Abstract
This study sought to investigate the effect of overexpression of SMAR1 (scaffold/matrix-associated region-binding protein 1) on cell radiosensitivity in breast cancer, as well as elucidate its regulatory mechanism. We constructed a lentiviral expression system to successfully overexpress SMAR1 in human breast cancer cell line MCF7. In addition, overexpression of SMAR1 in MCF7 cells enhanced the radiosensitivity to 89SrCl2. Moreover, overexpression of SMAR1 significantly induced cell apoptosis rate and G2/M phase arrest under the irradiation of 89SrCl2. In addition, Western blot analysis showed that overexpression of SMAR1 in MCF cells significantly increased the expression levels of pP53 (ser15), pP53 (ser20), acP53, and p21 and obviously decreased the expression of MDM2 under the irradiation of 89SrCl2. Notably, these expression changes could be neutralized by PFTα, an inhibitor of p53 signaling pathway that could inhibit p53-dependent transactivation of p53-responsive genes. Therefore, overexpression of SMAR1 may increase radiosensitivity to 89SrCl2 in breast cancer cell line MCF7 by p53-dependent G2/M checkpoint arrest and apoptosis. Enhanced expression of SMAR1 in tumors will help to improve the clinical efficiency of radiation therapy.
Key words: Breast cancer, Scaffold/matrix-associated region-binding protein 1 (SMAR1), Radiosensitivity, p53, G2/M checkpoint arrest, Apoptosis
INTRODUCTION
Breast cancer is one of the most common malignant tumors in women, accounting for approximately 29% of all new cancer cases annually among women (1,2). Radiation therapy is an important modality in the management of breast cancers. Several meta-analyses of individual patient data from large-scale randomized trials have shown that radiotherapy after surgery can reduce the risk of recurrence and breast cancer death (3,4). However, the development of radioresistance presents a significant problem over prolonged courses of treatment (5). Therefore, a better understanding of the molecular mechanisms behind radioresistance in breast cancer cells will help to greatly improve clinical outcomes.
Matrix-associated region-binding proteins (MARBPs) are shown to be implicated in regulation of various physiological processes, such as cell cycle progression, DNA damage repair, and apoptosis (6). SMAR1 (scaffold/matrix-associated region-binding protein 1) is a recently identified MARBP (7), which shows 99% homology with BANP (mapped to the 16q24 locus) in humans (8). It has been suggested that SMAR1 can function as a potent tumor suppressor via interaction with and activation of p53 and subsequently induce G2/M arrest and delay tumor growth in mice (9). Singh et al. also demonstrated that SMAR1 was downregulated in human breast cancers and could cross talk between p53 and TGF-β signaling pathways to regulate tumor growth and metastases (10). Besides tumor-suppressor function, SMAR1 can also function as a transcriptional repressor to repress the expression of cyclin D1, whose higher expression is a hallmark in breast cancer (11). Malonia et al. also demonstrated that SMAR1 could regulate NF-κB-dependent interleukin-8 transcription, which is important for the metastasis and angiogenesis of breast tumors (12). Despite these, the crucial role of SMAR1 in regulation of radiosensitivity or radioresistance in breast cancer has not been fully investigated.
In the present study, we constructed a lentiviral expression system to overexpress SMAR1 in human breast cancer cell line MCF7. MCF7 cells were then treated with different concentrations of strontium-89 chloride (89SrCl2). Clone formation assay, cell apoptosis, and cell cycle analyses were performed to detect whether overexpression of SMAR1 in MCF7 cells could influence radiosensitivity to 89SrCl2. Finally, the expression levels of p53 signaling pathway-related proteins were determined by Western blot analysis. Our study sought to investigate the effect of overexpression of SMAR1 on cell radiosensitivity in breast cancer, as well as elucidate its regulatory mechanism. A combination of radiation therapy and gene therapy may provide a new insight for improving the treatment outcomes of this disease.
MATERIALS AND METHODS
Cell Culture
Human breast cancer cell line MCF7 was obtained from the experimental center of clinical laboratory diagnostics at Bengbu Medical College (Anhui, China). Human embryonic kidney (HEK) 293FT cells were purchased from Invitrogen (Carlsbad, CA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37°C.
