Abstract
Calcitriol (1α,25-dihydroxyvitamin D3) has demonstrated anticancer activity against several tumors. However, the underlying mechanism for this activity is not yet fully understood. Our experiment was designed and performed to address one aspect of this issue in cervical cancer. HeLa S3 cells were cultured in media with various concentrations of calcitriol. Cell proliferation and cell cycle were assessed by spectrophotometry and flow cytometry, respectively. The mRNA and protein expression levels of human cervical cancer oncogene (HCCR-1) and p21 were determined by RT-PCR and Western blot, respectively. Results indicated that calcitriol inhibited HeLa S3 cell proliferation and induced cell cycle arrest at the G1 phase. Calcitriol decreased HCCR-1 protein expression in a dose- and time-dependent manner. Furthermore, promoter activity analyses revealed that transcriptional regulation was involved in the inhibition of HCCR-1 expression. Overexpression of HCCR-1 in HeLa S3 cells reversed the inhibition of cell proliferation and G1 phase arrest that resulted from calcitriol treatment. In addition, calcitriol increased p21 expression and promoter activity. HCCR-1 overexpression decreased p21 expression and promoter activity. Thus, our results suggested that calcitriol inhibited HeLa S3 cell proliferation by decreasing HCCR-1 expression and increasing p21 expression.
Key words: Calcitriol, HeLa S3 cells, Human cervical cancer oncogene (HCCR-1)
INTRODUCTION
Calcitriol (1α,25-dihydroxyvitamin D3), which has a long-recognized function of mineralizing the skeleton by maintaining serum calcium and phosphorus (1), has recently attracted increasing attention because of its anticancer effects. Several retrospective and prospective investigations revealed that serum vitamin D deficiency increases cancer incidence and lethality (2–5). Beneficial roles of vitamin D during cancer treatment have also been suggested (4,6,7). High concentration of serum vitamin D has been proven beneficial in the prevention and treatment of breast cancer (8), lung cancer (9), pancreatic cancer (10), prostate cancer (11), colon cancer, and leukemia (12). However, the mechanism underlying the anticancer effects of vitamin D has been barely elucidated.
Cervical cancer is the most common cause of death from cancer for women in the developing countries, with 529,512 new cases diagnosed per year worldwide (13). In 2010, a case-control study revealed an inverse association between dietary calcium and vitamin D intake and cervical neoplasia risk in Japanese women (14), suggesting that vitamin D can decrease the incidence rate of cervical carcinoma. However, little is known about the effect of vitamin D on cervical cancer development.
Human cervical cancer oncogene (HCCR-1), a gene first indentified in a carcinoma tissue of cervical cancer (15), is overexpressed in various human tumors, including leukemia, lymphoma, and carcinomas of the breast, kidney, ovary, stomach, colon, and uterine cervix. HCCR-1 can contribute to neoplastic cellular transformation and tumorigenesis (16), stimulate morphogenesis of epithelia or mesenchyme, and is involved in transdifferentiation processes of cancer stem cell. In vitro cell experiments showed that HCCR-1 can induce epithelial–mesenchymal transition and mesenchymal–epithelial transition in humans and mice, respectively (17). In addition, HCCR-1 is overexpressed in cervical cancer tissues and cell lines but undetectable in normal cervical tissue (16), suggesting that HCCR-1 might play an important role in cervical carcinoma development.
The p21 protein is a cyclin-dependent kinase inhibitor that inhibits the activity of cyclin-CDK2, -CDK1, and -CDK4/6 complexes through binding; thus, it regulates cell cycle progression at the G1 and S phases (18). p21 is involved in tumorigenesis as it is both a classical tumor suppressor and an oncogene, depending on cellular context (19). p21 has been shown to be downregulated by calcitriol in vitro and in vivo (20). The role of p21 in cervical cancer progression should be further explored.
In this investigation, we evaluated a possible mechanism for the anticancer effect of calcitriol on cervical carcinoma.
MATERIALS AND METHODS
Proliferation Assays
HeLa S3 cells obtained from the American Type Culture Collection were plated at a density of 1,000 cells/well in 96-well plates of Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), treated with 1% ethanol (control) or various concentrations of calcitriol (100, 200, and 500 nM) (Sigma-Aldrich Co., St. Louis, MO, USA) for 72 h. A Cell Counting Kit8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) was used to determine cell proliferation. At 24, 48, 72, 96, 120, and 144 h after culturing with 200 nM calcitriol, cells were harvested for analysis. Three independent experiments were performed in quadruplicate.
Cell Cycle Analysis
Cell cycle distribution was profiled through the cellular DNA content measured by flow cytometry. HeLa S3 cells (less than 70% confluence) were collected and then fixed with 70% (v/v) ice-cold ethanol for more than 2 h. Fixed cells were pelleted and resuspended in propidium iodide (PI) staining buffer consisting of 0.1% (v/v) Triton X-100, 10 µg/ml PI, and 100 µg/ml DNase-free RNase A in PBS for 30 min. Cells were then sorted by a FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed with ModFit LT software.
