Abstract
It is largely recognized that PDCD4 is frequently lost in tumors of various origins, including lung cancer, and its loss contributes to tumor progression. However, its role and molecular mechanism remain largely unexplored in non-small cell lung cancer (NSCLC). In this study, downregulated PDCD4 mRNA expression was found in NSCLC tissues compared to their corresponding paracarcinoma tissues and distal paracarcinoma tissues. Induced expression of PDCD4 inhibited cell growth and proliferation and cell cycle transition in vitro. Conversely, knocking down PDCD4 expression promoted cell growth and proliferation. Mechanistically, PDCD4 inactivated PI3K/Akt signaling and its downstream cell cycle factors CCND1 and CDK4 to regulate cell growth in NSCLC. Additionally, PI3K-specific inhibitor Ly294002 suppressed the expression of pPI3K (Tyr458), pAkt (Ser473), CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells. Furthermore, Akt-specific inhibitor MK2206 inhibited the expression of pAkt (Ser473), CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells. Taken together, our study provides evidence that PDCD4 inhibits cell growth through PI3K/Akt signaling in NSCLC and may be a potential therapeutic target for NSCLC.
Key words: PDCD4, Non-small cell lung cancer (NSCLC), Cell growth, PI3K/Akt
INTRODUCTION
Lung cancer is the most common cancer in the world, with a survival rate of 15% (1), of which 80% are non-small cell lung cancer (NSCLC). Surgical resection is known to be the most effective treatment for NSCLC. However, due to the fact that most diagnoses are confirmed in an advanced stage because of its deep location with no specificity of symptoms in its early stage, only a few patients can be cured by surgical treatment. Therefore, there is an urgent need to search for valuable factors allowing early diagnosis, prediction of prognosis, and novel therapeutic strategies.
Programmed cell death 4 (PDCD4) is localized to chromosome band 10q24 with two basic domains, at the N-terminus and C-terminus involving nuclear localization signals, and two conserved-helical MA-3 domains associated with the ATP-dependent RNA helicase eIF4A proposing the repression of translation initiation (2,3). PDCD4 expresses ubiquitously in normal tissues with highest levels in liver and is first identified as an upregulated gene during investigation of apoptosis (2). Accumulating evidence has shown that PDCD4 is frequently lost in tumors of various origins including lung cancer (4–9), and its loss contributes to tumor progression. Furthermore, PDCD4 is confirmed as a new tumor-suppressor gene involved in the apoptotic machinery, which suppresses cell transformation (6,10), tumorigenesis (2,11–13), cell growth (8,11,14–16), cell apoptosis (5,8), and tumor invasion at present (7). However, its roles and molecular mechanism are still unclear in NSCLC.
Thus, this study was designed to investigate the function and possible molecular basis of PDCD4 in the pathogenesis of NSCLC. Here we demonstrated that PDCD4 inhibited cell growth through PI3K/Akt signaling and its downstream cell cycle factors CCND1 and CDK4 in NSCLC, which in turn contributed to the pathogenesis of NSCLC.
MATERIALS AND METHODS
Cell Culture, Sample Collection, and Ethics Statement
NSCLC PC9 and A549 cells were maintained in DMEM medium supplemented with 10% fatal bovine serum (FBS) (PAA Laboratories, Inc, Pasching, Austria) and were incubated in a humidified chamber with 5% CO2 at 37°C. Twenty-eight fresh NSCLC tissues, and their corresponding paracarcinoma tissues (2–5 cm) and distal paracarcinoma tissues (≥5 cm), were obtained at the time of diagnosis before any therapy from the Affiliated Hospital of Guangdong Medical College, Zhanjiang City, China. Clinical processes were approved by the Ethics Committees of Affiliated Hospital of Guangdong Medical College. Patients provided informed consents.
RNA Isolation, Reverse Transcription, and qRT-PCR
RNA was extracted from 28 NSCLC tissues and their corresponding paracarcinoma tissues and distal paracarcinoma tissues using TRIzol (Takara, Shiga, Japan). For PDCD4 qRT-PCR, RNA was transcribed into cDNA and amplified with specific sense/antisense primers, which have been reported in our previous study (8). Assays were performed in accordance with manufacturer’s instructions (Takara, Shiga, Japan). PCR reaction for each gene was repeated three times. mRNA expression was normalized to ARF5, respectively, using the 2−ΔΔCt method (8,17).
