Abstract
The purpose of this study was to assess the effects of NBPF1 expression on cervical cancer cell invasion and apoptosis and to illustrate its potential mechanism. Human cervical cancer HeLa cells were transfected with the constructed siNBPF1 or pcDNA3.1-NBPF1 vectors. Effects of NBPF1 expression on cell invasion ability and cell apoptosis were analyzed using the Matrigel method and an Annexin V-FITC cell apoptosis kit, respectively. In addition, cell apoptosis-related proteins involved with the PI3K/mTOR signaling pathway were analyzed using Western blot. Remediation experiments were conducted to verify the effects of NBPF1 expression on cell invasion and apoptosis. Compared to the control, mRNA and protein expressions of NBPF1 were significantly decreased when cells were transfected with siNBPF1 (p < 0.05), which was contrary to the results of cells transfected with pcDNA3.1-NBPF1. Overexpression of NBPF1 significantly suppressed HeLa cell invasion but promoted cell apoptosis (p < 0.05). Overexpression of NBPF1 performed a significant inhibitory role on PI3K/mTOR signal pathway expression, while NBPF1 was silenced, showing contrary results. Our data suggested that NBPF1 overexpression may be a suppressor for cervical cancer via affecting cell invasion and apoptosis through regulating PI3K/mTOR signaling pathway. NBPF1 may be a potential therapeutic target for cervical cancer treatment.
Key words: Cervical cancer, Neuroblastoma breakpoint family member 1 (NBPF1), Cell apoptosis, Cell invasion, PI3K/mTOR pathway
INTRODUCTION
Cervical cancer is one of the most common malignancies among females, which has become a huge burden worldwide (1). Mortality for cervical cancer is high, and its incidence is second only to breast cancer among malignancies in females (2). Previous achievements in cervical cancer treatments were mainly surgery and chemotherapy. While surgery was reserved for patients who were diagnosed with cervical cancer at an early stage, cervical cancer patients with mid- or late stage disease could only receive chemotherapy treatment, which exerted huge side effects on their bodies (3,4). So far, there is no cure for cervical cancer in the clinical setting due to its complicated pathogenesis. Therefore, it is necessary to explore its mechanism for pathogenesis and to find several methods that would be effective in cervical cancer treatment.
Previous evidence has inferred that there are a variety of factors involved in cervical cancer pathogen mechanism such as human papillomavirus (HPV) infection, miRNAs, nutritional factors, biological factors, and immune and cancer-related genes. For instance, HPV has been considered to be the dominant sector for cervical cancer progression, and several new HPV types have been reported to be associated with cervical cancer development (5). TGF-β promotes cervical cancer by activating a signal pathway through suppressing the cell cycle at G1 stage (6).
Neuroblastoma breakpoint family member 1 (NBPF1) is a member of the NBPF family of proteins that consists of dozens of recently duplicated genes primarily located in segmental duplications on human chromosome 1 (7). NBPF1 has been implicated in a number of developmental and neurogenetic diseases such as microcephaly, macrocephaly, autism, schizophrenia, mental retardation, congenital heart disease, neuroblastoma, and congenital kidney and urinary tract anomalies (8,9). Recently, numerous evidence has reported that altered expression of NBPF1 was associated with several types of cancer pathogenesis, such as gastric cancer and neuroblastoma (10,11). Moreover, a recent article reported that NBPF1 acts as a tumor suppressor in neuroblastoma through inducing G1 cell cycle arrest (12). Although NBPF1 has been implicated in several kinds of diseases, there is no report on the association of NBPF1 in cervical cancer.
In this study, we used the human cervical cell line of HeLa cells to assess the effects of NBPF1 expression on cancer cell invasion and apoptosis based on the gene silencing method. Comprehensive experimental methods were used to analyze cell apoptosis, cell invasion, and cell apoptotic-related protein expressions in HeLa cells. This study aimed to investigate the potential mechanism of NBPF1 in cervical cancer development and progression. Our study may provide a basis for illustrating the potential pathogenic mechanism for cervical cancer and for the therapeutic possibility of NBPF1 in cervical treatment application.