Expression Vector Construction and Transfection
Human HCCR-1 cDNA clone (NM_015416) was purchased from (Shanghai Genechem Co., Ltd.), and the open reading frame of the HCCR-1 gene was amplified by PCR using pfu polymerase. The PCR product was inserted to pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) using a recombinant cloning technology (Gene script). Cells were cultured to 30% confluence, and transfection of the expression vectors into HeLa S3 cells was performed using FuGENE HD reagent (Promega Corp., Fitchburg, WI, USA) according to the manufacturer’s instructions.
Western Blot Analysis
Protein extracted from cells were separated on sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene fluoride membranes (Pall Corp., Port Washington, NY, USA). After blocking with 5% nonfat milk, the membranes were incubated with an appropriate dilution of primary antibodies against HCCR-1, p21, and β-actin (Santa Cruz, Dallas, TX, USA). This step was followed by incubation with horseradish peroxidase-conjugated secondary antibody (Santa Cruz) according to the manufacturer’s instructions. Immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, and subsequent luminescence signals were obtained by Kodak Image Station 4000.
Reverse Transcription and Real-Time PCR
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and 1 µg of total RNA was used for reverse transcription. The mRNA levels of HCCR-1 and GAPDH were examined by real-time PCR (Applied Biosystems 7500 Real-Time PCR System; Life Technologies, Carlsbad, CA, USA) using SYBR® Green PCR Master Mix (Life Technologies). The following thermal profile was used for PCR amplification: initial denaturation for 10 min at 95°C, 40 cycles of denaturation for 10 s at 95°C, and extension and annealing for 40 s at 60°C. The following primers were used, with the expected PCR product length given in terms of base pair (bp): HCCR-1 (118 bp): 5′-CAGTCACCCCTGGACATTTTGT-3′ (sense), 5′-AAGTTCTTCACATCTGCCTTTGGA-3′ (antisense); p21 (109 bp): 5′-TGGAGACTCTCAGGGTCGAA-3′ (sense), 5′-CTTCCTGTGGGCGGATTAGG-3′ (antisense); GAPDH (115 bp): 5′-TCTTTTGCGTCGCCAGCCGAG-3′ (sense), 5′-CAGAGTTAAAAGCAGCCCTGGTGAC-3′ (antisense). Relative expression levels of HCCR-1 in real time were analyzed using the 2−ΔΔCT method (14). Results were expressed in fold induction by comparing with the control (0 nM calcitriol), which was arbitrarily set as 1. Each sample was replicated twice from three independent sets of RNA preparations.
Promoter Activity Assay
The promoter regions of HCCR-1 (1,632 bp) and p21 (934 bp) were inserted into pGL3-Basic Vector (Promega Corp.) using a recombinant cloning technology (GenScript). Cells with 70% confluence were cotransfected with 1 µg of promoter/pGL3 basic chimerical plasmid (expressing firefly luciferase) and 0.02 µg of pRL-CMV plasmid (expressing Renilla luciferase) (Promega Corp.), following the instructions of FuGENE HD (Promega Corp.). Promoter activities were detected following the protocol of Dual-Luciferase® Reporter Assay System (Promega Corp.) on a Synergy H1 Hybrid Multi-Mode Microplate Reader (Biotek, Winooski, VT, USA). Firefly luciferase values were standardized to Renilla values.
Statistical Analysis
Error bars were generated by calculating the standard error of each independent experiment. Each independent data point was generated from three averaged RT-PCR reactions.
RESULTS
Calcitriol Inhibited HeLa S3 Cell Proliferation and Induced Cell Cycle Arrest at G1 Phase
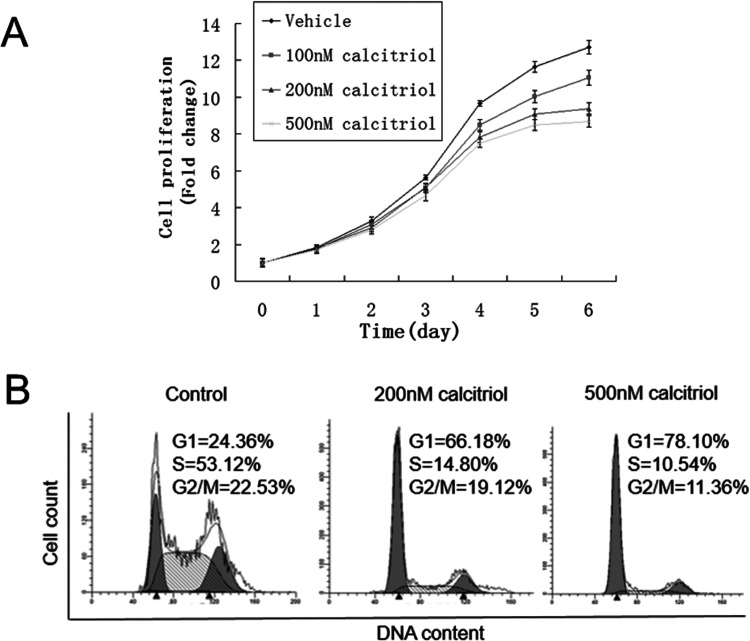
Calcitriol exerted antiproliferative effects on cervical cancer cells in vitro. As shown in Figure 1, cells decreased by 12.8% when treated with 100 nM calcitriol for 6 days, compared with control. Inhibition of cell proliferation became more pronounced with the increase in calcitriol concentration. The decrease was 26.1% and 31.6% for 200 and 500 nM calcitriol, respectively. These data suggested that calcitriol inhibited HeLa S3 cell proliferation in a dose-dependent manner. Given that cell cycle progression has an important effect on cell proliferation, we detected cell cycle distribution of HeLa S3 cells during calcitriol treatment. As shown in Figure 1B, treatment with calcitriol for 72 h induced an evident accumulation of cells in the G1 phase, with approximately 66.18% in 200 nM and 78.10% in 500 nM, compared with the control (24.36%).