Establishment of NSCLC PC9 and A549 Cells With Stable Overexpression of PDCD4 or PDCD4 Short Hairpin RNA
PC9 and A549 cells were infected by lentiviruses with pGC-FU-PDCD4-GFP vector or pLVTHM-shPDCD4-GFP vector, which have been well established in our previous study (8). Polyclonal cells with GFP signals were selected for further experiments using FACS flow cytometer assay.
Transient Transfection With PI3K Inhibitor Ly294002 or Akt Inhibitor MK2206
PI3K inhibitor Ly294002 and Akt inhibitor MK2206 were bought from Sigma-Aldrich (St. Louis, MO, USA). Twenty-four hours prior to transfection, PC9-shPDCD4 and A549-shPDCD4 cells were plated onto a six-well plate or a 96-well plate (Nest, Biotech, China) at 30–50% confluence. PI3K inhibitor Ly294002 or Akt inhibitor MK2206 was then transfected at a working concentration of 50 nM or 5 µM according to the manufacturer’s protocol. Cells were collected after 48–72 h for the further experiments.
MTT Assay
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to evaluate in vitro cell proliferation. Briefly, 1 × 103 cells were seeded into a 96-well plate with quadruplicate repeat for each condition. For pGC-FU-PDCD4 and shRNA-PDCD4, cells were incubated for 1, 2, 3, 4, 5, 6, and 7 days. For inhibitors, cells were incubated for 1, 2, and 3 days. Twenty microliters of MTT (5 mg/ml) (Sigma-Aldrich) was added to each well and incubated for 4 h. At the end of incubation, the supernatants were removed, and 150 µl of DMSO (Sigma-Aldrich) was added to each well. The absorbance value (OD) of each well was measured at 490 nm. Experiments were performed three times.
Edu Incorporation Analysis
Edu (5-ethynyl-2′-deoxyuridine) assay was also used to measure in vitro cell proliferation. Briefly, 4 × 103 cells were seeded into a 96-well plate with quadruplicate repeat for each condition. Cells were labeled with 50 µM Edu (RiboBio Inc, Guangzhou, China) for 2 h before they were formalin fixed and processed following the manufacturer’s instructions. Stained cells were observed under a fluorescent microscope.
Colony Formation Assay
Cells were plated in six-well culture plates at a density of 100 cells/well. Each cell group had two wells. After incubation for 14 days at 37°C, cells were washed twice with PBS and stained with the Giemsa solution. The number of colonies containing ≥50 cells was counted under a microscope. The colony formation efficiency was calculated as (number of colonies/number of cells inoculated) × 100%.
Cell Cycle Analysis
For cell cycle analysis, cells were seeded in 10-cm-diameter plates in DMEM containing 10% FBS. After incubation for 48 h, a total of 5 × 106 cells were harvested, rinsed with cold PBS, and fixed with 70% ice-cold ethanol for 48 h at 4°C. Fixed cells were rinsed with cold PBS followed by incubation with PBS containing 10 µg/ml propidium iodide and 0.5 mg/ml RNase A for 30 min at 37°C. The DNA content of labeled cells was acquired using FACS cytometry assay (BD Biosciences). Each experiment was performed in triplicate.
Western Blot Analysis
Cells were lysed in RIPA buffer (Beyotime, Beijing, China), and protein concentration was determined using the BCA assay (Beyotime). Total protein (30 µg) was resolved using a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel and electro-transferred to polyvinylidene fluoride membranes (Invitrogen, USA). Membranes were blocked with 5% non-fat dry milk (for phosphorylation antibody, adding BSA) in Tris-buffered saline (pH 7.5), followed by immunoblotting overnight at 4°C with anti-PDCD4 (1:500; Proteintech), anti-pPI3K (Tyr458), PI3K, pAkt (Ser473), and Akt antibody (1:1,000; Cell Signaling Technology), anti-CCND1 and CDK4 antibody (1:400; Santa Cruz Biotechnology), and anti-β-actin antibody (1:1,000; Proteintech). A HRP-conjugated anti-rabbit or anti-mouse IgG antibody was used as the secondary antibody (1:1,000; Proteintech). Signals were detected using enhanced chemiluminescence reagents (Merk Millipore).