MATERIALS AND METHODS
Cell Culture and siRNA Transfection
Human cervical cancer cell line HeLa cells (purchased from the Institute of Immunology of Medicine, Shanghai Jiaotong University) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic (Invitrogen, USA) in a saturation of 5% CO2 at 37°C.
The NBPF1 expression vector of pcDNA3.1-NBPF1 was constructed based on subcloning the full-length wild-type NBPF1 coding sequence into pcDNA3.1 (Sangon Biotech, Shanghai, China). The empty pcDNA3.1 plasma was transfected into HeLa cells as a control group. In addition, NBPF1-siRNA vector was constructed by Sangon Biotech (Shanghai). siRNA with no silencing NBPF1 target sequence was synthesized by (Sangon Biotech) and transfected into cells for the control. Cell transfections were conducted with Lipofectamine 2000 reagent (Invitrogen) based on the manufacturer’s protocol. G418 (Sigma-Aldrich, USA) was used for stable NBPF1 transfectant selection.
Cell Invasion Assay
The invasion ability of HeLa cells transfected with siNBPF1 or pcDNA3.1-NBPF1 plasmas was measured using the Matrigel method as previously described (13). Briefly, cells were cultured in six-well plates at the density of 5 × 105 per well and cultured for 48 h, followed by replacement of fresh serum-free RPMI-1640 medium containing FBS for 24 h. The upper layer of a Transwell was enveloped with serum-free RPMI-1640 medium supplemented with 50 mg/L Matrigel and then air dried at 4°C. After being sucked out of the medium, 50 µl fresh serum-free medium containing 10 g/L BSA was added and then cultured for 30 min at 37°C. After that, the Transwell was put into the 24-well plates and cultured with DMEM (Dulbecco’s modified Eagle medium) medium (Sigma-Aldrich, USA) mixed with 10% FBS. The cells in the Transwell were suspended with serum-free DMEM medium. After 48 h, the Transwell in each group was washed with PBS buffer to remove the upper cells on microporous membrane and then fixed in ice-cold alcohol. Finally, the Transwell from each group was stained with 0.1% crystal violet for 30 min and then decolorized with 33% acetic acid. The absorbance of eluents was observed at OD 570 nm using a microplate reader (Biotech, USA). The Transwell in the control group was treated without Matrigel.
Cell Apoptosis Assay
Apoptosis of HeLa cells that were transfected with siNBPF1 or pcDNA3.1-NBPF1 vectors was detected using an Annexin V-FITC cell apoptosis kit (Invitrogen) based on the manufacturer’s protocol (14). Briefly, HeLa cells were cultured for 36 h, followed with the replacement of cell medium with serum-free RPMI-1640. Cells were then harvested and washed with PBS buffer (pH 7.4) three times and then were resuspended in the staining buffer. Consequently, 5 µl of Annexin V-FITC and 5 µl of propidium iodide (PI) were mixed with the cells. After 10 min of cultivation at room temperature, cells were detected with the FACScan flow cytometry (BD, USA). Annexin V-positive and propidium iodide-negative cells were considered to be apoptotic cells.
RT-PCR
mRNA expressions of NBPF1 or the other proteins in HeLa cells were measured using RT-PCR analysis as previously described (15). Procedures were as follows. The HeLa cells were collected at 48 h and were ground in lysis buffer. Total RNA from HeLa cells was isolated using a TRIzol extraction reagent (Invitrogen) as previously described (16), followed with the addition of RNase-free DNase I (Promega, Biotech) to remove the mixed DNA. Concentration and purity of extracted RNA were detected with SMA4000 UV-VIS (Merinton, Shanghai, China) at 260 nm. Purified RNA of 0.5 µg/µl was used for cDNA synthesis with the PrimerScript 1st Strand cDNA Synthesis Kit (Invitrogen). Expressions of mRNA were measured using SYBR green-based quantitative RT-PCR (Invitrogen, USA). The total reaction system of 20 µl volume containing 1 µl cDNA from the above PCR, 10 µl SYBR Premix EX Taq, 1 µl each of the primers (10 µM), and 7 µl ddH2O. PCR reaction was carried out at 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one product was amplified and detected. Phosphoglyceraldehyde dehydrogenase (GAPDH) was chosen as the internal control. Primers used for target amplification were as follows: NBPF1 sense primer: 5′-GCGAGGCTGCCCGAGCTTCT-3′ and antisense primer: 5′-GACTTCGCGTAACTTCCCATTCA-3′; and GAPDH sense primer: 5′-GGGTGGAGCCAAACGGGTC-3′, and antisense primer: 5′-GGAGTTGCTGTTGAAGTCGCA-3′.
