. Apoptotic cells are expressed as a percentage of total cells. The expressions of Bax and Bcl-2 were analyzed by Western blotting using anti-Bax polyclonal antibody (ImmunoWay, Newark, DE, USA) and anti-Bcl-2 polyclonal antibody (Abcam, Cambridge, UK).
Cell Cycle Analysis
Cells were suspended in PBS and then fixed in 70% ethanol at 4°C for 18 h. After being washed with PBS, cells were resuspended in staining solution (50 µg/ml of PI, 1 mg/ml of RNase A, and 0.1% Triton X-100 in PBS). The stained cells (1 × 105) were then analyzed with flow cytometry. The percentage of cells in each phase of the cell cycle was determined using a Multicycle Cell-Cycle Analysis program. The expressions of cyclin D1 and PCNA were analyzed by Western blotting using anti-cyclin D1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-PCNA polyclonal antibody (Santa Cruz Biotechnology).
Cisplatin Treatment and Cell Viability Assay
Cells were seeded on 96-well plates at a density of 5 × 103 cells per well. Cisplatin was added to cells at a final concentration of 20 mM and incubated at 37°C, 5% CO2 for 24 h. Then cells were incubated in drug-free medium for an additional 0, 24, 48, and 72 h, respectively. Cell viability after cisplatin treatment was assessed by CCK-8 kit (Dojindo, Tokyo, Japan). Briefly, 10 µl of CCK-8 (5 mg/ml) solution was added to each well for 30 min, and absorbance was measured at 450 nm using a Microplate Reader. The cell viability in each group was expressed as a percentage of that in control cells. The assay was repeated three times. Meanwhile, the protein expression levels of LC3 I/II and Beclin 1 were detected by Western blotting using anti-Beclin 1 polyclonal antibody (Epitomics) and anti-LC3 polyclonal antibody (Abcam).
Statistical Analysis
Data are expressed as the means ± SD from at least three separate experiments. Statistical analysis was carried out using SPSS 15.0 software. The difference between two groups was analyzed by the Student’s t-test. A value of p < 0.05 was considered to indicate a statistically significant result.
RESULTS
The HMGB1 Expression Level in BUC Cells Is Much Higher Than in Normal Human Urethra Epithelial Cells
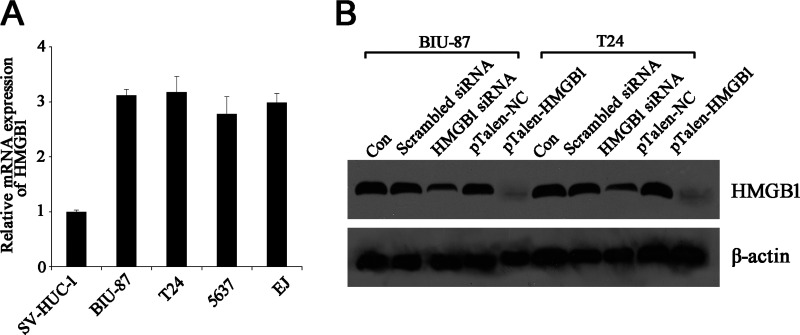
HMGB1 is known to be expressed in all eukaryotic cells and to be overexpressed in tumor cells. Therefore, four BUC cell lines were evaluated for expression of this “danger signal.” The quantitative RT-PCR (qRT-PCR) results demonstrated that mRNA levels of HMGB1 in all four BUC cell lines were significantly higher (around three times higher) than that in normal urethra epithelial cell line (Fig. 1A). Subsequently, the expression of HMGB1 was further analyzed by Western blotting using anti-HMGB1 monoclonal antibody, the result of which showed a similar trend as that of mRNA levels (data not shown). As BIU-87 and T24 cells exhibited higher expression of HMGB1, they were chosen as the targets for the downstream experiments.
Figure 1.
