Abstract
The quantitative measurement of water flow-induced swimming of fish species using a swimmill is a powerful method to evaluate motor ability of individual fish. Zebrafish is a commonly used vertebrate that enables the study of morphological, physiological and behavioral characteristics associated with genes. We here established a reproducible method that allows to measure the body length and the critical swimming speed of adult zebrafish using a swimmill.
Keywords: Motor, Speed, Swimmill, Swimming, Zebrafish
Background
To evaluate motor ability of fish, swimmill systems have been used for measurement of critical swimming speed (Ucrit), which is the maximum water velocity in which fish can keep swimming. Many different swimmill equipments and protocols have been used for zebrafish studies (Table 1) and thus, it was difficult to compare results of different experiments and different protocols due to the lack of detailed calibration methods and protocols. We applied commercially available swimmill equipment Swim Tunnel Respirometer, which is the major and standard one among zebrafish researchers, established a reproducible protocol for obtaining basic swimming characteristics of adult zebrafish, such as adequate water temperature and adequate length of caudal fin among various wild-type strains ( Wakamatsu et al., 2019 ). We here describe the detailed methods to monitor dissolved oxygen for the estimation of oxygen consumption in swimming, to measure body size of adult zebrafish, to conduct calibration of water velocity, to acclimate zebrafish to the narrow swimming chamber and to measure swimming speed. This protocol would be useful for researchers who use swimmill.
Table 1. Swimmill equipments.
The absolute value of Ucrit measured in adult wild-type zebrafish (3-12 months old) appears to vary by the difference of the swimmill equipments. This is probably due to the size difference of swimming chamber that generates uneven water flow between center and peripheral. Therefore, the comparison of Ucrit should be done in the assays that use the same equipment.
| Swimmill | Ucrit (cm/s) | References |
|
Massé et al., 2013 |
||
|
Swim Tunnel Respirometer model 170 ml |
Thomas et al., 2013 |
|
|
230 V, 50 Hz |
25-40 |
Lucas et al., 2016 |
|
Loligo Systems #SW10000 |
Parisi et al., 2018 |
|
|
Wakamatsu et al., 2019 |
||
|
Swim Tunnel Respirometer model 5 L 230 V, 50 Hz Loligo Systems #SW10050 |
70-90 |
Gemberling et al., 2015 Mokalled et al., 2016 |
|
Swim Tunnel Respirometer model 10 L 230 V, 50 Hz Loligo Systems #SW10100 |
40-80 |
Gilbert et al., 2014 Conradsen and McGuigan, 2015 Conradsen et al., 2016 Messerli et al., 2020 |