Western Blotting
HeLa cells incubated at 48 h in each group were lapped with radioimmunoprecipitation buffer (RIPA; Sangon Biotech) containing phenylmethanesufonyl fluoride (PMSF; Sigma-Aldrich), followed by centrifugation at 12,000 rpm for 10 min at 4°C. Supernatant was collected for the detection of protein concentrations using BCA protein assay kit (Pierce, Rockford, IL, USA). For Western blot analysis (17), 30 µg protein per cell lysate was subjected onto a 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidencefluoride (PVDF) membrane (Millipore). The PVDF membrane was blocked in Tris-buffered saline Tween (TBST) containing 5% nonfat milk for 1 h. After that, the membrane was incubated with rabbit anti-human antibodies (mTOR, PI3K, P70, 1:100 dilution; Invitrogen) and then overnight at 4°C. The membrane was then incubated with horseradish peroxidase-labeled goat anti-rat secondary antibody (1:1,000 dilution) at room temperature for 1 h. Finally, PVDF membrane was washed with 1× TBST buffer for 10 min three times. Detection was conducted using the development of X-ray after chromogenic substrate with an enhanced chemiluminescence (CEL) method. Additionally, GAPDH served as the internal control.
Statistical Analysis
All experiments were conducted three times independently in this study. Total data were presented as mean ± standard deviation (SD). Differences between the two groups were calculated using one-way analysis of variance (ANOVA). Statistical analysis was performed using graph prism 5.0 software (GraphPad Prism, San Diego, CA, USA). A value of p < 0.05 was considered as statistically significant.
RESULTS
NBPF1 Expression in Cervical Cancer HeLa Cell Lines
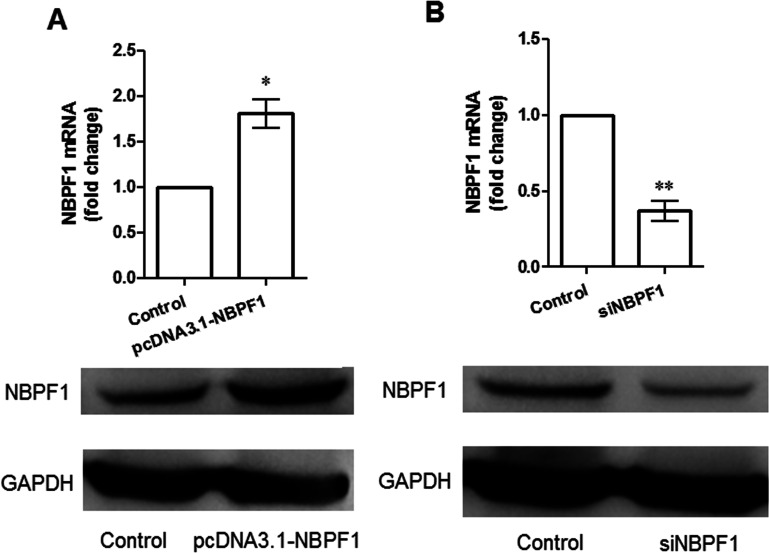
Effects of NBPF1 expression on its self-expression in HeLa cells were analyzed using RT-PCR and Western blot (Fig. 1). When HeLa cells were transfected with pcDNA-NBPF1, mRNA level and protein level of NBPF1 were both significantly increased compared with the control group (p < 0.05) (Fig. 1A). Otherwise, when cells were transfected with siRNA-NBPF1 plasma, its expression significantly declined compared with the control group (p < 0.01) (Fig. 1B).
Figure 1.
Expression of NBPF1 in HeLa cells transfected with different plasmas. (A) mRNA and protein expressions of NBPF1 were increased when cells were transfected with pcDNA-NBPF1. (B) mRNA and protein expressions of NBPF1 were decreased when cells were transfected with siRNA-NBPF1. *p < 0.05, compared with the control; **p < 0.01, compared with the control group.