Expression of HMGB1 in human BUC cell lines. (A) Analysis of relative expression of HMGB1 mRNA in human BUC cell lines and normal urethra epithelial cell line using qRT-PCR. (B) Western blotting assay of HMGB1 expression in knockdown and knockout BUC cell lines BIU-87 and T24. β-Actin was used as an internal control.
The differences in HMGB1 expression between BUC cells and normal urethra epithelial cells prompted us to investigate the role of HMGB1 in BUC cells. Consequently, RNA interference and Talen-mediated gene KO were used to knockdown or KO HMGB1 in BUC cell lines BIU-87 and T24. Western blotting results clearly demonstrated that the expression levels of HMGB1 in siRNA-treated cells were dramatically decreased, while in Talen-mediated KO cells, the expression of HMGB1 was barely detectable (Fig. 1B). This suggested that the expression of HMGB1 had been successfully knocked down or knocked out in BUC cell lines BIU-87 and T24.
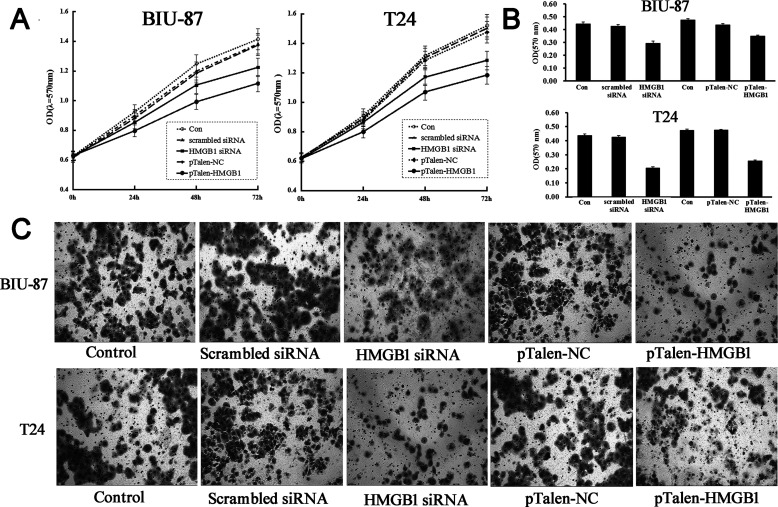
HMGB1 Knockdown/Out Inhibits Proliferation, Migration, and Invasion of BUC Cells
The effect of HMGB1 knockdown/out on proliferation of BUC cells was tested by MTT assay. The data showed that both knockdown and KO BUC cells showed a lower proliferative rate compared with that in control groups (Fig. 2A), which suggests that HMGB1 plays a positive role in the proliferation of BUC cells. Scratch assay demonstrated that the cell migration of HMGB1 knockdown and KO BUC cells was significantly decreased compared with the control group, indicating that HMGB1 plays a positive role in the regulation of migration in BUC cells (Fig. 2B). We next investigated the effect of HMGB1 knockdown/out on invasion of BUC cells. It demonstrated that the invasive capabilities of both HMGB1 knockdown/out BUC cells were significantly decreased compared with the control group (Fig. 2C, D), suggesting that the expression of HMGB1 can promote the invasion of BUC cells.
Figure 2.
The effects of HMGB1 knockdown/out on proliferation and invasion of human BUC cells. (A) MTT assay data showed that HMGB1 knockdown/out suppressed cellular proliferation in both BIU-87 and T24 cells. (B) Scratch assay data showed that HMGB1 knockdown/out notably suppressed cellular migration in BUC cells. (C, D) Transwell assay data showed that HMGB1 knockdown/out notably suppressed cell invasion in both BIU-87 and T24 cells. Control, BUC cells without any transfection; scrambled siRNA, BUC cells transfected with scrambled siRNA; HMGB1 siRNA, BUC cells transfected with HMGB1 siRNA; pTalen-NC, BUC cells transfected with blank vector; pTalen-HMGB1, BUC cells transfected with pTalen-HMGB1.