| Swimmill system made by the author | 50-65 | Plaut, 2000 |
| Swimmill system made by the authors | 55 | Palstra et al., 2010 |
| Swimmill system made by the authors | 25-30 | Leris et al., 2013 |
| Flume | 35-45 | Widrick et al., 2018 |
Materials and Reagents
50 ml conical tube (TrueLine, catalog number: TR2005)
Zebrafish wild-type strain 3-9 months old adult (Neos Pet Shop, catalog number: a2-1020v3)
Green fluorescent PE micro spheres (Loligo Systems, catalog number: AC10555)
Oxygen can 5 L (Pinole, catalog number: 4530896201169)
Sodium sulfite (Wako, catalog number: 192-03415)
Equipment
Swim Tunnel Respirometer model 170 ml, 230 V, 50 Hz (Loligo Systems, model: SW10000)
High-speed video camera (Imaging Development Systems, model: UI-3240CP-C-GL)
Green laser pointer 300 mW, 532 nm (Loligo Systems, model:AC10551)
Eheim pump 5 L/min, 230 V, 50 Hz (Loligo Systems, model: PU10050)
Aquarium heater 300 W (Nisso, model: R300W)
10 ml measurement cylinder (Iwaki, model:3022CYL10S)
Electric balance 0.01 g resolution (A&D, model: FZ-3000i)
Software
AutoRespTM version 2 (Loligo Systems #AR12600)
uEye Cockpit (Imaging Development Systems #UI-3240CP-C-GL)
Digital Particle Tracking Velocimetry (DPTV) (Loligo Systems #AC10550)
ImageJ (https://imagej.nih.gov/ij/)
Microsoft Excel (Microsoft)
Procedure
-
Calibration of water velocity
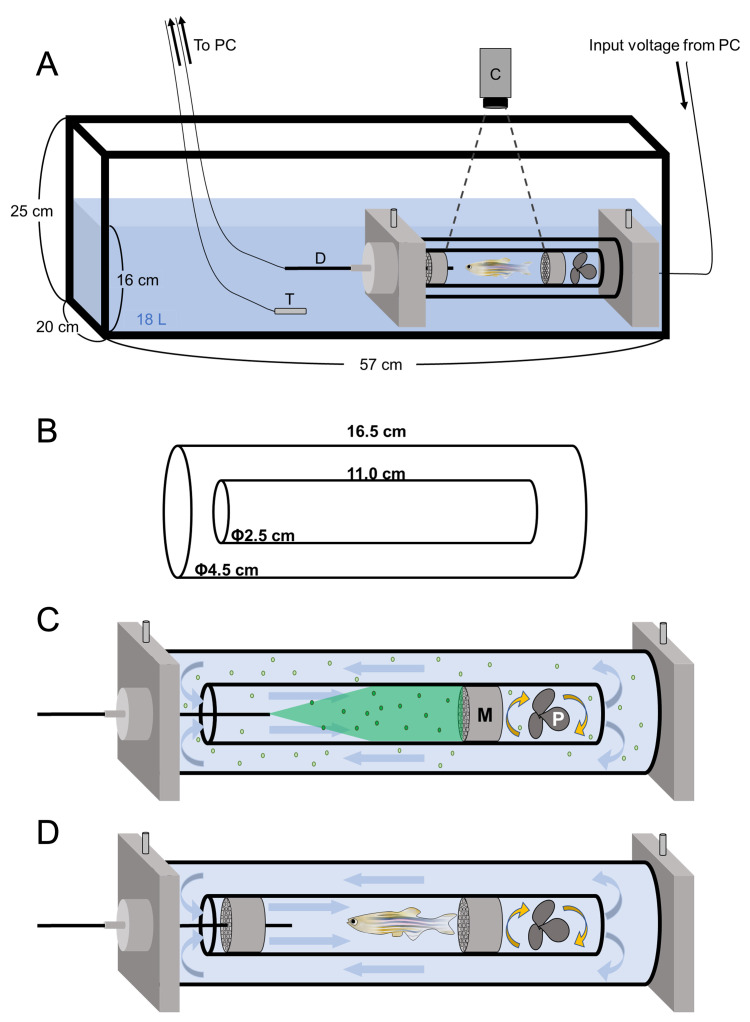
This system consists of an outside tank and a double cylinder (Figure 1A). The outside tank contributes to maintain water temperature of the double cylinder, which is composed of an external cylinder and an inner swimming chamber (Figure 1B). A propeller in the swimming chamber generates water flow in the double cylinder by controlling the propeller rotation depending on an input voltage controlled by the AutoResp software. It is necessary to calibrate the input voltage and water velocity. This calibration can be done by flowing green fluorescent micro beads in the swimming chamber using high-speed camera controlling software uEye Cockpit and analyzing velocity of moving beads using DPTV.
Set up the Swim Tunnel Respirometer with 18 L of water in the outside tank and inside double cylinder along with a heater that maintain the outside water temperature at 26 ± 0.2 °C (Video 1). Fish is not necessary for the calibration of water velocity.
Replace the dissolved oxygen densitometer, which is inserted into the inner swimming chamber, with fluorescent laser pointer for visualization of fluorescent beads (Video 2).
Suspend 1 mg of fluorescent beads in 2 ml of water and then apply it to the double cylinder through a water inlet at the top of the side cover of the double cylinder.