Figure 1.
Calcitriol decreased HeLa S3 cell proliferation and induced G1 phase accumulation. (A) HeLa S3 cells were cultured in DMEM with 10% FBS and treated with vehicle (0.1% alcohol), 100 nM, 200 nM, and 500 nM calcitriol. Cell count at day 0 was arbitrarily set as 1. Data shown are proliferation fold changes following 6 days of treatment. (B) HeLa S3 cells treated with 0.1% alcohol (control), 200 nM, or 500 nM calcitriol for 72 h were harvested and subjected to flow cytometry analysis to determine the proportion of cells at each cell cycle phase. ModFit software was used to analyze flow cytometry data.
Calcitriol Decreased HCCR-1 Expression in HeLa S3 Cells
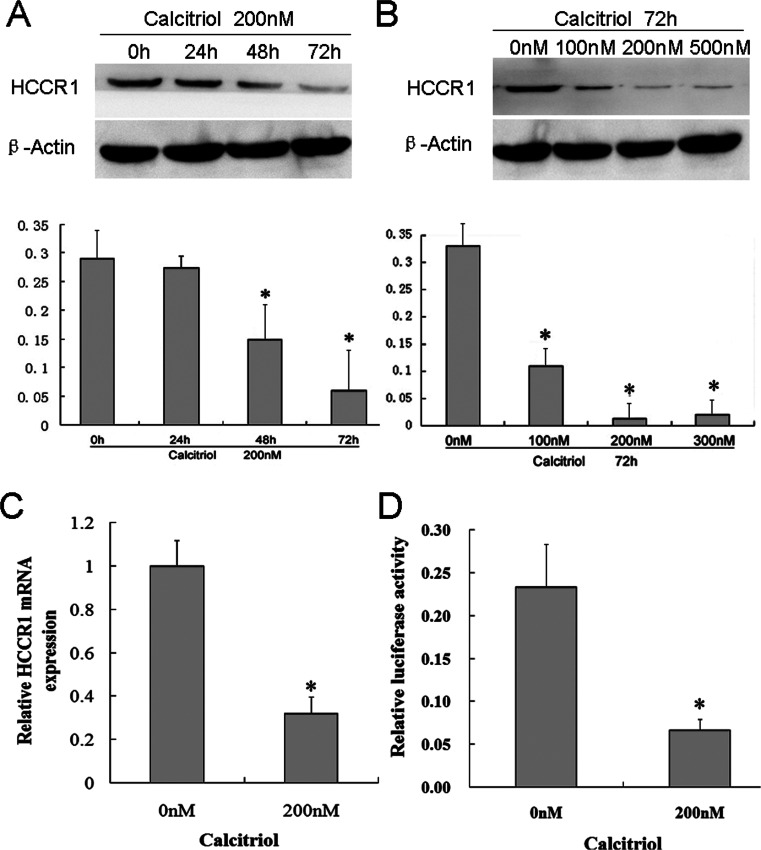
HCCR-1 is evidently overexpressed in cervical cancer, and its function is related to proliferation and cell cycle progression. Hence, we determined whether this gene is affected by calcitriol. As shown in Figure 2, calcitriol treatment significantly decreased HCCR-1 protein expression compared with the control in a time (Fig. 2A, except for that at 24 h)- and dose (Fig. 2B)-dependent manner. To clarify whether downregulation of HCCR-1 results from mRNA transcription, we analyzed HCCR-1 mRNA level and its promoter activity. Treatment of HeLa S3 cell with 200 nM calcitriol for 72 h decreased HCCR-1 mRNA to 32% (p < 0.05) (Fig. 2C). Dual-luciferase assay showed that 200 nM calcitriol significantly decreased HCCR-1 promoter activity to 28% compared with vehicle control (Fig. 2D).
Figure 2.