Statistical Analysis
All data were analyzed for statistical significance using SPSS 13.0 software. One-way ANOVA was applied to evaluate PDCD4 expression levels among different lung cancer tissues. Two-tailed Student’s t-test was used for comparisons of two independent groups. One-way ANOVA was used to determine differences between groups for all in vitro analyses. A value of p < 0.05 was considered statistically significant.
RESULTS
PDCD4 Is Lowly Expressed in NSCLC
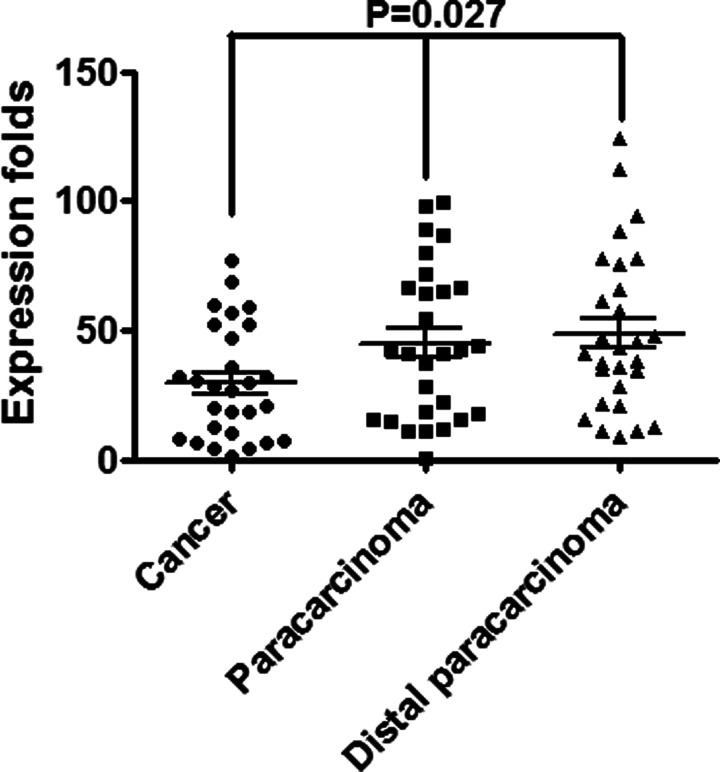
We measured the expression of PDCD4 transcripts and found that it was significantly decreased in NSCLC tissues compared to their corresponding paracarcinoma tissues and distal paracarcinoma tissues (p = 0.027) (Fig. 1). The data were similar to a previous study (9). PDCD4 was lost in human lung cancer and correlated with tumor progression and prognosis.
Figure 1.
PDCD4 was markedly decreased in 28 fresh NSCLC tissues compared to their corresponding paracarcinoma tissues and distal paracarcinoma tissues.
PDCD4 Inhibits Cell Growth, Proliferation, and Cell Cycle Transition
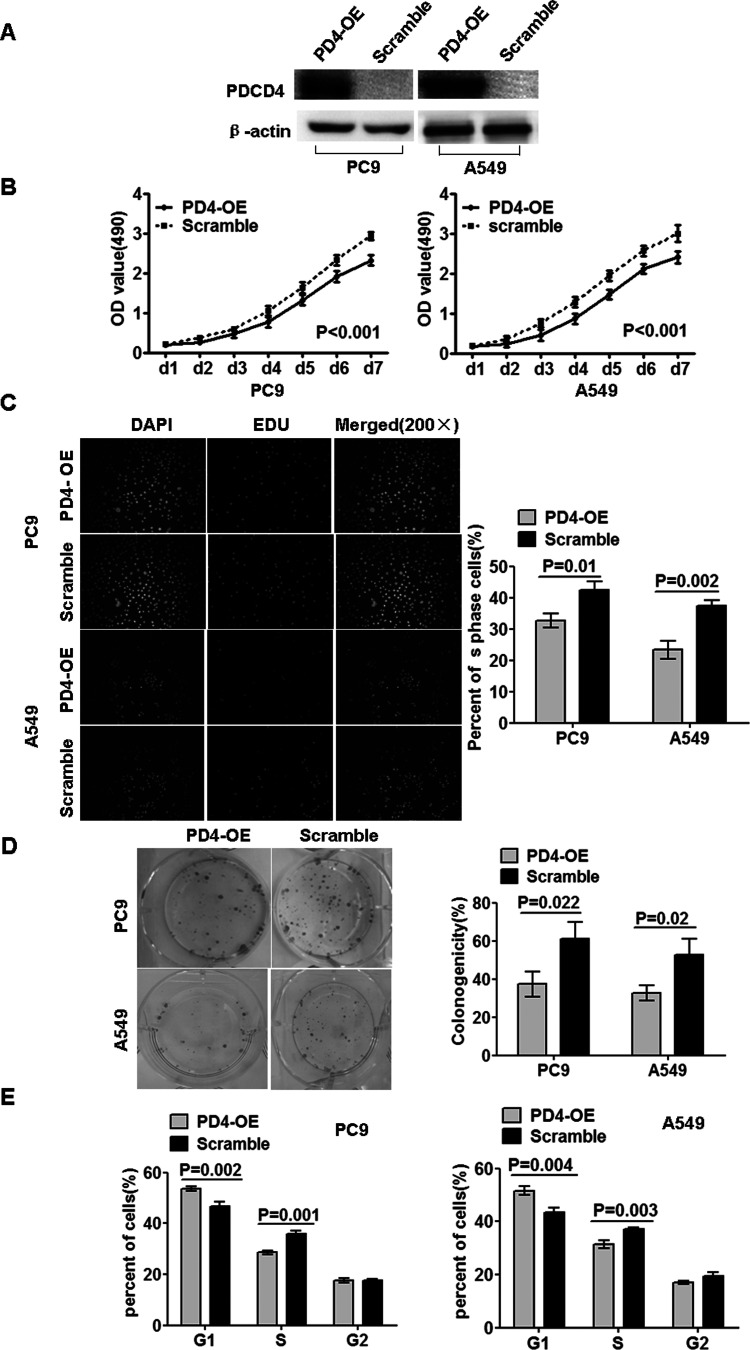
To study the biological functions of PDCD4, we introduced PDCD4 into PC9 and A549 cells using lentiviruses containing PDCD4 gene, and the polyclonal cells with GFP signals were selected for further experiments