Start the AutoResp software and set the input voltage at 0.5 V, initiating rotation of the propeller to generate water flow in the inner swimming chamber (Figure 1C) (https://loligosystems.com/FileCatalog/GetCatalogItemFileUpload?catalogItemId=82).
Start the uEye Cockpit software for video capturing and record the movement of fluorescent beads in the inner swimming chamber (https://en.ids-imaging.com/ueye-cockpit.html).
Repeat Steps A4 and A5 by changing input voltage from 0.5 to 4.5 V in a stepwise manner with a 0.25 V increment.
Analyze the speed of ~10,000 fluorescent beads in movies using the DPTV software and calculate the water velocity at each input voltage. The user manual of the DPTV software is available here.
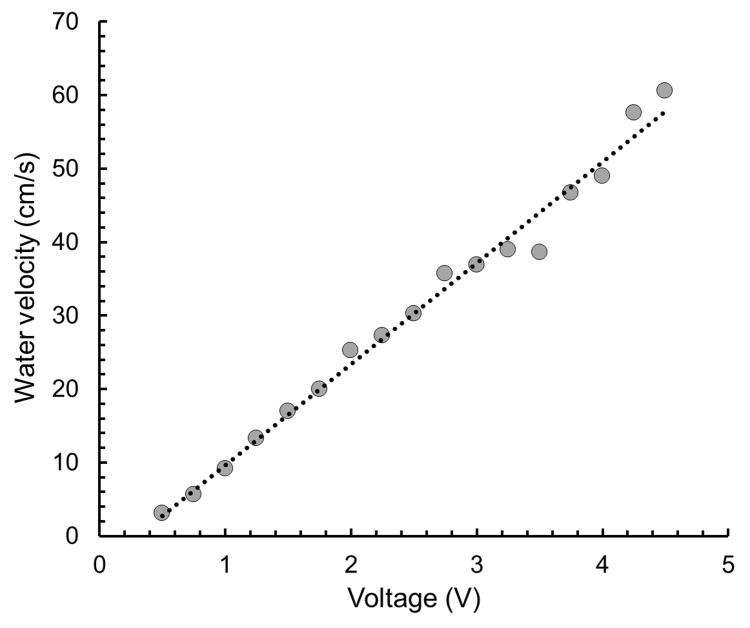
Make a graph of input voltage and water velocity with a best-fit approximate line using Microsoft Excel (Figure 2).
Input the graph parameters in the AutoResp software so as to automatically calibrate the input voltage and water velocity.
-
Calibration of dissolved oxygen concentration
The probe of dissolved oxygen densitometer, which can be inserted into the inner swimming chamber during swimmill assay, records the dissolved oxygen (%) per saturated dissolvable oxygen (constant) at the water temperature. This calibration of dissolved oxygen densitometer can be carried out in the AutoResp software using oxygen-saturated water (100%) and sodium sulfite solution (0%).
Place the probe about 5 cm depth in 40 ml of water in a 50 ml conical tubes and check the dissolved oxygen in the AutoResp software (Video 3).
Bubble ~5 L of oxygen from an oxygen can into water through a nozzle (inner diameter 1 mm) until the value of dissolved oxygen no longer increase.
Calibrate the probe for 100% by setting “Lock Hi” in the AutoResp software.
Dissolve 2 g of sodium sulfite, which is an oxygen scavenger agent, in 40 ml of water to make oxygen-free water.
Clean the probe and place it in the oxygen-free water then calibrate the probe for 0% by setting “Lock Lo” in the AutoResp software.
-
Measurement of swimming speed
Zebrafish (Danio rerio) are reared and maintained at 26-28 °C under a 14 h light and 10 h dark photoperiod and fed twice a day in the regular care. Zebrafish used for swimming assay are kept unfed for 20~24 h prior to the measurement, because swimming speed of zebrafish decreases after eating food. This measurement is done between AM 10:00 and PM 3:00.