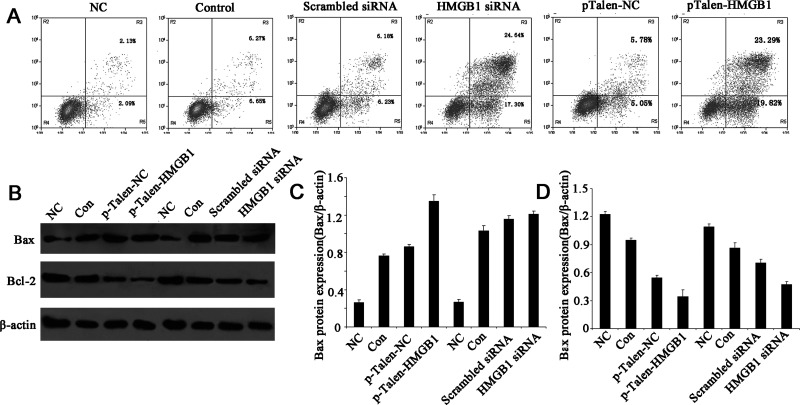
HMGB1 Knockdown/Out Induces Apoptosis via Bax/Bcl-2 in Human BUC Cell Lines
To determine whether the decrease in cell viability caused by HMGB1 knockdown/out was due to an increase in apoptosis, we determined the number of early apoptotic cells in BUC cell lines with annexin V-FITC and PI labeling followed by fluorescence-activated cell sorting (FACS). The data showed that after siRNA transfection for 48 h, the number of apoptotic cells was increased significantly in both BIU-87 and T24 cells compared with control groups (Fig. 3A). Similar results were also observed in Talen-mediated HMGB1 KO cells (Fig. 3A). Thus, it indicated that the decrease in cell viability caused by HMGB1 knockdown/out was due to an increase in apoptosis.
Figure 3.
The effects of HMGB1 knockdown/out on apoptosis in human BUC cells. (A) Flow cytometric analysis of BUC apoptotic cells after annexin V-FITC/PI staining. (B) Western blotting analysis of the expressions of Bcl-2 and Bax proteins. (C, D) The relative expression levels of Bax and Bcl-2 proteins. The expression of Bcl-2 was noticeably decreased in HMGB1 knockdown/out BUC cells, while the expression of Bax protein was steadily increased. β-Actin was used as an internal control.
We then determined the protein levels of the important molecules involved in this apoptotic pathway by Western blotting. The expression level of Bax (Fig. 3B, C) was increased, whereas the expression level of Bcl-2 (Fig. 3B, D) was decreased in both HMGB1 knockdown and KO BUC cell lines.
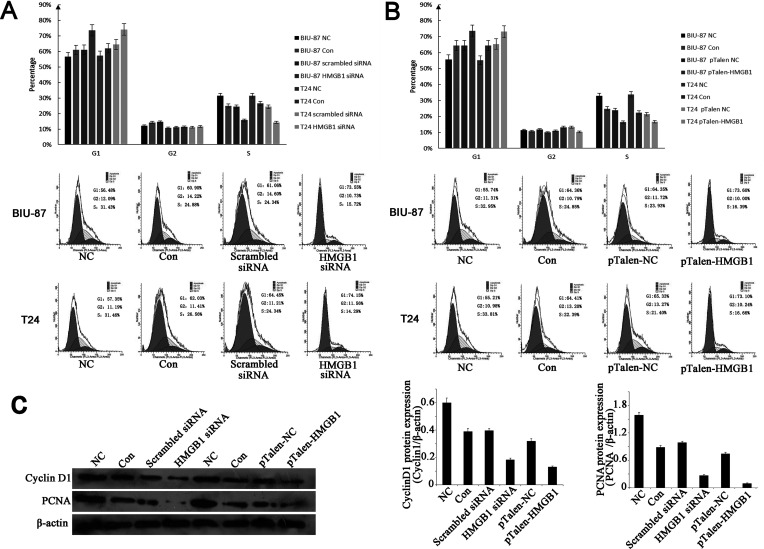
HMGB1 Knockdown/Out Inhibits the Cell Cycle G1/S Transition of Human BUC Cells
We then analyzed the effects of HMGB1 knockdown/out cell cycle of T24 BUC cell line by using flow cytometry. The data showed that HMGB1 knockdown/out significantly enhanced the cell proportion in G1 phase but reduced the cell proportion in S phase (Fig. 4A, B). This indicated that HMGB1 knockdown/out could inhibit the cell cycle G1/S transition.