Calcitriol decreased HCCR-1 expression in HeLa S3 cells at transcriptional and protein levels. (A) HeLa S3 cells were treated with 200 nM calcitriol. At the indicated time point, total protein was isolated and subjected to Western blot analysis to determine HCCR-1 expression. (B) HeLa S3 cells were treated with vehicle (0.1% alcohol), 100 nM, 200 nM, or 500 nM calcitriol for 2 days. Western blot analysis showed that calcitriol inhibited HCCR-1 expression in a dose-dependent manner. (C) HeLa S3 cells were treated with 200 nM calcitriol for 72 h. Real-time PCR was performed to analyze HCCR-1 mRNA expression. (D) Dual-luciferase assay showed that HCCR-1 promoter activity decreased to 28% following 3 days of treatment with 200 nM calcitriol.
Overexpression of HCCR-1 Blocked Calcitriol-Induced Proliferation Inhibition and G1 Phase Accumulation
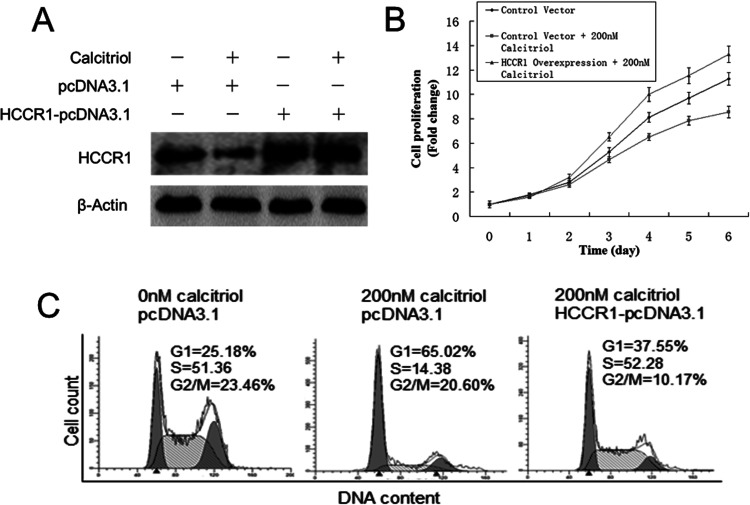
To clarify whether HCCR-1 is a downstream effector of calcitriol, we enforced the expression of HCCR-1 in HeLa S3 cells treated with calcitriol. Cell proliferation was assessed by CCK-8 assay. As shown in Figure 3A, with transfection of control vector (pcDNA3.1), calcitriol also inhibited HCCR-1 expression, which demonstrated that transfection of the control vector had no influence on calcitriol-induced HeLa S3 proliferation defect. Overexpression of HCCR-1 was achieved by delivery of HCCR-1-pcDNA3.1 vector to HeLa S3 cells even when cells were treated with 200 nM calcitriol. Figure 3B shows that 200 nM calcitriol inhibited HeLa S3 cell proliferation by 24% with transfection of the control vector. Overexpression of HCCR-1 through HCCR-1-pcDNA3.1 vector transfection not only blocked calcitriol-induced proliferation inhibition but also increased cell proliferation by 55% compared with cells transfected with control vector following 6 days of treatment. Figure 3C shows that overexpression of HCCR-1 arrested calcitriol-induced G1 phase arrest.
Figure 3.
HCCR-1 overexpression blocked calcitriol-induced HeLa S3 cell proliferation defect and G1 phase accumulation. (A) With transfection of the control vector (pcDNA3.1), 200 nM calcitriol decreased HCCR-1 expression following 3 days of treatment. Delivery of HCCR-1-pcDNA3.1 to HeLa S3 cells enforced HCCR-1 overexpression compared with pcDNA3.1 empty vector (control), even under 200 nM calcitriol treatment. (B) Cell count at day 0 was arbitrarily set as 1. Data shown are proliferation fold changes following 6 days of treatment. (C) HeLa S3 cells were treated with 1% alcohol (control) or 200 nM calcitriol and then transfected with pcDNA3.1 or HCCR-1-pcDNA3.1 as indicated. After 72 h, cells were harvested and subjected to flow cytometry analysis to determine the proportion of cells at each cell cycle phase. ModFit software was used to analyze flow cytometry data.