Lentiviral Production and Infection of Human Breast Cancer Cell Line MCF7
Recombinant expression plasmid pEGFP-SMAR1 and blank vector pEGFP were provided by Beijing Genomics Institute (BGI, Shenzhen, Guangdong, China). To establish MCF7 cells that could express pEGFP-SMAR1 and a control pEGFP, we used a lentiviral expression system. In brief, the lentivirus packaging vectors pMD2.G (encoding VSV-G), pMDLg/pRRE (encoding gag/pol), and pRSV-Rev (encoding rev) were obtained from Addgene (http://www.addgene.org/). Then the lentivirus packaging vectors pMD2.G, pMDLg/pRRE, pRSV-Rev, and recombinant expression plasmid pEGFP-SMAR1or blank vector pEGFP were cotransfected into 293FT cells using the Lipofectamine2000 (Invitrogen)-mediated transfection method. Forty-eight after transfection, the culture supernatants were collected and filtered through 0.22-µm pore-size filters, and viral particles were concentrated by ultracentrifugation. After the titer of virus was tested using large-scale real-time titration (LaSRT), a multiplicity of infection (MOI) 10 of virus was applied to infect MCF7 cells. Forty-eight after infection, the detection rate of GFP+ cells was measured by fluorescence microscope and flow cytometry. Meanwhile, the total protein was extracted from these infected MCF7 cells to determine whether pEGFP-SMAR1 or pEGFP was successfully expressed using Western blot. MCF7 cells that can successfully express pEGFP-SMAR1 were defined as the MCF7-SMAR1 group, and MCF7 cells that can successfully express pEGFP were the MCF7-c group. Notably, MCF7 cells without any treatment were defined as the control group.
Clone Formation Assay
A specified number of cells (n = 200 cells/each well) of each group were seeded into 24-well culture plates and cultured in DMEM medium with different concentrations (0.01, 0.1, 1, 10 µCi/ml, respectively) of Metastron (89SrCl2), which was obtained from Chengdu Gaotong Isotope Co., Ltd, China. Cells continued to be incubated for colony formation for 14–21 days. Colonies were then fixed with methanol and stained with 0.5% crystal violet. The number of colonies containing at least 50 cells was determined to calculate surviving fractions, and survival curves were then drawn using Kaleidagraph version3.51 (SynergySoftware, Reading, PA, USA).
Cell Apoptosis Analysis
Flow cytometry is an effective method to detect cell apoptosis after drug treatment (13). A specified number of cells of each group were seeded into six-well culture plates. Then cells were treated with 10 µCi/ml of 89SrCl2 for 24 h. Cells were collected and then resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Cell apoptosis analysis was then performed using Annexin-V Apoptosis Detection Kit APC (eBioscience, San Diego, CA, USA) according to the instructions of the manufacturer. Finally, apoptotic cells were analyzed at 488 nm by flow cytometry on a Beckman Coulter FC200 (Fullerton, CA, USA).
Cell Cycle Analysis
A specified number of cells of each group were seeded into six-well culture plates. Then cells were treated with 10 µCi/ml of 89SrCl2 for 24 h. Cells (1 × 106 cells/ml) were harvested by trypsinization, washed by ice-cold PBS, and fixed in ice-cold 70% ethanol. Cells were then incubated with bovine pancreatic RNAase A (500 U/ml; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 min, and then 0.25% propidium iodide (PI; Sigma-Aldrich) was added to stain cells for 30 min at room temperature. Finally, cells in each group were analyzed at 488 nm by flow cytometry on a Beckman Coulter FC200.
p53 Signaling Pathway Analysis
Cells in a different group were treated with 10 µCi/ml of 89SrCl2 for 24 h, and cells without treatment with 89SrCl2 were set as control. Moreover, cells that were exposed with 10 µCi/ml of 89SrCl2 were further treated with 10 µM of pifithrin-α (PFTα), an inhibitor of p53 signaling pathway that could inhibit p53-dependent transactivation of p53-responsive genes (14). Therefore, to further explore the regulatory mechanism involved in SMAR1-enhanced radiosensitivity to 89SrCl2, the expression levels of related proteins in p53 signaling pathway, such as phospho-P53 (pP53, Ser15), pP53 (Ser20), acetylated-P53 (acP53), total P53 (tP53), P21, and MDM2 were determined by Western blot. In addition, clone formation assay was performed to determine the effect of inhibition of p53 signaling pathway.