Figure 4.
The effects of HMGB1 knockdown/out on cell cycle distribution in human BUC cells. (A) HMGB1 knockdown increased the percentages of G1 phase cells in BUC cell lines. (B) HMGB1 knockout increased the percentages of G1 phase cells in BUC cell lines. (C) Effect of HMGB1 knockdown/out on the expression of cyclin D1 and PCNA proteins. Total cellular proteins were prepared, and the expressions of cyclin D1 and PCNA proteins were analyzed using Western blotting. The expressions of cyclin D1 and PCNA proteins were noticeably decreased in HMGB1 knockdown/out BUC cells. β-Actin was used as an internal control.
As HMGB1 knockdown/out induced cell cycle arrest, we investigated the expression of G1/S phase-related molecules, particularly PCNA and cyclin D1 by using Western blotting, both of which were significantly lower in HMGB1 knockdown/out BUC cells, compared to controls (Fig. 4C).
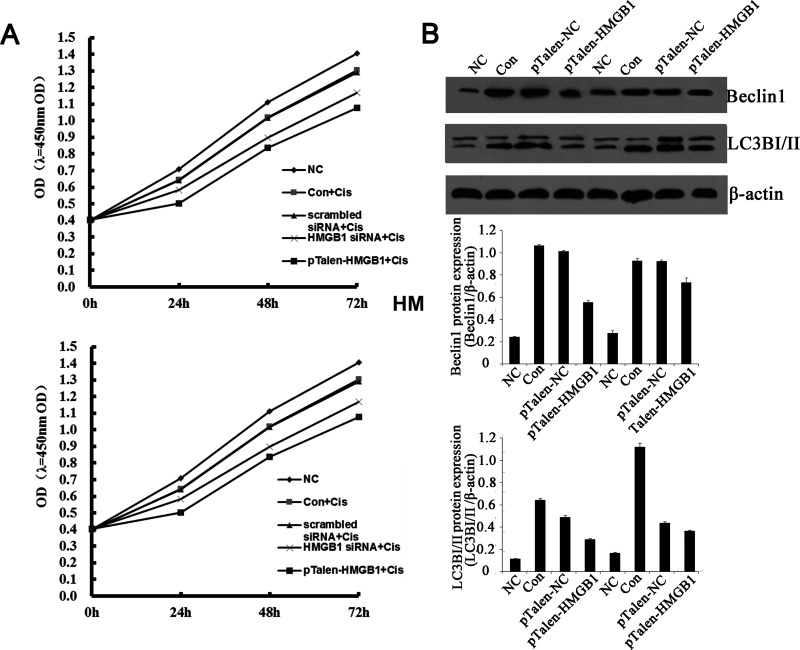
HMGB1 Knockdown/Out Renders Human BUC Cells More Sensitive to Cisplatin Probably Because of the Decreased Levels of Autophagy
Cisplatin is one of the most widely used anticancer drugs for the treatment of a variety of human malignancies. HMGB1 has been linked to cisplatin activity in a number of studies. Whether knockdown/out of HMGB1 might affect cisplatin sensitivity in BUC cells aroused our great interest. First, we assayed the effect of cisplatin on the expression of HMGB1, which exhibited significantly enhanced expression of HMGB1 in BUC cell lines BIU-87 and T24 after treatment with 20 mM cisplatin for 24 h (Fig. 5A). Real-time PCR revealed that HMGB1 mRNA was increased after treatment with cisplatin (data not shown). These findings show that HMGB1 is upregulated during chemotherapy in BUC cells. CCK assay demonstrated that knockdown or KO of HMGB1 in BUC cells rendered them significantly more sensitive to cisplatin (Fig. 5A), suggesting that HMGB1 increases the resistance of BUC cells to an anticancer agent.