p21 Was Involved in HCCR-1 Downregulation-Mediated Proliferation Defect and G1 Phase Accumulation
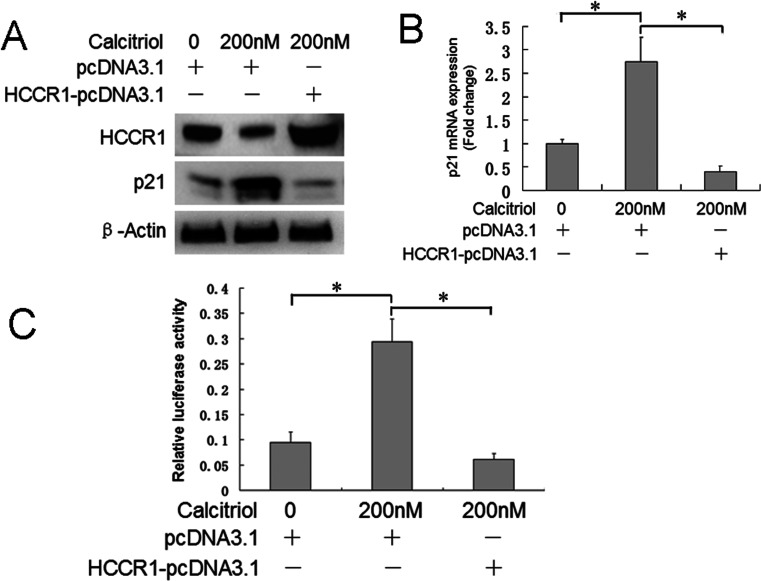
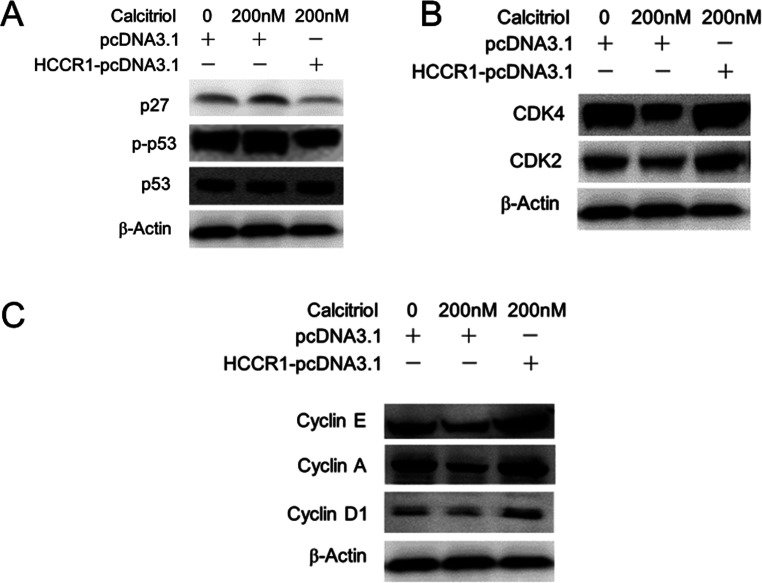
P21 is a potent inhibitor of cell cycle progression and has important roles in cell proliferation. Thus, we determined whether p21 is involved in this process. As shown in Figure 4A, calcitriol increased p21 mRNA, whereas overexpression of HCCR-1 blocked calcitriol-induced p21 protein upregulation. Figure 4B shows that p21 mRNA increased by 2.7-fold after calcitriol treatment, and HCCR-1 overexpression decreased p21 mRNA to 15%. Figure 4C shows that calcitriol enhanced p21 promoter activity by 3.1-fold, and HCCR-1 overexpression decreased p21 promoter activity to 20%. Calcitriol treatment resulted in decreased expression of p27, which is also a G1 CDK inhibitor, as well as decreased upstream factor phosphorylated p53 (Fig. 5A). G1 phase CDKs (CDK2 and CDK4) and cyclins (cyclin D1, cyclin A, and cyclin E) were upregulated by 200 nM calcitriol treatment (Fig. 5B and C). Overexpression of HCCR-1 also reversed the effect of calcitriol on these cell cycle progression-related factors.
Figure 4.
HCCR-1 overexpression blocked calcitriol-induced p21 expression and promoter activation. HeLa S3 cells were treated with 0.1% alcohol (control) or 200 nM calcitriol, transfected with pcDNA3.1 or HCCR-1-pcDNA3.1 as indicated, and cultured for 72 h. (A) Western blot analysis demonstrated that calcitriol-induced p21 protein expression was blocked by HCCR-1 overexpression. (B) Real-time PCR showed that calcitriol-induced p21 mRNA upregulation was blocked by HCCR-1 overexpression. (C) Dual-luciferase assay showed that calcitriol-induced p21 promoter activation was blocked by HCCR-1 overexpression.
Figure 5.
Overexpression of HCCR-1 reversed the effect of calcitriol on cell cycle progression-related factors. HeLa S3 cells were treated with 0.1% alcohol (control) or 200 nM calcitriol, transfected with pcDNA3.1 or HCCR-1-pcDNA3.1 as indicated, and cultured for 72 h. (A) Western blot analysis demonstrated that calcitriol-induced p27 and phosphorylated p53 protein expression was blocked by HCCR-1 overexpression. (B) G1 phase CDKs (CDK2 and CDK4) were upregulated by calcitriol and blocked by HCCR-1 overexpression. (C) G1 phase cyclins (cyclin D1, cyclin A, and cyclin E) were upregulated by calcitriol and blocked by HCCR-1 overexpression.