Effects of NBPF1 Expression on Cell Migration
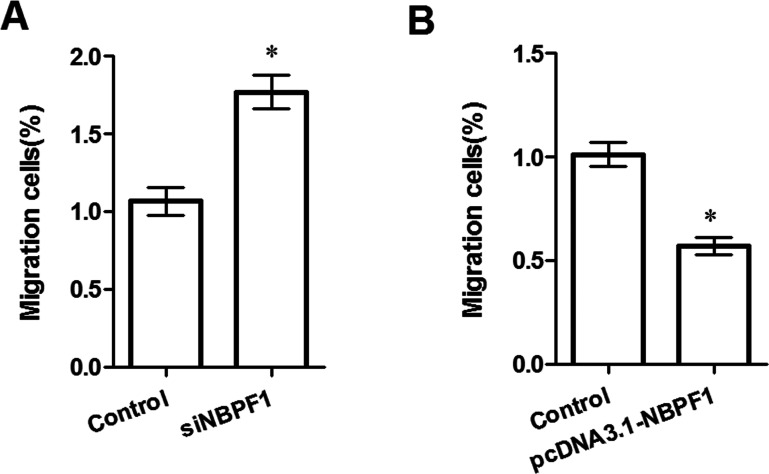
The Matrigel method was used to assess whether or not NBPF1 expression could influence HeLa cell migration ability (Fig. 2). The results showed that silenced NBPF1 could significantly enhance HeLa cell migration ability when compared to the control group (p < 0.05) (Fig. 2A), while NBPF1 overexpression significantly decreased the migrated HeLa cells (p < 0.05) (Fig. 2B), indicating that NBPF1 expression may be correlated to HeLa cell migration ability.
Figure 2.
Effects of NBPF1 expression on cell migration ability. (A) Downregulation of NBPF1 increased cell migration. (B) NBPF1 overexpression suppressed cell migration ability. *p < 0.05, compared with the control.
Effects of NBPF1 Expression on Cell Apoptosis
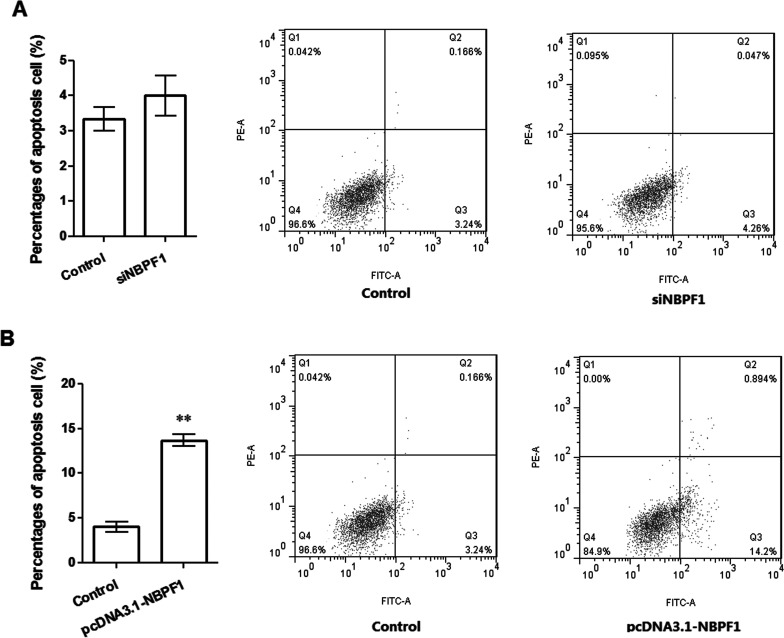
Flow cytometry was used to assess the effects of NBPF1 expression on cell apoptosis (Fig. 3). When NBPF1 was silenced, apoptotic cells in the siNBPF1 group were slightly increased with the percentage of 3.24%, but there was no significant difference between the two groups (Fig. 3A). On the other hand, when NBPF1 was overexpressed, apoptotic cells in the experimental group were significantly increased, to 14.2%, compared with the control (3.24%, p < 0.05) (Fig. 3B). These results suggested that there may be a correlation between NBPF1 overexpression and cell apoptosis.