Figure 5.
The effects of HMGB1 knockdown/out on the sensitivity of human BUC cells to cisplatin. (A) CCK assay demonstrated the effect of HMGB1 knockdown/out on BUC cells sensitive to cisplatin. (B) Effect of HMGB1 knockdown/out on the expression of Beclin 1 and LC3 proteins after cisplatin treatment. Upper panel: Western blotting analysis of the expressions of Beclin 1 and LC3 proteins. Middle panel: the relative expression levels of Beclin 1 protein. Lower panel: the relative expression levels of LC3 protein. The expressions of Beclin 1 and LC3 proteins were noticeably decreased in HMGB1 knockdown/out BUC cells after cisplatin treatment. β-Actin was used as an internal control.
Previous studies demonstrated that autophagy was crucial for cancer cells conferring resistance to chemotherapy, radiation therapy, and immunotherapy. HMGB1 was reported to be an important regulator of autophagy (11). We then detected microtubule-associated protein light chain 3 (LC3) conversion (LC3-I/LC3-II) by immunoblot analysis. When compared with normal urethra epithelial cells, the expression of LC3-II expression in both BUC cell lines was dramatically increased (around fivefold) after treatment with cisplatin. The expression of LC3-II in HMGB1 knockdown/out BUC cells after treatment with cisplatin was also elevated; however, the increased levels were much slighter (Fig. 5B).
As it was reported that Beclin 1 was important for the localization of autophagic proteins to phagophore assembly sites, which are required for the initiation of the formation of the autophagasome (12), we then detected the expression of Beclin 1 by Western blotting. The downregulation of Beclin 1 expression was observed in both HMGB1 knockdown/out BUC cell lines (Fig. 5B).
DISCUSSION
In this study, we found that HMGB1 was upregulated in BUC cells compared with their matched normal cells. More important, the level of HMGB1 was correlated with proliferative activities, invasion potential, and chemotherapy sensitivity. Taken together, the data suggested that HMGB1 may play a potential role in the progression of human bladder cancer.
The HMGB1 pathway is closely associated with tumorigenesis, expansion, and invasion of multiple cancers, and plays a critical role in the development and progression of many malignant tumors (7). The relation of HMGB1 overexpression with lymph node metastasis presence and advanced stage in hepatocellular carcinoma, head–neck and esophagus squamous cell carcinoma, cervix uteri, and ovary carcinoma was demonstrated. It was reported that HMGB1 expression was significantly increased in bladder cancer compared with normal bladder tissues, and it was also closely correlated with pathological grade and distant metastasis (2). To study the molecular mechanisms of HMGB1 in BUC cells, RNA interference and Talen-mediated gene KO were used to constructed HMGB1 knockdown and KO BUC cell lines, respectively. Since Talen is a very popular gene KO technology, we used it to construct a HMGB1 KO BUC cell line to compare with the data obtained from the HMGB1 knockdown BUC cell line, which was constructed by conventional siRNA interference. There was no clue why sometimes the KO BUC cell line did not show as good results as the knockdown BUC cell line. However, data obtained from HMGB1 knockdown and KO BUC cell lines did not show a statistically significant difference. The present data showed that knockdown or KO of HMGB1 gene inhibited the proliferative activities and invasion potential via induction of apoptosis and G1 cell cycle arrest in BUC cells.