using FACS flow cytometer assay. Further, expression of PDCD4 protein was confirmed by Western blot in these cells compared to scramble (Fig. 2A). The growth curves, Edu assay, and colony formation assay showed that PDCD4 significantly inhibited cell growth and proliferation of PC9-PD4-OE and A549-PD4-OE cells in comparison to their corresponding scramble cells (Fig. 2B–D). Furthermore, we also observed that PDCD4 blocked cell cycle transition from G1 to S and G2 phase in PC9-PD4-OE and A549-PD4-OE cells in comparison to their corresponding scramble cells (Fig. 2E).
Figure 2.
Overexpressed PDCD4 suppressed cell growth, proliferation, and cell cycle transition. (A) Restoration of PDCD4 protein expression was examined in polyclonal and scramble cells by Western blot. Because of the fusion protein from PDCD4 and green fluorescent protein (GFP), the fusion protein of scramble cannot be detected. β-Actin served as the internal control. (B) MTT cell viability assay was performed on PD4-OE and scramble cells of PC9 and A549. (C) Edu proliferation assay of PD4-OE and scramble cells of PC9 and A549. (D) Colony formation assay was performed on PD4-OE and scramble cells of PC9 and A549. (E) Cell cycle analysis of PD4-OE and scramble cells of PC9 and A549. Data were presented as mean ± SD for three independent experiments.