Western Blot Analysis
Cells in each group were washed three times with ice-cold PBS and resuspended in lysis buffer (iNtRON Biotechnology, Seoul, Korea). Proteins were collected by centrifugation, and the protein concentration was measured using bicinchoninic acid assay (BCA). Equal amounts of protein were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA, USA). After blocked, membranes were probed with primary antibodies [phospho-P53 (Ser15), phospho-P53 (Ser20), acetylated-P53, total P53, P21, and MDM2, respectively]. Membranes were washed and incubated with secondary antibody conjugated with horseradish peroxidase (HRP) at 1:2,000 dilution for 1 h. Antibody binding was detected using an ECL detection kit (Amersham Biosciences, Piscataway, NJ, USA). Notably, the expression level of these proteins was normalized to β-actin.
Statistics Analyses
All the measurement data are presented as the mean ± SD, and the statistical analyses were analyzed with SPSS 20.0 (SPSS Inc., Chicago, IL, USA). A t-test or one-way ANOVA as appropriate was performed for comparisons between groups. A value of p ≤ 0.05 represents statistical significance.
RESULTS
SMAR1 Was Successfully Overexpressed in MCF7 Cells
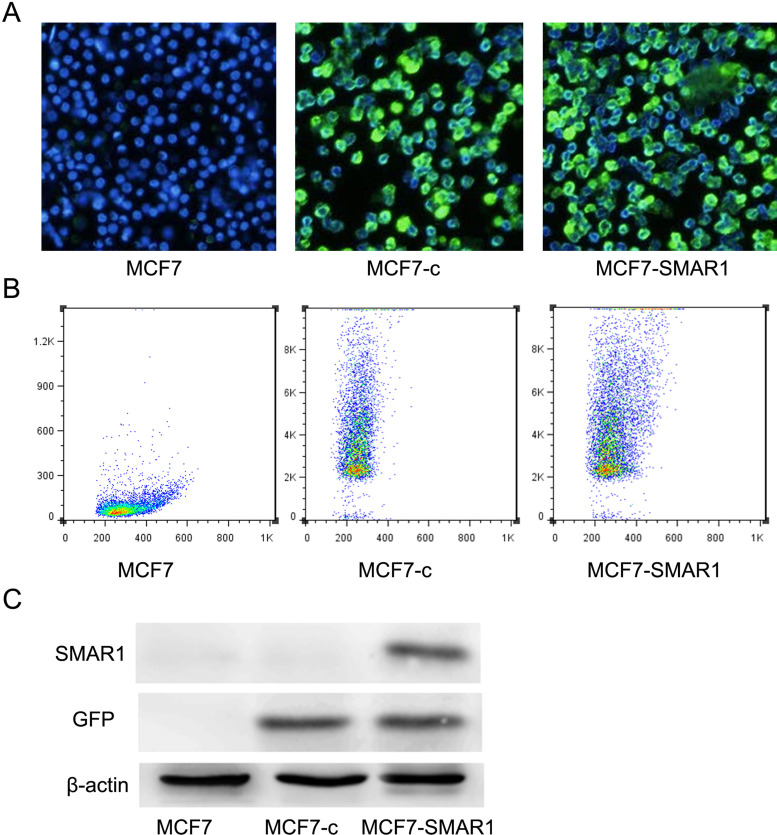
A lentivirus system could be used for the overexpression of SMAR1 in human breast cancer cell line MCF7. The results of fluorescence microscope and flow cytometry analysis showed that the detection rate of GFP+ cells in both MCF7-c group and MCF7-SMAR1 group were higher than 85% (Fig. 1A, B). Meanwhile, Western blot analysis displayed that SMAR1 was not expressed in MCF7 group, and the lentivirus was able to express GFP in both MCF7-c group and MCF7-SMAR1 group (Fig. 1C). Notably, SMAR1 was successfully overexpressed in MCF7-SMAR1 group (Fig. 1C).
Figure 1.
SMAR1 was successfully overexpressed in human breast cancer cell line MCF7. Fluorescence microscope (A) and flow cytometry analysis (B) showed that the detection rate of GFP+ cells in both MCF7-c group and MCF7-SMAR1 group were higher than 85%. Western blot analysis (C) displayed that SMAR1 was successfully overexpressed only in MCF7-SMAR1 group.