One of the major determinants of cell survival is the balance between the antiapoptotic (Bcl-2, Bcl-XL, and Mcl-1) and proapoptotic members (Bid, Bax, and Bad) of the Bcl-2 family (13). In the Bcl-2 protein family, proapoptotic member Bax and antiapoptotic member Bcl-2 are the active effectors and regulators, and the ratio between Bcl-2 and Bax affects apoptosis induction (14). We evaluated Bcl-2 and Bax after HMGB1 knockdown/out and found that Bax increased sharply and Bcl-2 acted in the opposite way when compared with those of controls. These findings suggest that the Bcl-2 and Bax are involved in apoptosis in HMGB1 knockdown/out BUC cells.
The G1/S transition is one of the two main checkpoints used by cells to regulate the cell cycle progress and thus the cell proliferation. G1/S progression is highly regulated by cyclin D1 and cyclin-dependent kinase 4 (CDK4) (15). Cyclin D1 is the major G1 phase cyclin and is overexpressed in most cancer cells (16). PCNA is an acidic nuclear protein that has been recognized as a histological marker for the G1/S phase in the cell cycle (17). Therefore, the expression of PCNA and cyclin D1 can reflect the proliferation state of BUC cells to some extent. We found that the expressions of cyclin D1 and PCNA were significantly increased in the BUC cells, which, however, could be significantly inhibited by HMGB1 knockdown/out.
Moreover, our experimental data demonstrated that HMGB1 knockdown/out significantly enhanced chemotherapy sensitivity, suggesting that HMGB1 might be considered as a potential therapeutic target for bladder cancer. Our results were in line with previous prospective studies that knockdown of HMGB1 by RNA interference increased the sensitivity of leukemia cells to chemotherapeutic agents. HMGB1 contributed to chemotherapy resistance though the upregulation of autophagy in leukemia (18). Autophagy has been recognized as a programmed cell survival mechanism that is involved in the response to cytotoxic chemotherapy or irradiation and is responsible for drug resistance (19). Since LC3 and Beclin 1 are involved in the formation of autophagosomes, the expression of both markers was examined by Western blot. During the process of autophagy, LC3 modification from the cytosolic form, LC3-I, to a lipidated, membrane bound form, LC3-II, are required. LC3-II is integrated into membranes of preautophagosomes and autophagosomes. Therefore, LC3 serves mostly as a molecular autophagosomal marker. Previous studies reported that HMGB1 directly regulated autophagy in leukemia cells by controlling LC3 conversion (LC3-I/LC3-II) (20). Beclin 1 was also shown to be a marker of autophagosomes. Beclin 1 is the mammalian ortholog of the yeast Atg 6 and a component of the class III PI3 kinase complex (PI3KC3), which is involved in the autophagosome formation. Overexpression of Beclin 1 promotes autophagy, and its underexpression prevents cell death (21). A recent study demonstrated that endogenous HMGB1 regulates autophagy by directly interacting with the autophagy protein Beclin 1 and displacing Bcl-2, supporting the role of HMGB1 in autophagy (22). Here we found that knockdown or KO of HMGB1 decreased the expression levels of LC3-II and Beclin 1, which reflects the decreased levels of autophagy.
In summary, we found that HMGB1 was upregulated in BUC cell lines. Knockdown/out of HMGB1 suppressed cell growth, migration, and induced cell apoptosis. In addition, knockdown/out of HMGB1 rendered BUC cells more sensitive to the anticancer drug cisplatin. All these novel findings provide the first evidence that HMGB1 exhibits tumor-promoting activity in human BUC cell lines. Our results give new insights into the function of HMGB1 in the development and progression of bladder cancer and provide novel targets for therapy of this malignancy.