DISCUSSION
Vitamin D, a fat-soluble vitamin, has long been known for its classic role in bone health and calcium homeostasis. Activities of this steroid hormone are not confined to the maintenance of bone health and mineral metabolism. Vitamin D can be taken in the diet or synthesized in the skin, and it undergoes two hydroxylation steps to generate calcitriol, which is the most active form of vitamin D. Vitamin D and its receptor have been implicated in cancer prevention and treatment. Several observational studies have demonstrated the inverse association between vitamin D serum levels and cancer incidence and mortality (21,22), such as in breast cancer, colorectal cancer, and prostate cancer (23).
Vitamin D or its analogs can enhance anticancer activity of some antineoplastic agents, such as 5-fluorouracil (24), irinotecan oxaliplatin (25), and cisplatin (26). VDR gene polymorphism has been associated with cancer incidence (27,28), such as breast cancer risk (29), ovarian cancer risk (30,31), and pancreatic cancer (32). In vitro cell models showed that vitamin D inhibits the proliferation of numerous cancer cells, such as human pancreatic cancer cells (33) and lung cancer cells (34). These data suggested that the antitumor effect of vitamin D is multifold. In the present study, we showed that calcitriol inhibited HeLa S3 cell proliferation in a dose- and time-dependent manner. Moreover, calcitriol induced HeLa S3 cell arrest at the G1 phase, indicating that cell cycle arrest might be responsible for calcitriol-induced proliferation disability. This in vitro cell experiment provided a possible mechanism for the clinical observation that vitamin D deficiency contributes to high mortality from colorectal cancer.
To date, sufficient evidence indicated that HCCR-1 is a tumorigenic gene. Overexpression of HCCR-1 in NIH3T3 cells induces elevated transformation efficiency and colony formation in soft agar (16). Silencing of HCCR-1 induces apoptosis and suppresses the aggressive phenotype of hepatocellular carcinoma cells in culture (35). HCCR-1 functions as a negative regulator of p53 tumor suppressor, decreasing the expression of the p53-responsive gene, such as CDKNIA, MDM2, and BAX (16). Through direct protein interaction, HCCR-1 mediates HCCRBP3- and HCCRBP2-induced tumorigenesis (36,37). In addition, HCCR-1 is overexpressed in cervical cancer tissue but cannot be detected by RT-PCR in normal cervical tissues (16), suggesting that HCCR-1 might play an important role during cervical tumor development. These data suggested that HCCR-1 is a possible target for antitumor therapy, particularly for cervical carcinoma.
Similar to other nuclear receptors, VDR modulates cell biological behavior by activating or repressing the transcription of target genes. Currently, only a limited number of target genes are regulated by calcitriol. Our data showed that HCCR-1 protein in HeLa cells clearly decreased under calcitriol treatment. Similarly, promoter activity assay indicated that decreased transcription was the mechanism involved in the calcitriol-induced suppression of HCCR-1 expression. These findings suggested that HCCR-1 might be a target of calcitriol. In this investigation, calcitriol treatment decreased HCCR-1 expression in HeLa S3 cells, promoted proliferation, and induced G1 phase accumulation. Enhanced HCCR-1 expression blocked calcitriol-induced proliferation defect and cell cycle arrest, indicating that HCCR-1 downregulation might mediate calcitriol-induced proliferation inhibition and cell cycle arrest.
p21 is a potent cell cycle inhibitor involved in tumorigenesis (19). Our results showed that calcitriol increased p21 expression. However, p21 has been shown to be downregulated by calcitriol in vitro and in vivo (20), which is contrary to our results. The discrepancy in the results may be due to different cell lines used or different cell harvesting times. A previous study demonstrated that HCCR-1 overexpression downregulates p21 expression in NCI-H460 cells and RKO cells (16). We found a similar result; overexpression of HCCR-1 blocked calcitriol-induced p21 upregulation, indicating that p21 was a downstream effector for calcitriol and HCCR-1 to exert control over cell cycle progression. Calcitriol also upregulated other cell cycle progression effectors, and overexpression of HCCR-1 also reversed the effect of calcitriol on cell cycle-related factors.
In summary, cell culture data in HeLa S3 cells suggested that calcitriol could play a beneficial role in the prevention and treatment of cervical cancer in women.