Overexpression of SMAR1 in MCF7 Cells Enhanced the Radiosensitivity to 89SrCl2
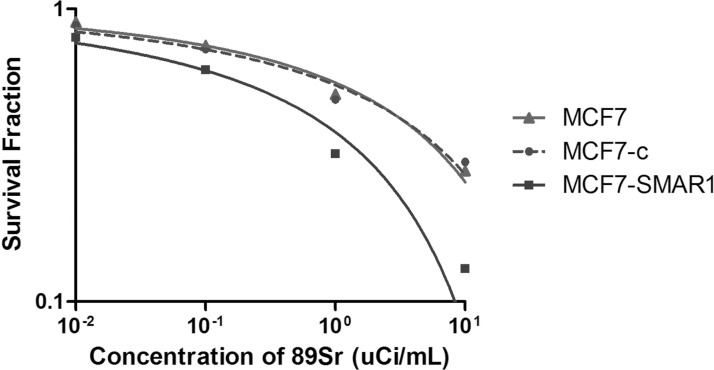
As shown in Figure 2, the survival curves of different groups were fitted using shoe model lining. The results showed that the survival curves of MCF7 group and MCF7-c group almost overlapped, indicating that the lentivirus blank vector had no obvious toxicity to cells. In addition, compared with the survival curve of MCF7 group or MCF7-c group, the shoulder area of the survival curve of MCF7-SMAR1 group was increased and broadened, indicating that overexpression of SMAR1 in MCF7 cells enhanced the radiosensitivity to 89SrCl2.
Figure 2.
Clone formation assay showed the survival curves of MCF7 group, MCF7-c group, and MCF7-SMAR1 group under the treatment of a different concentration of 89SrCl2.
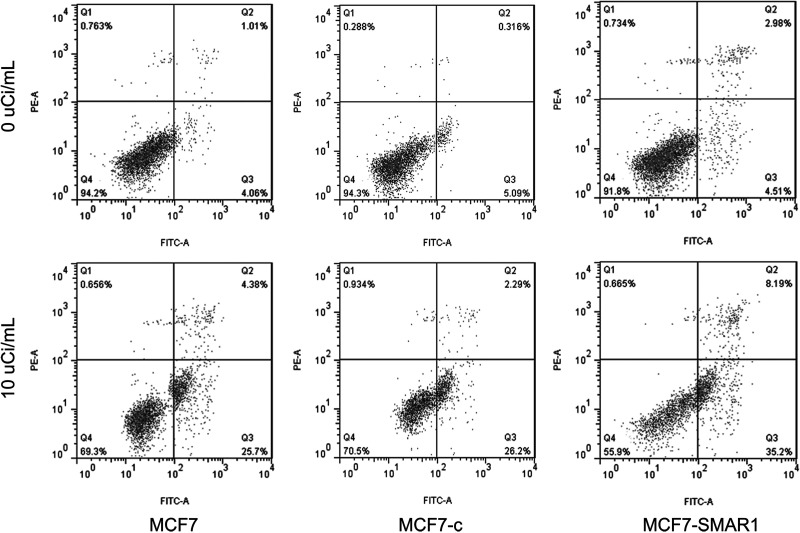
Overexpression of SMAR1 Induced Cell Apoptosis After Exposure to 89SrCl2
Flow cytometry analysis displayed cell apoptosis of different groups after exposure to 10 µCi/ml of 89SrCl2 (Fig. 3). Compared with no exposure to 89SrCl2, 10 µCi/ml of 89SrCl2 could markedly induce cell apoptosis no matter which MCF7 group they were in, the MCF7-c group or the MCF7-SMAR1 group. In addition, under the same dose irradiation, the apoptosis rate of the MCF7-SMAR1 group was higher than in the MCF7 group or the MCF7-c group, indicating that overexpression of SMAR1 enhanced cell radiosensitivity to 89SrCl2 via inducing cell apoptosis.
Figure 3.
Flow cytometry analysis displayed cell apoptosis of a different group after exposure to 10 µCi/ml of 89SrCl2. Under the same dose irradiation of 89SrCl2, the apoptosis rate of MCF7-SMAR1 group was higher than MCF7 group or MCF7-c group.