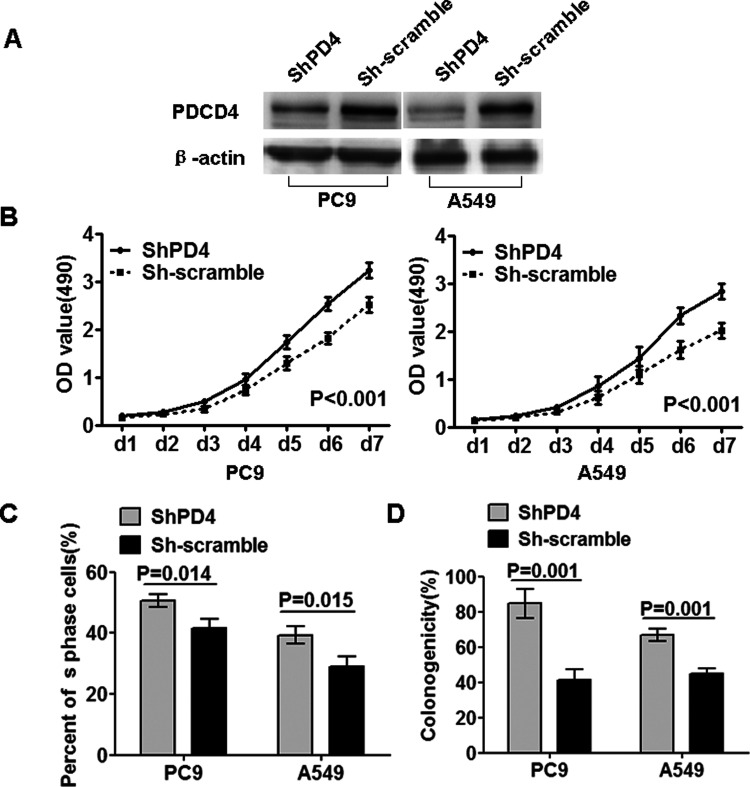
To further confirm the inhibitory biological functions of PDCD4, we did the loss-of-function study. We used shRNA specifically and stably knocking down the expression of PDCD4 in PC9 and A549 cell lines (Fig. 3A). Interestingly, inversed results were also observed that suppressing PDCD4 significantly elevated cell growth and proliferation in PC9-shPDCD4 and A549-shPDCD4 cells in comparison to their corresponding shScramble cells (Fig. 3B–D).
Figure 3.
Knocking down PDCD4 expression by shRNA promoted cell growth and proliferation. (A) Inhibition of PDCD4 protein expression was examined in polyclonal and scramble cells by Western blot. β-Actin served as the internal control. (B) MTT cell viability assay was performed on shPDCD4 and sh-scramble cells of PC9 and A549. (C) Edu proliferation assay of shPDCD4 and sh-scramble cells of PC9 and A549. (D) Colony formation assay was performed on shPDCD4 and sh-scramble cells of PC9 and A549. Data were presented as mean ± SD for three independent experiments.
PDCD4 Regulated PI3K/Akt Signaling and its Downstream Cell Cycle Factors CCND1 and CDK4 in NSCLC Cells
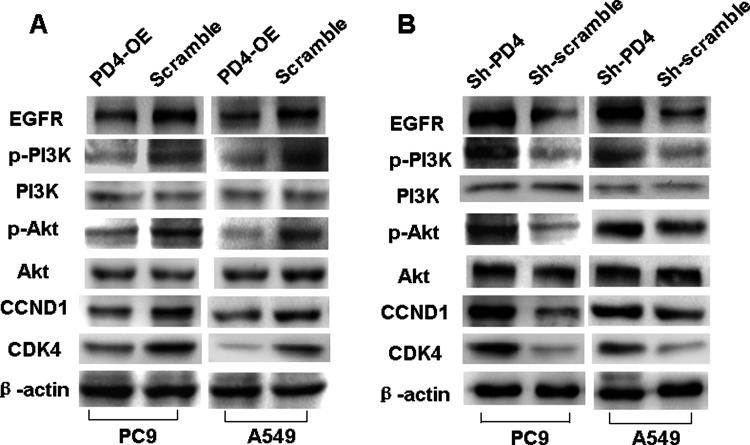
Attributed to the fact that PDCD4 suppressed cell cycle progression, we examined the expression of cell cycle G1 to S checkpoint proteins CCND1 and CDK4, in both PDCD4-overexpressed and PDCD4-knocked down cells. We found that introduction of PDCD4 or knocking down endogenous PDCD4 expression blocked or induced the expression of CCND1 and CDK4 (Fig. 4A, B).
Figure 4.
PDCD4 modulated PI3K/Akt signaling and its downstream cell cycle factors CCND1 and CDK4 in NSCLC cells. (A) Overexpressed PDCD4 suppressed the expression of pPI3K (Tyr458), pAkt (Ser473), CCND1, and CDK4 in PD4-OE compared to scramble cells of PC9 and A549, whereas total levels of PI3K and Akt remained unchanged. β-Actin served as the internal control. (B) Reduced PDCD4 elevated the expression of pPI3K (Tyr458), pAkt (Ser473), CCND1, and CDK4 in shPDCD4 PC9 and A549 cells compared to their respective controls, whereas total levels of PI3K and Akt remained unchanged. β-Actin served as the internal control.
Many studies indicate that cell cycle is a downstream target of the PI3K/Akt signaling pathway (8). In this study, we also examined the effect of PDCD4 on PI3K/Akt signaling pathway and found that introduction of PDCD4 or knocking down endogenous PDCD4 expression reduced or induced the expression of pPI3K (Tyr458) and pAkt (Ser473), respectively (Fig. 4A, B), whereas their total levels remained unchanged (Fig. 4A, B).