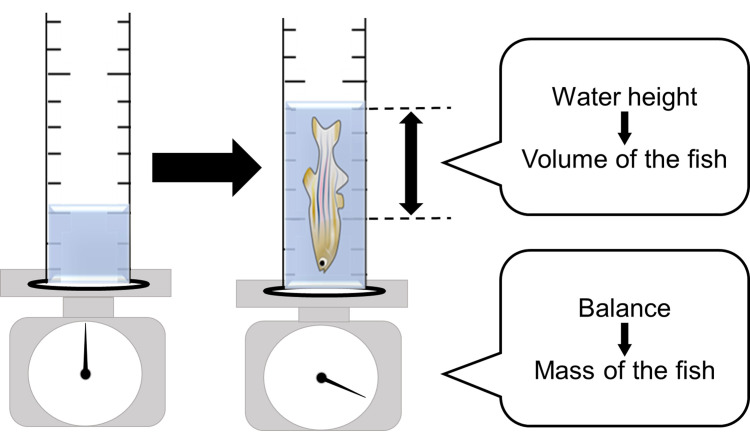
Put an adult zebrafish into a 10 ml measurement cylinder (Φ 11 mm) that contains 5 ml of water and measure the volume (increase of water height) and the mass of the fish by an electric balance (Figure 3).
Transfer the zebrafish into a 50 ml plastic tube filled with water and put it in the outside tank for 10 min to acclimate the fish to the narrow cylinder and water temperature (26 ± 0.2 °C).
Pre-mark 3 cm distance lines on the inner swimming chamber using an oil-based marker. This helps to measure total length and standard length of fish in the analysis of swimming movies (see Data analysis B).
Add a single zebrafish in the inner swimming chamber of the double cylinder (Video 4).
Set the double cylinder in the Swim Tunnel Respirometer.
Start the AutoResp and uEye Cockpit software to be ready for the control of input voltage and video-recording, respectively.
Initiate propeller rotation at 10 cm/s water flow and video-recording simultaneously (Video 5).
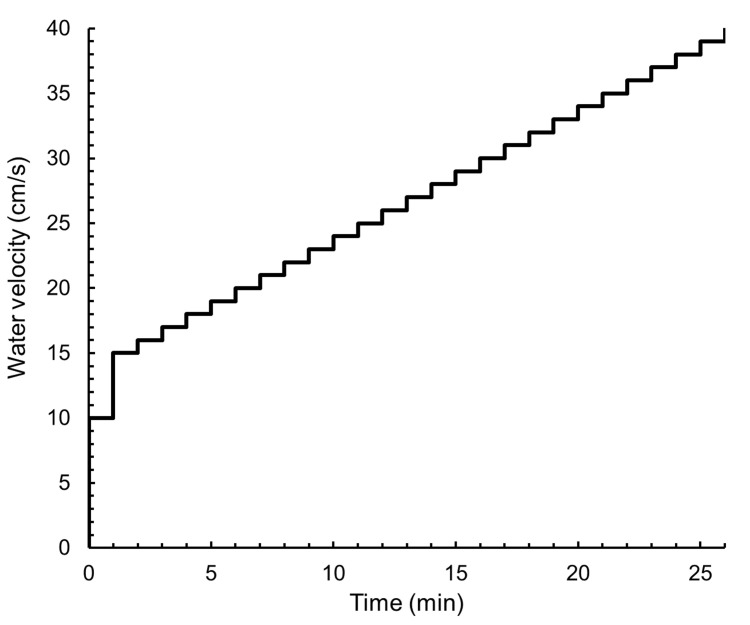
After 1 min of warming-up at 10 cm/s water flow, the water velocity is set at 15 cm/s to start automatic step protocol in the AutoResp software (Figure 4).
After the fish get fatigued and failed to continue swimming (Video 6), stop the propeller rotation and video-recording.
-
Record the water temperature and dissolved oxygen concentration as well as water velocity and time when the fish failed to keep swimming.
Note: If you want to change the water temperature, you can increase the temperature by altering the setting of thermal heater or decrease it by putting an ice bag in the outside water tank.
Figure 1. Structure of the swimmill.