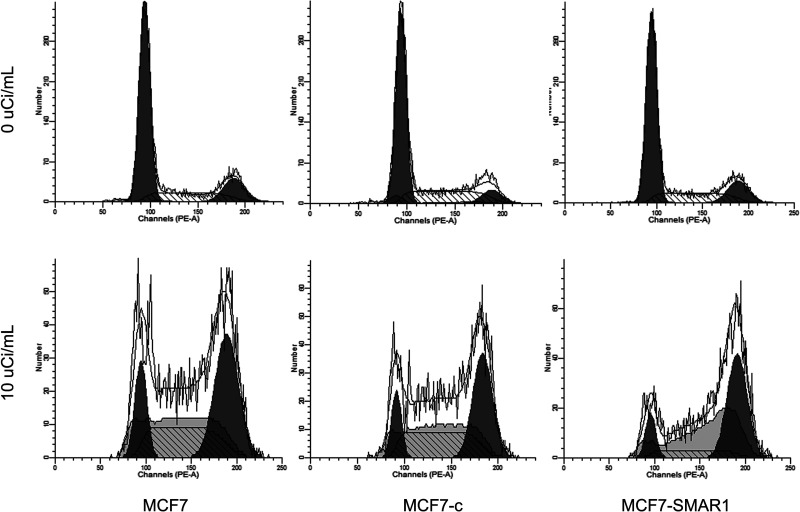
Overexpression of SMAR1 Induced G2/M Phase Arrest After Exposure to 89SrCl2
Flow cytometry analysis also displayed the cell cycle of different groups after exposure to 10 µCi/ml of 89SrCl2 (Fig. 4). The results showed that cells at G2/M phase transition in MCF7-SMAR1 group were significantly increased when compared to the MCF7 group or the MCF7-c group, while cells at S phase in the MCF7-SMAR1 group were obviously decreased, indicating that overexpression of SMAR1 induced G2/M phase arrest after exposure to 89SrCl2.
Figure 4.
Flow cytometry analysis displayed a cell cycle of different group after exposure to 10 µCi/ml of 89SrCl2. The results showed that cells at G2/M phase transition in the MCF7-SMAR1 group were significantly increased than that in the MCF7 group or the MCF7-c group, while cells at S phase in MCF7-SMAR1 group were obviously decreased.
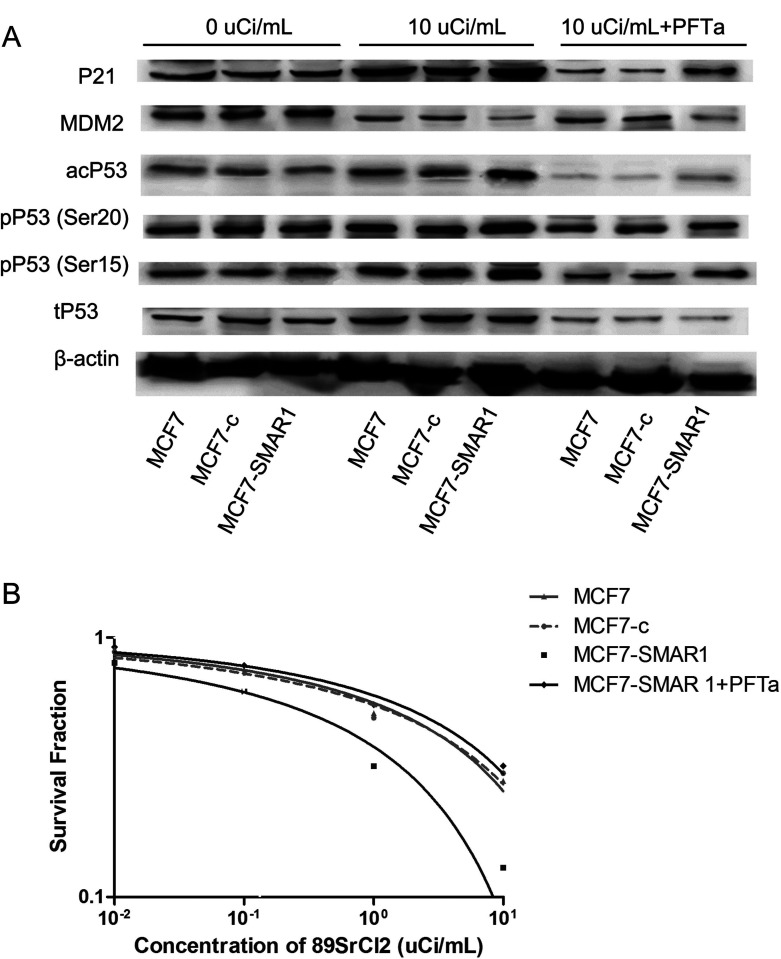
Overexpression of SMAR1 May Enhance the Radiosensitivity to 89SrCl2 via Activation of p53 Signaling Pathway
Western blot analysis showed whether p53 signaling pathway was a key regulatory mechanism involved in SMAR1-enhanced radiosensitivity to 89SrCl2 (Fig. 5A). The results showed that under normal condition (cells were not treated with 89SrCl2 or PFTα), the expression levels of p53 signaling pathway-related proteins in MCF7-SMAR1 group had no change compared with that in the MCF7 group or MCF7-c group. However, under the irradiation of 89SrCl2, the expression level of p53 signaling pathway-related proteins, such as pP53 (ser15), pP53 (ser20), acP53, and p21 in the MCF7-SMAR1 group were significantly increased, while the expression level of MDM2 was decreased compared with that in the MCF7 group or the MCF7-c group, suggesting that activation of p53 signaling pathway might be a key mechanism contributing to SMAR1-enhanced radiosensitivity to 89SrCl2. Notably, after cells were treated with both 89SrCl2 and PFTα (an inhibitor of p53 signaling pathway that could inhibit p53-dependent transactivation of p53-responsive genes), the expression levels of p53 signaling pathway-related proteins in the MCF7-SMAR1 group had no change compared with that in the MCF7 group or the MCF7-c group, indicating that PFTα could effectively inhibit the radiation-activated p53 signaling pathway in the MCF7-SMAR1 group (Fig. 5A).