ACKNOWLEDGMENTS
This work was supported by the Provincial Natural Science Foundation Research Project of Shanxi Province (No. 2013JM4030). The authors are grateful to Mr. Shaoli Cheng (Medicine School of Xi’an Jiaotong University, China) in critically revising the manuscript. Guoqing Wang and Kejun Nan conceived and designed the experiments. Guoqing Wang, Xixia Zhao, Jun Zhang, Min Zhou, and Lei Lei performed the experiments. Guoqing Wang and Kejun Nan analyzed the data. Kejun Nan contributed reagents/materials/analysis tools. Guoqing Wang wrote the paper.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. DeLuca H. F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80:1689S–1696S; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Giovannucci E.; Liu Y.; Rimm E. B.; Hollis B. W.; Fuchs C. S.; Stampfer M. J.; Willett W. C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 98:451–459; 2006. [DOI] [PubMed] [Google Scholar]
- 3. Bulathsinghala P.; Syrigos K. N.; Saif M. W. Role of vitamin D in the prevention of pancreatic cancer. J. Nutr. Metab. 2010:721365; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krishnan A. V.; Trump D. L.; Johnson C. S.; Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. North Am. 39:401–418; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garland C. F.; Garland F. C.; Gorham E. D.; Lipkin M.; Newmark H.; Mohr S. B.; Holick M. F. The role of vitamin D in cancer prevention. Am. J. Public Health 96:252–261; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luong K.; Nguyen L. T. The beneficial role of vitamin D and its analogs in cancer treatment and prevention. Crit. Rev. Oncol. Hematol. 73:192–201; 2010. [DOI] [PubMed] [Google Scholar]
- 7. Holick M. F. Vitamin D: Its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 92:49–59; 2006. [DOI] [PubMed] [Google Scholar]
- 8. Krishnan A. V.; Swami S.; Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 77:1107–1112; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norton R.; O’Connell M. A. Vitamin D: Potential in the prevention and treatment of lung cancer. Anticancer Res. 32:211–221; 2012. [PubMed] [Google Scholar]
- 10. Chiang K. C.; Chen T. C. Vitamin D for the prevention and treatment of pancreatic cancer. World J. Gastroenterol. 15:3349–3354; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen T. C.; Holick M. F. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol. Metab. 14:423–430; 2003. [DOI] [PubMed] [Google Scholar]
- 12. Beer T. M.; Myrthue A. Calcitriol in cancer treatment: From the lab to the clinic. Mol. Cancer Ther. 3:373–381; 2004. [PubMed] [Google Scholar]
- 13. Kesic V.; Poljak M.; Rogovskaya S. Cervical cancer burden and prevention activities in Europe. Cancer Epidemiol. Biomarkers Prev. 21:1423–1433; 2012. [DOI] [PubMed] [Google Scholar]
- 14. Hosono S.; Matsuo K.; Kajiyama H.; Hirose K.; Suzuki T.; Kawase T.; Kidokoro K.; Nakanishi T.; Hamajima N.; Kikkawa F.; Tajima K.; Tanaka H. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur. J. Clin. Nutr. 64:400–409; 2010. [DOI] [PubMed] [Google Scholar]
- 15. Kim T. E.; Kim Y. W.; Hwang S. Y.; Shin S. M.; Shin J. W.; Lee Y. H.; Shin S. Y.; Han K. T.; Lee J. M.; Namkoong S. E.; Kim J. W. Candidate tumor suppressor, hccs-1, is downregulated in human cancers and induces apoptosis in cervical cancer. Int. J. Cancer 97:780–786; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Ko J.; Lee Y. H.; Hwang S. Y.; Lee Y. S.; Shin S. M.; Hwang J. H.; Kim J.; Kim Y. W.; Jang S. W.; Ryoo Z. Y.; Kim I. K.; Namkoong S. E.; Kim J. W. Identification and differential expression of novel human cervical cancer oncogene hccr-2 in human cancers and its involvement in p53 stabilization. Oncogene 22:4679–4689; 2003. [DOI] [PubMed] [Google Scholar]
- 17. Ha S. A.; Kim H. K.; Yoo J.; Kim S.; Shin S. M.; Lee Y. S.; Hur S. Y.; Kim Y. W.; Kim T. E.; Chung Y. J.; Jeun S. S.; Kim D. W.; Park Y. G.; Kim J.; Shin S. Y.; Lee Y. H.; Kim J. W. Transdifferentiation-inducing hccr-1 oncogene. BMC Cell Biol. 11:49; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gartel A. L.; Radhakrishnan S. K. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65:3980–3985; 2005. [DOI] [PubMed] [Google Scholar]
- 19. Warfel N. A.; El-Deiry W. S. P21waf1 and tumourigenesis: 20 years after. Curr. Opin. Oncol. 25:52–58; 2013. [DOI] [PubMed] [Google Scholar]
- 20. Hershberger P. A.; Modzelewski R. A.; Shurin Z. R.; Rueger R. M.; Trump D. L.; Johnson C. S. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res. 59:2644–2649; 1999. [PubMed] [Google Scholar]