Figure 3.
Influence of NBPF1 expressions on cell apoptosis. (A) Silenced NBPF1 had no significant influence on cell apoptosis. (B) NBPF1 overexpression promoted cell apoptosis. **p < 0.01, compared with the control group.
Remediation Experiments
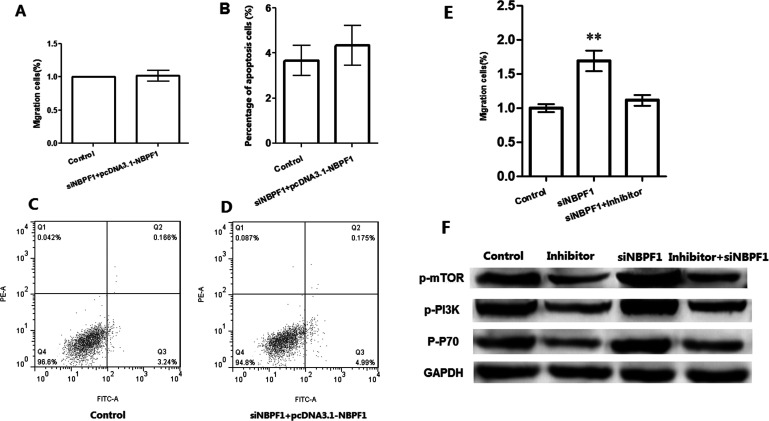
In order to verify the effects of NBPF1 on cell migration and apoptosis, siNBPF1 and pcDNA-NBPF1 were both transfected in HeLa cells (Fig. 4). The results showed that when cells were transfected with either siNBPF1 or with pcDNA3.1-NBPF1, there was no significance for cell migration between the two groups with the addition of rescue plasma of pcDNA3.1-NBPF1/siNBPF1 (Fig. 4A), or with cell apoptosis (Fig. 4B–D), indicating that coexpression of silenced NBPF1 and overexpression had no significant effects on cell migration and apoptosis. In addition, silenced NBPF1 was inhibited with the addition of inhibitor in HeLa cells, along with the decreasing migrating cells in the inhibitor group (Fig. 4E). Also, cell apoptotic-related proteins such as phosphorylated mammalian target of rapamycin (p-mTOR), phosphorylated phosphoinositide 3-kinase (p-PI3K), and p-P70 were decreased with silenced NBPF1 but were increased when silenced NBPF1 was inhibited (Fig. 4F).
Figure 4.
Remediation experiments of NBPF1 expression on cell biological process. (A) Coexpression of silencing and overexpression of NBPF1 had no significant effects on cell migration. (B) Coexpression of silenced and overexpression of NBPF1 had no significant effects on cell apoptosis. (C, D) Cell apoptosis in control and experimental group. (E) Migrating cells in each group when siNBPF1 expression was inhibited by inhibitor. (F) Cell apoptosis-related protein expression in each group; **p < 0.01, compared with the control group.
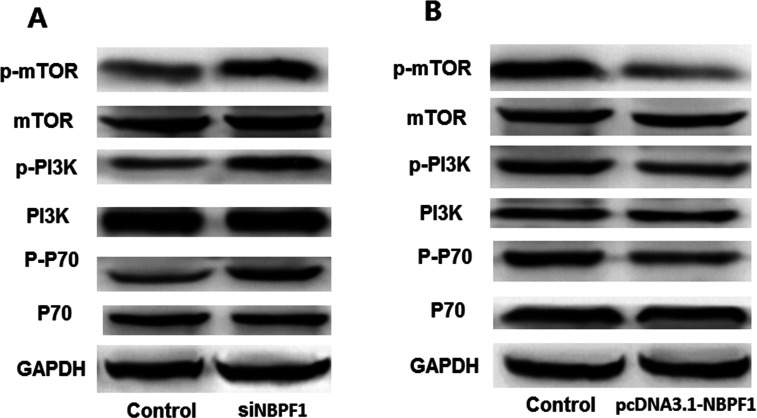
Effects of NBPF1 Silencing on Cell Apoptotic-Related Proteins
In order to assess the potential mechanism of NBPF1 expression on HeLa cell migration and apoptosis, the cell apoptotic-related proteins were analyzed using Western blotting (Fig. 5). As the results show, silenced NBPF1 increased the expression of p-mTOR, p-PI3K, and p-P70 in cells compared to the control group (Fig. 5A). By contrast, when cells were transfected with pcDNA3.1-NBPF1, overexpression of NBPF1 decreased the expressions of p-mTOR, p-PI3K, and p-P70 (Fig. 5B). These results suggested that NBPF1 expression may be correlated to HeLa cell apoptosis through affecting the cell apoptotic-related protein expression in PI3K/mTOR pathway.