Figure 5.
p53 signaling pathway analysis by Western blot analysis (A) and clone formation assay (B). (A) Under the irradiation of 89SrCl2, the expression levels of pP53 (ser15), pP53 (ser20), acP53, and p21 in the MCF7-SMAR1 group were significantly increased, while the expression level of MDM2 was decreased compared with that in the MCF7 group or the MCF7-c group. However, after cells were treated with both 89SrCl2 and PFTα, the expression levels of p53 signaling pathway-related proteins in MCF7-SMAR1 group had no change compared with that in the MCF7 group or the MCF7-c group. (B) After cells were treated with both 89SrCl2 and PFTα, PFTα could reverse the SMAR1-enhanced radiosensitivity to 89SrCl2 in the MCF7-SMAR1 group.
Furthermore, the results of clone formation assay showed that after cells were treated with both 89SrCl2 and PFTα, PFTα could reverse the SMAR1-enhanced radiosensitivity to 89SrCl2 in the MCF7-SMAR1 group (Fig. 5B), implying that SMAR1-enhanced radiosensitivity to 89SrCl2 may be associated with activation of the p53 signaling pathway.
DISCUSSION
Breast cancer in women is commonly associated with bone metastases (15). Systemic radionuclide therapy with few side effects has drawn much attention in the treatment of bone metastasis. Currently, 89SrCl2 has been widely and successfully used for the treatment of bone metastases (16,17). To improve the clinical efficiency of radiation therapy, the molecular mechanism underlying radioresistance was explored in our study. Recently, MARBPs are reported to play a significant role in tumor-specific metabolism. In the present study, SMAR1 was successfully overexpressed in human breast cancer cell line MCF7. Moreover, we found that overexpression of SMAR1 in MCF7 cells enhanced the radiosensitivity to 89SrCl2 via inducing cell apoptosis and G2/M phase arrest. In addition, the changes of p53 signaling pathway-related proteins expression implied that activation of the p53 signaling pathway might be a key mechanism involved in SMAR1-enhanced radiosensitivity to 89SrCl2.
In a previous study, SMAR1 was shown to inhibit p53-dependent apoptosis in response to DNA damage (18). Also, Sykes et al. suggested that acetylation of p53 was indispensable for p53-dependent activation of apoptotic targets, such as BAX and PUMA (19). In addition, Jalota et al. confirmed that SMAR1 can interact with p53 and stabilize it through displacing its negative regulator MDM2 (20). p53 can modulate both apoptosis and radiosensitivity, thus to regulate radiotherapy response (21). It has also been confirmed that p53 contributes to the radiosensitivity of lung cancer cells through regulating autophagy and apoptosis (22). In our study, the apoptosis rate of MCF7-SMAR1 group was higher than in the MCF7 group or the MCF7-c group under the irradiation of 89SrCl2, suggesting that SMAR1 may increase radiosensitivity to 89SrCl2 via inducing the apoptosis of breast cancer cells. Furthermore, Western blot analysis showed that the expression level of p53 signaling pathway-related proteins, such as pP53 (ser15), pP53 (ser20), acP53, and p21 in the MCF7-SMAR1 group were significantly increased, while the expression of MDM2 was obviously decreased compared with that in the MCF7 group or the MCF7-c group under the irradiation of 89SrCl2. Moreover, these expression changes could be neutralized by PFTα, an inhibitor of the p53 signaling pathway. Therefore, our results are in line with previous findings and strongly suggest that p53 signaling pathway-related proteins may provoke apoptosis in response to DNA damage after irradiation in breast cancer, and SMAR1 may responsible for the enhanced radiosensitivity to 89SrCl2 in breast cancer cells by p53-dependent cell apoptosis.