- 21. Manson J. E.; Mayne S. T.; Clinton S. K. Vitamin D and prevention of cancer—Ready for prime time? N. Engl. J. Med. 364:1385–1387; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Ross A. C.; Manson J. E.; Abrams S. A.; Aloia J. F.; Brannon P. M.; Clinton S. K.; Durazo-Arvizu R. A.; Gallagher J. C.; Gallo R. L.; Jones G.; Kovacs C. S.; Mayne S. T.; Rosen C. J.; Shapses S. A. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 96:53–58; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen C. J.; Adams J. S.; Bikle D. D.; Black D. M.; Demay M. B.; Manson J. E.; Murad M. H.; Kovacs C. S. The nonskeletal effects of vitamin D: An endocrine society scientific statement. Endocr. Rev. 33:456–492; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milczarek M.; Psurski M.; Kutner A.; Wietrzyk J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 13:294; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milczarek M.; Rosinska S.; Psurski M.; Maciejewska M.; Kutner A.; Wietrzyk J. Combined colonic cancer treatment with vitamin D analogs and irinotecan or oxaliplatin. Anticancer Res. 33:433–444; 2013. [PubMed] [Google Scholar]
- 26. Jorgensen A.; Blomberg J. M.; Nielsen J. E.; Juul A.; Rajpert-De M. E. Influence of vitamin D on cisplatin sensitivity in testicular germ cell cancer-derived cell lines and in a NTera2 xenograft model. J. Steroid Biochem. Mol. Biol. 136:238–246; 2013. [DOI] [PubMed] [Google Scholar]
- 27. Huang J.; Huang J.; Ma Y.; Wang H.; Yang J.; Xiong T.; Du L. The Cdx-2 polymorphism in the VDR gene is associated with increased risk of cancer: A meta-analysis. Mol. Biol. Rep. 40:4219–4225; 2013. [DOI] [PubMed] [Google Scholar]
- 28. Huang J.; Yang J.; Wang H.; Xiong T.; Zhang H.; Ma Y.; Wang X.; Huang J.; Du L. The association between the poly(a) polymorphism in the VDR gene and cancer risk: A meta-analysis. Tumour Biol. 34:1833–1838; 2013. [DOI] [PubMed] [Google Scholar]
- 29. Wang H.; Wang W.; Yang D.; Wang S. TaqI polymorphism of VDR gene contributes to breast cancer risk. Tumour Biol. 35:93–102; 2014. [DOI] [PubMed] [Google Scholar]
- 30. Qin X.; Lu Y.; Qin A.; Chen Z.; Peng Q.; Deng Y.; Xie L.; Wang J.; Li R.; Zeng J.; Li S.; Zhao J. Vitamin D receptor Bsmcapital I, ukrainian polymorphism and ovarian cancer risk: A meta-analysis. Int. J. Gynecol. Cancer 23:1178–1183; 2013. [DOI] [PubMed] [Google Scholar]
- 31. Lurie G.; Wilkens L. R.; Thompson P. J.; Carney M. E.; Palmieri R. T.; Pharoah P. D.; Song H.; Hogdall E.; Kjaer S. K.; DiCioccio R. A.; McGuire V.; Whittemore A. S.; Gayther S. A.; Gentry-Maharaj A.; Menon U.; Ramus S. J.; Goodman M. T. Vitamin D receptor rs2228570 polymorphism and invasive ovarian carcinoma risk: Pooled analysis in five studies within the ovarian cancer association consortium. Int. J. Cancer 128:936–943; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L.; Wu B.; Yang L.; Yin G.; Wei W.; Sui S.; Liu J. Association of vitamin D receptor gene polymorphisms with pancreatic cancer: A pilot study in a North China population. Oncol. Lett. 5:1731–1735; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanemaru M.; Maehara N.; Chijiiwa K. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 involves upregulation of cyclin-dependent kinase inhibitor p21 in human pancreatic cancer cells. Hepatogastroenterology 60:1199–1205; 2013. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Q.; Kanterewicz B.; Buch S.; Petkovich M.; Parise R.; Beumer J.; Lin Y.; Diergaarde B.; Hershberger P. A. Cyp24 inhibition preserves 1alpha,25-dihydroxyvitamin D(3) anti-proliferative signaling in lung cancer cells. Mol. Cell. Endocrinol. 355:153–161; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo J.; Yang L.; Zhang Y.; Wang J.; Wan S.; Xia S.; Yang S.; Wang R.; Fang D. Silencing of the HCCR2 gene induces apoptosis and suppresses the aggressive phenotype of hepatocellular carcinoma cells in culture. J. Gastrointest. Surg. 15:1807–1813; 2011. [DOI] [PubMed] [Google Scholar]
- 36. Ha S. A.; Kim H. K.; Yoo J. A.; Kim S.; Shin S. M.; Gong G. H.; Lee Y. K.; Kim J. W. HCCRBP-3 induces tumorigenesis through direct interaction with hccr-1 in human cancers. Mol. Carcinog. 53:30–37; 2014. [DOI] [PubMed] [Google Scholar]
- 37. Ha S. A.; Shin S. M.; Lee Y. J.; Kim S.; Kim H. K.; Namkoong H.; Lee H.; Lee Y. S.; Cho Y. S.; Park Y. G.; Jeon H. M.; Oh C.; Kim J. W. HCCRBP-1 directly interacting with HCCR-1 induces tumorigenesis through p53 stabilization. Int. J. Cancer 122:501–508; 2008. [DOI] [PubMed] [Google Scholar]