Figure 5.
Effects of NBPF1 expression on cell apoptotic-related proteins in HeLa cells. (A) Silencing NBPF1 contributed to expressions of p-mTOR, p-PI3K, p-P70 compared with the controls. (B) NBPF1 overexpression suppressed expressions of p-mTOR, p-PI3K, p-P70 compared with the controls.
DISCUSSION
Numerous studies have reported the pivotal role of NBPF1 in many diseases, but there is no research focused on the association between NBPF1 expression and cervical cancer. In this study, we assessed the effect of NBPF1 expression on cervical cancer cell invasion and apoptosis using the gene silencing method. The present study determined that silenced NBPF1 downregulated mRNA expression of NBPF1 in HeLa cells, promoted cell invasion, and contributed to cell apoptosis-related protein expression including PI3K, mTOR, and P70. Furthermore, NBPF1 overexpression significantly suppressed cell invasion, promoted cell apoptosis, and inhibited the cell apoptosis signal pathway-related protein expressions, indicating the important role of NBPF1 in cervical cancer metastasis.
Our data showed that mRNA expression of NBPF1 in cervical cancer was suppressed by transfection with siNBPF1 vector, which was contrary to the results in cells transfected with pcDNA3.1-NBPF1. Vandepoele and his colleagues proved that expression of NBPF1 was decreased in neuroblastoma cell lines (12,18). Comparable to previous evidence, our results suggested that NBPF1 expression may be correlated with cervical cancer in certain aspects. NBPF1 overexpression significantly suppressed cell invasion but contributed to HeLa cell apoptosis. As we know, strong cell invasion ability and slight cell apoptosis ability would play crucial roles in cancer cell development and metastasis (19,20). Li and colleagues found that NBPF1 (as the subunit for eukaryotic translation initiation factor 3, EIF2AK3) played a certain role in colon cancer cell proliferation and invasion through RhoA/ROCK signaling pathway (21). Similarly, NBPF1 can be targeted by Chibby and can then suppress oncogenic processes, including migration and proliferation in cervical cancer (22). On the other hand, Schütz et al. demonstrated that chromosome instability of cell-free DNA including NBPF1 abnormal expression was found to be correlated with cell apoptosis in prostate cancer (23). Therefore, we speculated that high expression of NBPF1 may be a suppressor for cervical HeLa via inducing tumor cell apoptosis. Additionally, remediation experiments performed showed that there was no significant difference between NBPF1 expression on HeLa cell invasion and apoptosis when cells were transfected with both siNBPF1 and pcDNA3.1-NBPF1, indicating that NBPF1 expression was correlated with HeLa cell invasion and apoptosis.
We detected the cell apoptosis-related signal pathway protein expression to investigate whether or not NBPF1 expression could affect cell invasion and apoptosis through the PI3K/mTOR signal pathway. p-PI3K catalyzes the production of phosphatidylinositol-3,4,5-triphosphate in cell survival pathways (24), while mTOR (belonging to the PIKK family of proteins) could function in numerous biological processes including proliferation, cell growth, and cell cycle during cancer development and progression (25). Previous studies have indicated that activation of p-PI3K and mTOR was closely associated with cancer progression in diseases such as breast cancer and ovarian cancer (26,27). Activation of the mTOR signaling pathway promotes cervical cancer cell survival (28), and p-mTOR expression has been considered to be an independent prognostic factor for cervical cancer (29). Thus, activation of p-PI3K and p-mTOR was positively correlated with cancer progression. Moreover, it has been indicated that NBPF1 may be a cancer suppressor for breast cancer via targeting PI3K/mTOR pathway (30). The correlation of NBPF1 expression in cervical cancer cell apoptosis has not been fully discussed. But in this study, NBPF1 overexpression significantly decreased the expression of p-PI3K and p-mTOR, indicating that overexpression of NBPF1 may contribute to cell apoptosis via suppressing the PI3K/mTOR signal pathway.