Furthermore, it has been suggested that SMAR1 can positively regulate the onset of ionizing radiation-induced G2/M checkpoint (23). Kaul et al. suggested that SMAR1 could cause G2/M delay to retard cell growth via activating p53 through direct interaction (9). In addition, p53 can activate p21 in response to DNA damage, and p21 expression can augment G2/M arrest in human breast cancer cells via a p53-independent mechanism (24). Also, inhibition of MDM2–p53 interaction is reported to augment radiation response in human tumors (25). The modulation of cell cycle progression is shown to be one possibility to improve therapeutic strategy. It is a fact that the radiation-induced G2 phase block is a universal event in cancer cells, rendering the application prospects of G2/M checkpoint arrest in breast cancer cells for improved efficacy of radiation therapy (26). Anastasov et al. also confirmed that the extent of the G2/M arrest following irradiation is associated with the survival of tumor cells, and a potent G2/M checkpoint inhibitor may help to overcome radiation resistance of breast tumors (27). In our study, overexpression of SMAR1 induced G2/M phase arrest after exposure to 89SrCl2. In addition to the changes of p53 signaling pathway-related protein expression, it is thus intriguing to speculate that SMAR1 may induce p53-dependent G2/M checkpoint arrest to increase radiosensitivity to 89SrCl2 and could function as a potent G2/M checkpoint inhibitor to overcome radiation resistance of breast tumors.
In conclusion, our findings indicate that SMAR1 may increase radiosensitivity to 89SrCl2 in breast cancer cell line MCF7 by p53-dependent G2/M checkpoint arrest and apoptosis. Enhanced expression of SMAR1 in tumors will help to improve the clinical efficiency of radiation therapy.
ACKNOWLEDGMENT
This study was supported by Anhui Province Department of Education Fund (KJ2013B148, 2012SQRL099).
REFERENCES
- 1. Stebbing J.; Slater S.; Slevin M. Breast cancer (metastatic). Clin. Evid. 15:2331–2359; 2006. [PubMed] [Google Scholar]
- 2. Siegel R.; Naishadham D.; Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 63:11–30; 2013. [DOI] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S.; McGale P.; Correa C.; Taylor C.; Arriagada R.; Clarke M.; Cutter D.; Davies C.; Ewertz M.; Godwin J.; Gray R.; Pierce L.; Whelan T.; Wang Y.; Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 378:1707–1716; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwong D.; McGale P.; Taylor C.; Correa C.; Cutter D.; Duane F.; Ewertz M.; Gray R.; Mannu G.; Peto R. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127–2135; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jameel J.; Rao V.; Cawkwell L.; Drew P. Radioresistance in carcinoma of the breast. Breast 13:452–460; 2004. [DOI] [PubMed] [Google Scholar]
- 6. Galande S. Chromatin (dis)organization and cancer: BUR-binding proteins as biomarkers for cancer. Curr. Cancer Drug Targets 2:157–190; 2002. [DOI] [PubMed] [Google Scholar]
- 7. Chattopadhyay S.; Kaul R.; Charest A.; Housman D.; Chen J. SMAR1, a novel, alternatively spliced gene product, binds the Scaffold/Matrix-associated region at the T cell receptor beta locus. Genomics 68:93–96; 2000. [DOI] [PubMed] [Google Scholar]
- 8. Birot A.-M.; Duret L.; Bartholin L.; Santalucia B.; Tigaud I.; Magaud J.-P.; Rouault J.-P. Identification and molecular analysis of BANP. Gene 253:189–196; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Kaul R.; Mukherjee S.; Ahmed F.; Bhat M. K.; Chhipa R.; Galande S.; Chattopadhyay S. Direct interaction with and activation of p53 by SMAR1 retards cell-cycle progression at G2/M phase and delays tumor growth in mice. Int. J. Cancer 103:606–615; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Singh K.; Mogare D.; Giridharagopalan R. O.; Gogiraju R.; Pande G.; Chattopadhyay S. p53 target gene SMAR1 is dysregulated in breast cancer: Its role in cancer cell migration and invasion. PloS One 2:e660–616; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rampalli S.; Pavithra L.; Bhatt A.; Kundu T. K.; Chattopadhyay S. Tumor suppressor SMAR1 mediates cyclin D1 repression by recruitment of the SIN3/histone deacetylase 1 complex. Mol. Cell. Biol. 25:8415–8429; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malonia. S. K.; Yadav B.; Sinha S.; Lazennec G.; Chattopadhyay S. Chromatin remodeling protein SMAR1 regulates NF-κB dependent interleukin-8 transcription in breast cancer. Int. J. Biochem. Cell Biol. 55:220–226; 2014. [DOI] [PubMed] [Google Scholar]