A. The schematic diagram of the Swim Tunnel Respirometer. C: high speed video camera; D: dissolved oxygen densitometer, T: thermometer. B. Size of the double cylinder. C. An enlarged view of the double cylinder. The propeller rotation generates a directional water flow in the double cylinder. Fluorescent beads flow in the double cylinder and glitter by receiving the excitation light from the green laser pointer. M: mesh; P: propeller. D. In the presence of water flow, zebrafish keep swimming by the same speed with water velocity to stay at the same position. Two plastic meshes were put in the swimming chamber to avoid the fatigued fish hit the propeller.
Video 1. Set up the Swim Tunnel Respirometer.
Video 2. Calibration of water velocity.

Figure 2. Voltage-Water velocity plot.
The approximate line becomes straight because water velocity is proportional to the input voltage.
Video 3. Calibration of dissolved oxygen concentration.

Figure 3. Measurement of the volume and mass of adult fish.
The increment of the water height by putting a zebrafish in the measurement cylinder is the volume of the fish. The weight of the fish is simultaneously measurable using an electric balance.
Video 4. Loading zebrafish into Swim Tunnel Respirometer.

Video 5. A zebrafish could keep swimming in 10 cm/s water velocity.

Figure 4. A protocol of the swimming time and water velocity.
The water velocity is initially set at 10 cm/s for 1 min and eventually increased to 15 cm/s for 1 min. Thereafter step protocol increases the water velocity 1 cm/s every 1 min.
Video 6. A zebrafish failed to keep swimming in 33 cm/s water velocity.
Data analysis
-
Calculation of the critical swimming speed
The critical swimming speed ( Ucrit) is the maximum water velocity in which fish can keep swimming. The Ucrit of fish is calculated according to the established formula (Brett, 1964). Ucrit = Umax + T/60. Umax (cm/s): the highest water velocity when zebrafish continued to swim for whole 1 min. T (s): time elapsed when fish failed to keep swimming in 1 min.
-
Measurement of the body size
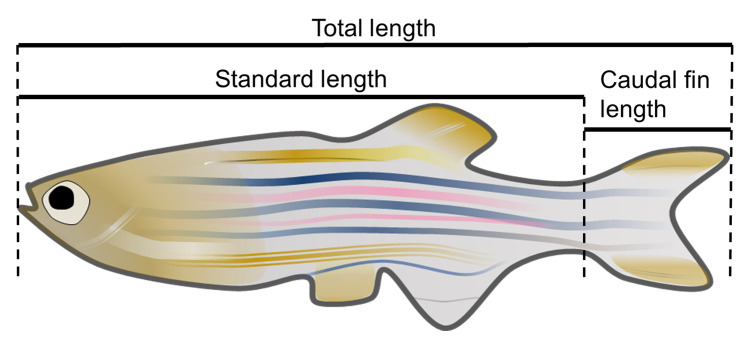
Three different definitions of body size have been established; total length, standard length and caudal fin length (Figure 5) (Plaut, 2000). The total length and standard length are measurable in comparison with pre-marked 3 cm distance lines in a frame of swimming movies (dorsal view). In ImageJ software, draw a line of 3 cm distance and measure the length by pixel using the Analyze/Measure function. Similarly, draw a line along the midline of the fish from the head to the tail and measure the length by pixel and then calculate the actual length by hands. The standard length is calculated by the line from the head to the root of caudal fin. The difference of total and standard lengths is the caudal fin length. The motor ability of zebrafish varies by the length of caudal fin ( Wakamatsu et al., 2019 ).
Figure 5. The definition of body size of zebrafish.
Three different parameters of body size are established in fish studies.
Notes
We could obtain reproducible results using this protocol ( Wakamatsu et al., 2019 ).
Acknowledgements
We thank Hirata Lab members for helpful comments. This work was supported by a Grant-in-Aid for Scientific Research B (16H04657, 19H03329) and Scientific Research on Innovative Areas (17H05578) from the MEXT, Japan, the Naito Foundation and the Japan Epilepsy Research Foundation.