In conclusion, the data presented in this study suggest that NBPF1 overexpression plays an important cancer-inhibiting role in cervical cancer progression with involvement in cell invasion and apoptosis through the PI3K/mTOR signal pathway. Overexpression of NBPF1 suppresses cell invasion and induces cell apoptosis, as well as suppressing the PI3K/mTOR signal pathway protein expression. This study may provide a basis for the potential therapeutic possibility of NBPF1 in cervical cancer treatment. However, further research is still needed to investigate the potential mechanism.
REFERENCES
- 1. Centers for Disease Control and Prevention: Cervical cancer screening among women aged 18–30 years-United States, 2000–2010. Morb. Mortal. Wkly. Rep. 61:1038; 2013. [PubMed] [Google Scholar]
- 2. Siegel R.; Ma J.; Zou Z.; Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 64:9–29; 2014. [DOI] [PubMed] [Google Scholar]
- 3. Angioli R.; Plotti F.; Montera R.; Aloisi A.; Luvero D.; Capriglione S.; Terranova C.; Nardone C. D. C.; Muzii L.; Benedetti-Panici P. Neoadjuvant chemotherapy plus radical surgery followed by chemotherapy in locally advanced cervical cancer. Gynecol. Oncol. 127:290–296; 2012. [DOI] [PubMed] [Google Scholar]
- 4. Katsumata N.; Yoshikawa H.; Kobayashi H.; Saito T.; Kuzuya K.; Nakanishi T.; Yasugi T.; Yaegashi N.; Yokota H.; Kodama S. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: A Japan Clinical Oncology Group trial (JCOG 0102). Br. J. Cancer 108:1957–1963; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halec G.; Alemany L.; Lloveras B.; Schmitt M.; Alejo M.; Bosch F.; Tous S.; Klaustermeier J.; Guimera N.; Grabe N.; Lahrhamm B.; Gissmann L.; Quint W.; Bosch F. X.; De Sanjose S.; Pawlita M. Pathogenic role of eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J. Pathol. 234:441–451; 2014. [DOI] [PubMed] [Google Scholar]
- 6. Noordhuis M. G.; Fehrmann R. S.; Wisman G. B. A.; Nijhuis E. R.; van Zanden J. J.; Moerland P. D.; van Themaat E. V. L.; Volders H. H.; Kok M.; Klaske A. Involvement of the TGF-β and β-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin. Cancer Res. 17:1317–1330, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Andries V.; van Roy F.; Vandepoele K. The NBPF Gene Family. INTECH Open Access Publisher, 2012. [Google Scholar]
- 8. Carmeliet P. Queen Elisabeth Medical Foundation. Thesis, Ghent University; 2009. [Google Scholar]
- 9. Vandepoele K.; Andries V.; Van Roy F. The NBPF1 promoter has been recruited from the unrelated EVI5 gene before simian radiation. Mol. Biol. Evol. 26:1321–1332; 2009. [DOI] [PubMed] [Google Scholar]
- 10. Wang C.; Li L.; Chen G.; Yang S.; Jing B.; Ren B.; Feng L. Cloning associated special genes in gastric cancer of North Sichuan Area. China Medical Herald 5:011; 2011. [Google Scholar]
- 11. Andries V. Functional analysis of the NBPF1 gene in cancer. Thesis, Ghent University; 2012. [Google Scholar]
- 12. Andries V.; Vandepoele K.; Staes K.; Berx G.; Bogaert P.; Van Isterdael G.; Ginneberge D.; Parthoens E.; Vandenbussche J.; Gevaert K. NBPF1, a tumor suppressor candidate in neuroblastoma, exerts growth inhibitory effects by inducing a G1 cell cycle arrest. BMC Cancer 15:1–25; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y.; Han N.; Yin T.; Huang L.; Liu S.; Liu D.; Xie C.; Zhang M. Lentivirus-mediated Nox4 shRNA invasion and angiogenesis and enhances radiosensitivity in human glioblastoma. Oxid. Med. Cell. Longev. 2014:581732, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nahata A.; Saxena A.; Suri N.; Saxena A. K.; Dixit V. K. Sphaeranthus indicus induces apoptosis through mitochondrial-dependent pathway in HL-60 cells and exerts cytotoxic potential on several human cancer cell lines. Integrative cancer therapies. Integr. Cancer Ther. 12:236–247, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Li J.