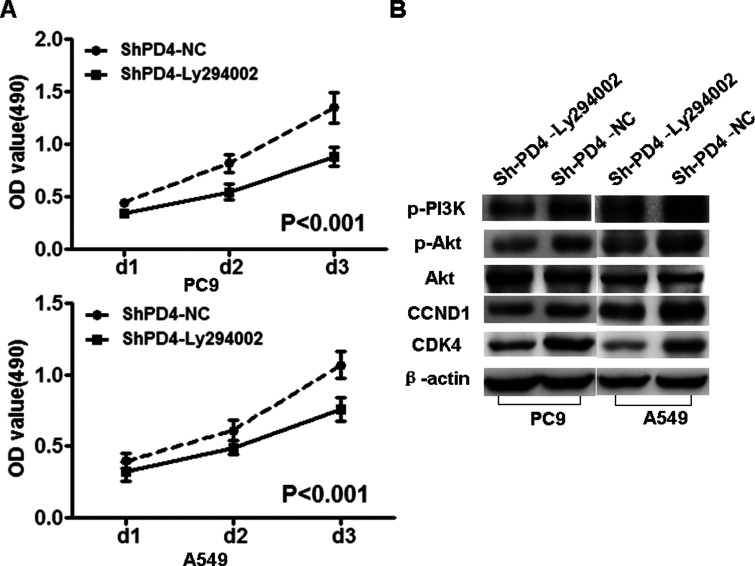
Subsequently, we used specific inhibitor Ly294002 of PI3K to suppress the expression of PI3K and observed that the protein expression of pPI3K (Tyr458), pAkt (Ser473), CCND1, and CDK4 was decreased in PC9-shPDCD4 and A549-shPDCD4 cells (Fig. 5).
Figure 5.
PI3K inhibitor Ly294002 reduced the expression of pPI3K, pAkt, CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells. PI3K inhibitor Ly294002 reduced the expression of pPI3K (Tyr458), pAkt (Ser473), CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells, but did not change the expression of Akt. β-Actin served as the internal control.
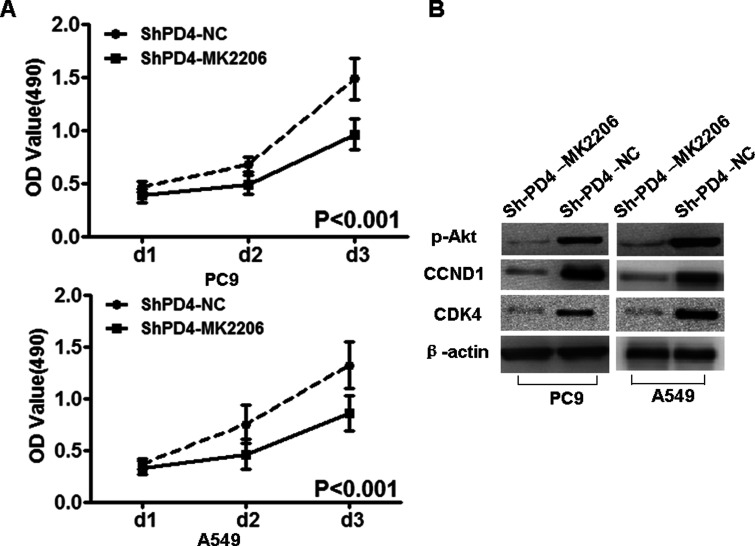
Furthermore, Akt-specific inhibitor MK2206 was used to inhibit the expression of Akt, and the protein expression of pAkt (Ser473), CCND1, and CDK4 was downregulated in PC9-shPDCD4 and A549-shPDCD4 cells (Fig. 6).
Figure 6.
Akt inhibitor MK2206 reduced the expression of pAkt, CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells. Akt inhibitor MK2206 suppressed the expression of pAkt (Ser473), CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells. β-Actin served as the internal control.
Taken together, our results demonstrated that PDCD4 inhibited cell growth through PI3K/Akt signaling and its downstream cell cycle factors CCND1 and CDK4 in NSCLC.
DISCUSSION
PDCD4, a cytoplasmic and nuclear expression protein, plays a significant role in suppression tumor growth (8,18). However, its role and molecular mechanism remain largely unknown in NSCLC.