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments described in this manuscript and guidelines for use of zebrafish have been approved by Animal Care and Use Committee at Aoyama Gakuin University (A9: valid until March 2021).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Brett J.(1964). The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Research Board of Can 21: 1183-1226. [Google Scholar]
- 2. Conradsen C. and McGuigan K.(2015). Sexually dimorphic morphology and swimming performance relationships in wild-type zebrafish Danio rerio . J Fish Biol 87(5): 1219-1233. [DOI] [PubMed] [Google Scholar]
- 3. Conradsen C., Walker J. A., Perna C. and McGuigan K.(2016). Repeatability of locomotor performance and morphology-locomotor performance relationships. J Exp Biol 18): 2888-2897. [DOI] [PubMed] [Google Scholar]
- 4. Gemberling M., Karra R., Dickson A. L. and Poss K. D.(2015). Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 4: e05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert M. J. H., Zerulla T. C. and Tierney K. B.(2014). Zebrafish(Danio rerio) as a model for the study of aging and exercise: physical ability and trainability decrease with age . Experimental Gerontology 50: 106-113. [DOI] [PubMed] [Google Scholar]
- 6. Leris I., Sfakianakis D. G. and Kentouri M.(2013). Are zebrafish Danio rerio males better swimmers than females? J Fish Biol 83(5): 1381-1386. [DOI] [PubMed] [Google Scholar]
- 7. Lucas J., Percelay I., Larcher T. and Lefrancois C.(2016). Effects of pyrolytic and petrogenic polycyclic aromatic hydrocarbons on swimming and metabolic performance of zebrafish contaminated by ingestion. Ecotoxicol Environ Saf 132: 145-152. [DOI] [PubMed] [Google Scholar]
- 8. Massé A. J., Thomas J. K. and Janz D. M.(2013). Reduced swim performance and aerobic capacity in adult zebrafish exposed to waterborne selenite. Comp Biochem Physiol C Toxicol Pharmacol 157(3): 266-271. [DOI] [PubMed] [Google Scholar]
- 9. Messerli M., Aaldijk D., Haberthur D., Ross H., Garcia-Poyatos C., Sande-Melon M., Khoma O. Z., Wieland F. A. M., Fark S. and Djonov V.(2020). Adaptation mechanism of the adult zebrafish respiratory organ to endurance training. PLoS One 15(2): e0228333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mokalled M. H., Patra C., Dickson A. L., Endo T., Stainier D. Y. and Poss K. D.(2016). Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354(6312): 630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palstra A. P., Tudorache C., Rovira M., Brittijn S. A., Burgerhout E., den Thillart G. E., Spaink H. P. and Planas J. V.(2010). Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One 5(12): e14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parisi A., Blattmann P., Lizzo G., Stutz V., Strohm L., Richard J., Civiletto G., Charpagne A., Raymond F., Gobet C., et al. (2018). PGC1a and Exercise Adaptations in Zebrafish. BioRxiv 483784. [Google Scholar]
- 13. Plaut I.(2000). Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio . J Exp Biol 4): 813-820. [DOI] [PubMed] [Google Scholar]
- 14. Thomas J. K., Wiseman S., Giesy J. P. and Janz D. M.(2013). Effects of chronic dietary selenomethionine exposure on repeat swimming performance, aerobic metabolism and methionine catabolism in adult zebrafish(Danio rerio) . Aquatic Toxicology 130: 112-122. [DOI] [PubMed] [Google Scholar]
- 15. Wakamatsu Y., Ogino K. and Hirata H.(2019). Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Sci Rep 9(1): 16307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Widrick J. J., Gibbs D. E., Sanchez B., Gupta V. A., Pakula A., Lawrence C., Beggs A. H. and Kunkel L. M.(2018). An open source microcontroller based flume for evaluating swimming performance of larval, juvenile, and adult zebrafish. PLoS One 13(6): e0199712. [DOI] [PMC free article] [PubMed] [Google Scholar]