- 13. Wlodkowic D.; Skommer J.; Darzynkiewicz Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 559:19–32; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komarov P. G.; Komarova E. A.; Kondratov R. V.; Christov-Tselkov K.; Coon J. S.; Chernov M. V.; Gudkov A. V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285:1733–1737; 1999. [DOI] [PubMed] [Google Scholar]
- 15. Baczyk M.; Czepczynski R.; Milecki P.; Pisarek M.; Oleksa R.; Sowinski J. 89Sr versus 153Sm-EDTMP: Comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl. Med. Commun. 28:245–250; 2007. [DOI] [PubMed] [Google Scholar]
- 16. Falkmer U.; Jarhult J.; Wersall P.; Cavallin-Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol. 42:620–633; 2003. [DOI] [PubMed] [Google Scholar]
- 17. Giammarile F.; Mognetti T.; Resche I. Bone pain palliation with Strontium-89 in cancer patients with bone metastases. Q. J. Nucl. Med. 45:78–83; 2001. [PubMed] [Google Scholar]
- 18. Sinha S.; Malonia S. K.; Mittal S. P.; Mathai J.; Pal J. K.; Chattopadhyay S. Chromatin remodelling protein SMAR1 inhibits p53 dependent transactivation by regulating acetyl transferase p300. Int. J. Biochem. Cell Biol. 44:46–52; 2012. [DOI] [PubMed] [Google Scholar]
- 19. Sykes S. M.; Mellert H. S.; Holbert M. A.; Li K.; Marmorstein R.; Lane W. S.; McMahon S. B. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell 24:841–851; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jalota A.; Singh K.; Pavithra L.; Kaul-Ghanekar R.; Jameel S.; Chattopadhyay S. Tumor suppressor SMAR1 activates and stabilizes p53 through its arginine-serine-rich motif. J. Biol. Chem. 280:16019–16029; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Leszczynska K. B.; Foskolou I. P.; Abraham A. G.; Anbalagan S.; Tellier C.; Haider S.; Span P. N.; O’Neill E. E.; Buffa F. M.; Hammond E. M. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J. Clin. Invest. 125:2385–2398; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng G.; Kong D.; Hou X.; Liang B.; He M.; Liang N.; Ma S.; Liu X. The tumor suppressor, p53, contributes to radiosensitivity of lung cancer cells by regulating autophagy and apoptosis. Cancer Biother. Radiopharm. 28:153–159; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaudhary N.; Nakka K.; Chavali P.; Bhat J.; Chatterjee S.; Chattopadhyay S. SMAR1 coordinates HDAC6-induced deacetylation of Ku70 and dictates cell fate upon irradiation. Cell Death Dis. 5:e1447; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han J.; Kim S.; Yang J.-H.; Nam S. J.; Lee J. E. TPA-induced p21 expression augments G2/M arrest through a p53-independent mechanism in human breast cancer cells. Oncol. Rep. 27:517–522; 2012. [DOI] [PubMed] [Google Scholar]
- 25. Werner L. R.; Huang S.; Francis D. M.; Armstrong E. A.; Ma F.; Li C.; Iyer G.; Canon J.; Harari P. M. Small molecule inhibition of MDM2-p53 interaction augments radiation response in human tumors. Mol. Cancer Ther. 14(9):1994–2003; 2015. [DOI] [PubMed] [Google Scholar]
- 26. Strunz A.; Peschke P.; Waldeck W.; Ehemann V.; Kissel M.; Debus J. Preferential radiosensitization in p53-mutated human tumour cell lines by pentoxifylline-mediated disruption of the G2/M checkpoint control. Int. J. Radiat. Biol. 78:721–732; 2002. [DOI] [PubMed] [Google Scholar]
- 27. Anastasov N.; Hofig I.; Vasconcellos I. G.; Rappl K.; Braselmann H.; Ludyga N.; Auer G.; Aubele M.; Atkinson M. J. Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat. Oncol. 7:206; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]