In this study, we measured the expression of PDCD4 transcripts in NSCLC and found that PDCD4 was significantly downregulated in NSCLC tissues compared to their corresponding paracarcinoma tissues and distal paracarcinoma tissues, which was similar to a previous study that PDCD4 is lost in human lung cancer and correlates with tumor progression and prognosis (9). Our data also hinted that lost expression of PDCD4 was involved in the pathogenesis of NSCLC.
Subsequently, we presented the evidence that PDCD4 inhibited cell growth and proliferation in NSCLC. To specifically determine the contributions of PDCD4 protein in the regulation of NSCLC cell phenotypes, stably increased PDCD4 expression in NSCLC PC9 and A549 cells by lentiviral-delivered PDCD4 markedly reduced the cell growth, proliferation and retarded cell cycle progression. On the contrary, stably knocking down PDCD4 expression in PC9 and A549 cells significantly elevated cell growth and proliferation. These results together suggested that PDCD4 suppressed cell growth and proliferation in NSCLC. Biological functions of PDCD4 found in this study were consistent to previous studies (8,13,15).
Biological functions of PDCD4 found in this study provide a mechanistic basis for NSCLC. It is well known that high proliferative activity of tumor cells is associated with the increased cell cycle transition (19). CCND1, a classic oncogenic protein of cell cycle signal, promoted cell proliferation and the beginning of S phase in the cell cycle in many cancers (20,21). CDK4, part of the cyclin-dependent kinase family, is important for cell cycle G1–S phase progression (22). Here we found that PDCD4-mediated growth suppression attributed to cell cycle transition obstacle by repressing the expression of cell cycle G1/S checkpoint proteins CCND1 and CDK4. Our results were consistent with some reports about knocking down PDCD4-induced Myc and CCND1 expression in tumor cells (8,23).
PI3K/Akt, a classical signal pathway, induces cell cycle progression (8,24). In this study, we found that introduction of PDCD4 or knocking down endogenous PDCD4 expression reduced or induced the expression of pPI3K, pAkt, CCND1, and CDK4, respectively.
In order to validate the effects of PI3K and Akt on PDCD4-mediated cell growth suppression, experiments were performed wherein PI3K inhibitor Ly294002 or Akt inhibitor Mk2206 was used in PC9-shPDCD4 and A549-shPDCD4 cells. We observed that PI3K inhibitor Ly294002 suppressed the expression of pPI3K, pAkt, CCND1, and CDK4, and Akt-specific inhibitor Mk2206 inhibited the expression of pAkt, CCND1, and CDK4 in PC9-shPDCD4 and A549-shPDCD4 cells.
Taken together, this study provided evidence that PDCD4 suppressed cell growth by inactivating PI3K/Akt signaling and its downstream cell cycle signals CCND1 and CDK4 in NSCLC.
ACKNOWLEDGMENTS
This study was supported by National Nature Science Fund of China (No. 81372184, No. 81272268, No. 81401906) (http://www.nsfc.gov.cn), Nature Science Fund of Guangdong Province (No. S2013010014724), Science and Technology Planning Project of Guangdong Province (No. 10301kpkjt20120013), and Guangdong Medical College Key Discipline (XZ1120).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R.; Ma J.; Zou Z.; Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 64:9–29; 2014. [DOI] [PubMed] [Google Scholar]
- 2. Lankat-buttgereit B.; Goke R. Programmed cell death protein 4 (pdcd4): A novel target for antineoplastic therapy? Biol. Cell 95:515–519; 2003. [DOI] [PubMed] [Google Scholar]
- 3. Goke A.; Goke R.; Knolle A.; Trusheim H.; Schmidt H.; Wilmen A.; Carmody R.; Goke B.; Chen Y. H. DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem. Biophys. Res. Commun. 297:78–82; 2002. [DOI] [PubMed] [Google Scholar]
- 4. Baffa R.; Fassan M.; Volinia S.; O’hara B.; Liu C. G.; Palazzo J. P.; Gardiman M.; Rugge M.; Gomella L. G.; Croce C. M.; Rosenberg A. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J. Pathol. 219:214–221; 2009. [DOI] [PubMed] [Google Scholar]