REFERENCES
- 1. Jemal A.; Bray F.; Center M. M.; Ferlay J.; Ward E.; Forman D. Global cancer statistics. CA Cancer J. Clin. 61:69–90; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Yang G. L.; Zhang L. H.; Bo J. J.; Huo X. J.; Chen H. G.; Cao M.; Liu D. M.; Huang Y. R. Increased expression of HMGB1 is associated with poor prognosis in human bladder cancer. J. Surg. Oncol. 106:57–61; 2012. [DOI] [PubMed] [Google Scholar]
- 3. Han Y.; Chen J.; Zhao X.; Liang C.; Wang Y.; Sun L.; Jiang Z.; Zhang Z.; Yang R.; Chen J. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PloS. One 6:e18286; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianchi M. E.; Agresti A. HMG proteins: Dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15:496–506; 2005. [DOI] [PubMed] [Google Scholar]
- 5. Štros M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 1799:101–113; 2010. [DOI] [PubMed] [Google Scholar]
- 6. Yanai H.; Ban T.; Wang Z.; Choi M. K.; Kawamura T.; Negishi H.; Nakasato M.; Lu Y.; Hangai S.; Koshiba R. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99–103; 2009. [DOI] [PubMed] [Google Scholar]
- 7. Wild C. A.; Brandau S.; Lotfi R.; Mattheis S.; Gu X.; Lang S.; Bergmann C. HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral. Oncol. 48:409–416; 2012. [DOI] [PubMed] [Google Scholar]
- 8. Chung H. W.; Lee S. G.; Kim H.; Hong D. J.; Chung J. B.; Stroncek D.; Lim J. B. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J. Transl. Med. 7:38–48; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong Y. D.; Cui L.; Peng C. H.; Cheng D. F.; Han B. S.; Huang F. Expression and clinical significance of HMGB1 in human liver cancer: Knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol. Rep. 29:87–94; 2013. [DOI] [PubMed] [Google Scholar]
- 10. Baskić D.; Popović S.; Ristić P.; Arsenijević N. N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 30:924–932; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Tang D.; Kang R.; Livesey K. M.; Cheh C. W.; Farkas A.; Loughran P.; Hoppe G.; Bianchi M. E.; Tracey K. J.; Zeh H. J. Endogenous HMGB1 regulates autophagy. J. Cell. Biol. 190:881–892; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang X. H.; Jackson S.; Seaman M.; Brown K.; Kempkes B.; Hibshoosh H.; Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676; 1999. [DOI] [PubMed] [Google Scholar]
- 13. Xu C.; Wu A.; Zhu H.; Fang H.; Xu L.; Ye J.; Shen J. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomed. Pharmacother. 67:133–139; 2013. [DOI] [PubMed] [Google Scholar]
- 14. Duo J.; Ying G. G.; Wang G. W.; Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 5:1453–1456; 2012. [DOI] [PubMed] [Google Scholar]
- 15. Bertoli C.; Skotheim J. M.; de Bruin R. A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell. Biol. 14:518–528; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jirawatnotai S.; Hu Y.; Michowski W.; Elias J. E.; Becks L.; Bienvenu F.; Zagozdzon A.; Goswami T.; Wang Y. E.; Clark A. B. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature 474:230–234; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao H.; Lo Y. H.; Ma L.; Waltz S. E.; Gray J. K.; Hung M. C.; Wang S. C. Targeting tyrosine phosphorylation of PCNA inhibits prostate cancer growth. Mol. Cancer Ther. 10:29–36; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L.; Yang M.; Kang R.; Wang Z.; Zhao Y.; Yu Y.; Xie M.; Yin X.; Livesey K.; Lotze M. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia 25:23–31; 2011. [DOI] [PubMed] [Google Scholar]
- 19. Yang Z. J.; Chee C. E.; Huang S.; Sinicrope F. A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 10:1533–1541; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez J.; Almendinger J.; Oberst A.; Ness R.; Dillon C. P.; Fitzgerald P.; Hengartner M. O.; Green D. R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 108:17396–17401; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang R.; Zeh H.; Lotze M.; Tang D.; The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18:571–580; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang R.; Livesey K. M.; Zeh H. J.; Lotze M. T.; Tang D. HMGB1: A novel Beclin 1-binding protein active in autophagy. Autophagy 6:1209–1211; 2010. [DOI] [PubMed] [Google Scholar]