-M.; Wang Y.-Y.; Zhao M.-X.; Tan C.-P.; Li Y.-Q.; Le X.-Y.; Ji L.-N.; Mao Z.-W. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 33:2780–2790, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Taliouri E.; Vrekoussis T.; Vergetaki A.; Agorastos T.; Makrigiannakis A. Corticotropin-releasing hormone (CRH) is expressed in the human cervical carcinoma cells (HeLa) and upregulates the expression of Fas ligand. Tumour Biol. 34:125–130, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Kurien B. T.; Scofield R. H. Western blotting of high and low molecular weight proteins using heat. Methods Mol. Biol. 247–255, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vandepoele K.; Staes K.; Andries V.; Van Roy F. Chibby interacts with NBPF1 and clusterin, two candidate tumor suppressors linked to neuroblastoma. Exp. Cell Res. 316:1225–1233, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Waggoner S. E. Cervical cancer. Lancet 361:2217–2225, 2003. [DOI] [PubMed] [Google Scholar]
- 20. De Schutter T.; Andrei G.; Topalis D.; Duraffour S.; Mitera T.; van den Oord J.; Matthys P.; Snoeck R. Reduced tumorigenicity and pathogenicity of cervical carcinoma SiHa cells selected for resistance to cidofovir. Mol. Cancer 12:1–16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li N.; Tang A.; Huang S.; Li Z.; Li X.; Shen S.; Ma J.; Wang X. MiR-126 suppresses colon cancer cell proliferation and invasion via inhibiting RhoA/ROCK signaling pathway. Mol. Cell. Biochem. 380:107–119, 2013. [DOI] [PubMed] [Google Scholar]
- 22. Huang Y.-L. The role of chibby as a potential tumor suppressor gene in human cervical cancer; 2010. Retrieved from http://etd.lib.nsysu.edu.tw/ETD-db/ETD-search/view_etd?URN=etd-0902110-170133
- 23. Schütz E.; Akbari M. R.; Beck J.; Urnovitz H.; Zhang W. W.; Bornemann-Kolatzki K.; Mitchell W. M.; Nam R. K.; Narod S. A. Chromosomal instability in cell-free DNA is a serum biomarker for prostate cancer. Clin. Chem. 61(1):239–248; 2015. [DOI] [PubMed] [Google Scholar]
- 24. Engelman J. A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 9:550–562, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Hay N.; Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 18:1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Fujita T.; Doihara H.; Kawasaki K.; Takabatake D.; Takahashi H.; Washio K.; Tsukuda K.; Ogasawara Y.; Shimizu N. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br. J. Cancer 94:247–252, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ou C.-C.; Hsu S.-C.; Hsieh Y.-H.; Tsou W.-L.; Chuang T.-C.; Liu J.-Y.; Kao M.-C. Downregulation of HER2 by RIG1 involves the PI3K/Akt pathway in ovarian cancer cells. Carcinogenesis 29:299–306, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Ji J.; Zheng P.-S. Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol. Oncol. 117:103–108, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Faried L. S.; Faried A.; Kanuma T.; Aoki H.; Sano T.; Nakazato T.; Tamura T.; Kuwano H.; Minegishi T. Expression of an activated mammalian target of rapamycin in adenocarcinoma of the cervix: A potential biomarker and molecular target therapy. Mol. Carcinog. 47:446–457, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Vandepoele K.; Andries V.; Van Roy N.; Staes K.; Vandesompele J.; Laureys G.; De Smet E.; Berx G.; Speleman F.; Van Roy F. A constitutional translocation t (1; 17)(p36. 2; q11. 2) in a neuroblastoma patient disrupts the human NBPF1 and ACCN1 genes. PloS One 3:e2207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]