- 5. Zhang H.; Ozaki I.; Mizuta T.; Hamajima H.; Yasutake T.; Eguchi Y.; Ideguchi H.; Yamamoto K.; Matsuhashi S. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25:6101–6112; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Mudduluru G.; Medved F.; Grobholz R., Jost C.; Gruber A.; Leupold J. H.; Post S.; Jansen A.; Colburn N. H.; Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 110:1697–1707; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Wang Q.; Sun Z.; Yang H. S. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene 27:1527–1535; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Zhen Y.; Liu Z.; Yang H.; Yu X.; Wu Q.; Hua S.; Long X.; Jiang Q.; Song Y.; Cheng C.; Wang H.; Zhao M.; Fu Q.; Lyu X.; Chen Y.; Fan Y.; Liu Y.; Li X.; Fang W. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 4:e872; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y.; Knosel T.; Kristiansen G.; Pietas A.; Garber M. E.; Matsuhashi S.; Ozaki I.; Petersen I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J. Pathol. 200:640–646; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Bohm M.; Sawicka K.; Siebrasse J. P.; Brehmer-fastnacht A.; Peters R.; Klempnauer K. H. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene 22:4905–4910; 2003. [DOI] [PubMed] [Google Scholar]
- 11. Afonja O.; Juste D.; Das S.; Matsuhashi S.; Samuels H. H. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 23:8135–8145; 2004. [DOI] [PubMed] [Google Scholar]
- 12. Bitomsky N.; Bohm M.; Klempnauer K. H. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene 23:7484–7493; 2004. [DOI] [PubMed] [Google Scholar]
- 13. Jansen A. P.; Camalier C. E.; Colburn N. H. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 65:6034–6041; 2005. [DOI] [PubMed] [Google Scholar]
- 14. Dorrello N. V.; Peschiarol A.; Guardavaccaro D.; Colburn N. H.; Sherman N. E.; Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314:467–471; 2006. [DOI] [PubMed] [Google Scholar]
- 15. Bitomsky N.; Wethkamp N.; Marikkannu R.; Klempnauer K. H. siRNA-mediated knockdown of Pdcd4 expression causes upregulation of p21(Waf1/Cip1) expression. Oncogene 27:4820–4829; 2008. [DOI] [PubMed] [Google Scholar]
- 16. Carayol N.; Katsoulidis E.; Sassano A.; Altman J. K.; Druker B. J.; Platanias L. C. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J. Biol. Chem. 283:8601–8610; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408; 2001. [DOI] [PubMed] [Google Scholar]
- 18. Kalinichenko S. V.; Kopantzev E. P.; Korobko E. V.; Palgova I. V.; Zavalishina L. E.; Bateva M. V.; Petrov A. N.; Frank G. A.; Sverdlov E. D.; Korobko I. V. Pdcd4 protein and mRNA level alterations do not correlate in human lung tumors. Lung Cancer 62:173–180; 2008. [DOI] [PubMed] [Google Scholar]
- 19. Huang L.; Wang H. Y.; Li J. D.; Wang J. H.; Zhou Y.; Luo R. Z.; Yun J. P.; Zhang Y.; Jia W. H.; Zheng M. KPNA2 promotes cell proliferation and tumorigenicity in epithelial ovarian carcinoma through upregulation of c-Myc and downregulation of FOXO3a. Cell Death Dis. 4:e745; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang W.; Kahn S. M.; Zhou P.; Zhang Y. J.; Cacace A. M.; Infante A. S.; Doi S.; Santella R. M.; Weinstein I. B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 8:3447–3457; 1993. [PubMed] [Google Scholar]
- 21. Baldin V.; Lukas J.; Marcote M. J.; Pagano M.; Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7:812–821; 1993. [DOI] [PubMed] [Google Scholar]
- 22. Wu A.; Wu B.; Guo J.; Luo W.; Wu D.; Yang H.; Zhen Y.; Yu X.; Wang H.; Zhou Y.; Liu Z.; Fang W.; Yang Z. Elevated expression of CDK4 in lung cancer. J. Transl. Med. 9:38; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo X.; Li W.; Wang Q.; Yang H. S. AKT Activation by Pdcd4 knockdown up-regulates cyclin D1 expression and promotes cell proliferation. Genes Cancer 2:818–828; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H.; Wu Q.; Liu Z.; Luo X.; Fan Y.; Liu Y.; Zhang Y.; Hua S.; Fu Q.; Zhao M.; Chen Y.; Fang W.; Lv X. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 5:e1155; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]