Abstract
Expansion of fibrous connective tissue and abnormal deposition of extracellular matrix (ECM) are at the basis of many fibrotic diseases. Fibrosis can occur in response to both physiological and pathological cues, including wound healing, tissue remodeling/repair and inflammation. Chronic fibrosis can lead to severe tissue damage, organ failure and death. Assessing the extent of organ fibrosis is crucial for accurate diagnosis of this condition. The use of Masson’s trichrome staining of tissue sections from skeletal muscle is a fast method for detection of morphological alterations indicative of a fibrotic phenotype in this organ. This staining method detects the extent of collagen fibers deposition and, because it employs the combination of three dyes, can also distinguish muscle fibers (red), from collagen (blue) and nuclei (black), simultaneously.
Keywords: Masson’s Trichrome, Tissue section, Collagen, Fibrosis, Skeletal muscle, Fibroblasts
Background
Fibrosis is the formation of excessive fibrous connective tissue in an organ as a result of chronic inflammation, tissue damage/remodeling (for instance, following chemical or radiation therapy), persistent infections, autoimmune disease, allergic responses and cancer. During this process excessive extracellular matrix (ECM) components, including collagens, are deposited and accumulate. If progressive, fibrosis becomes chronic, ultimately leading to organ failure and even death ( Rockey et al., 2015 ). Several types of fibrotic diseases have been described in humans, many of which are of unknown etiology. The organs most commonly affected are the lungs, kidneys, liver, heart and skeletal muscle ( Hinderer et al., 2019 ; Majo et al., 2019 ; Mahdy, 2019). Idiopathic pulmonary fibrosis, for instance, is a common, progressive and often fatal disease associated with scarring of the lung tissue that gradually looses the capacity to oxygenate, leading to the patient’s inability to breathe properly ( Lederer et al., 2018 ).
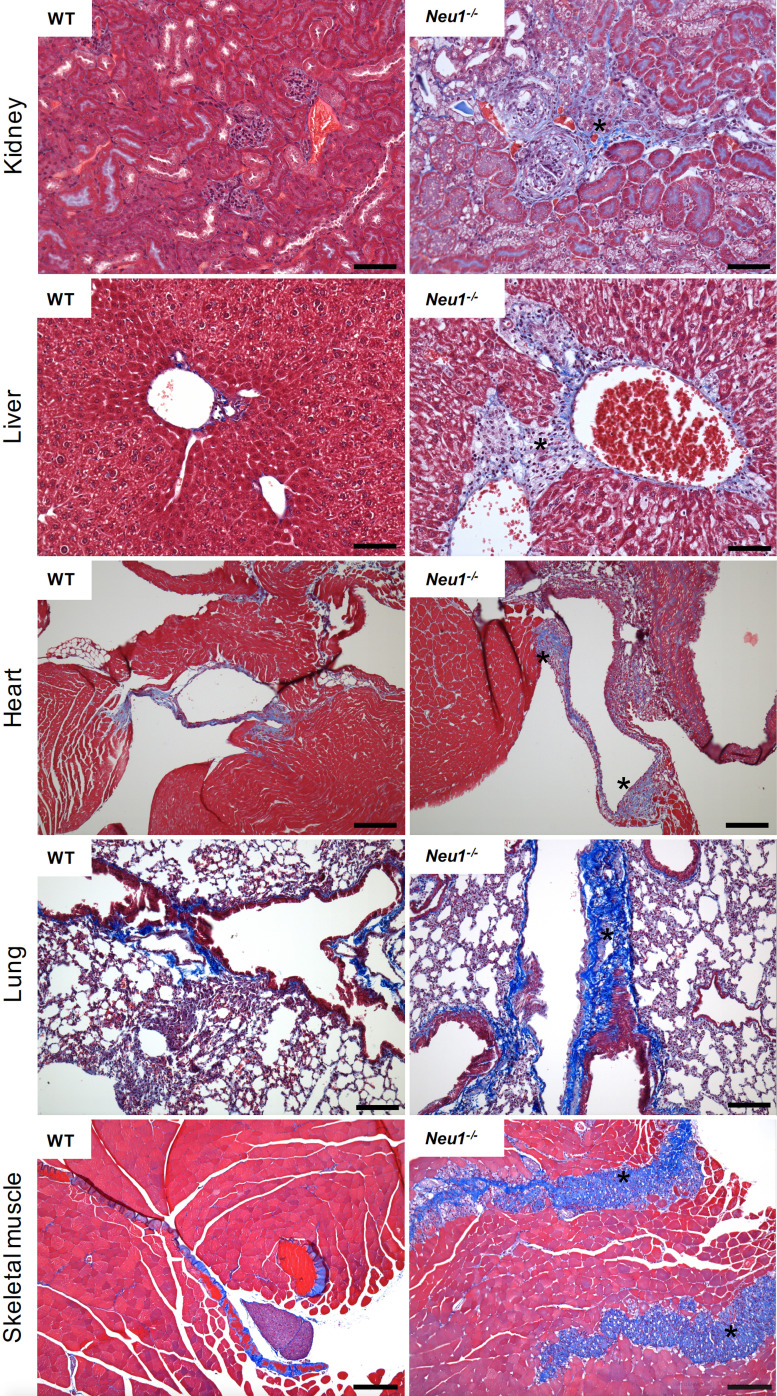
A fibrotic process entails the activation of fibroblasts into myofibroblasts, cells that acquire a stellate or spindle-shape morphology, are motile and contractile, express α-smooth muscle actin, and secrete/remodel the ECM, altering the stiffness, morphology and composition of the tissue ( Gattazzo et al., 2014 ; Kendall et al., 2014 ; Rockey et al., 2015 ). In the skeletal muscle under healthy conditions fibrosis occurs in the form of scar tissue during the healing process following muscle injury. This is usually associated with infiltration of inflammatory cells that induce satellite cells to proliferate and differentiate into new myotubes and myofibers while simultaneously the ECM undergoes remodeling. In disease conditions, such as muscular dystrophies or myopathies, progressive expansion of the connective tissue and abnormal deposition of ECM components, consequent to myofiber degeneration, ultimately results in full-blown fibrosis (Mahdy, 2019). We have recently discovered that deficiency of the sialic acid processing enzyme neuraminidase 1 (NEU1) in the mouse model of the rare pediatric lysosomal storage disease sialidosis, triggers a persistent expansion of the connective tissue, leading to generalized fibrosis in several organs including the liver, kidney, heart and skeletal muscle ( Zanoteli et al., 2010 ; van de Vlekkert et al., 2019 ). Detection of the fibrotic disease was carried out using Masson’s trichrome staining of tissue sections from skeletal muscle, lung, heart, kidney and liver (Figure 1). This method is generally more accurate and informative than the standard hematoxilin & eosin staining because Masson’s trichrome staining not only maintains intact the overall morphology of the tissues, but also has the advantage of utilizing three dyes that allow for the identification of multiple tissue structures. In skeletal muscle the three dyes, Weigert’s iron hematoxylin, Biebrich scarlet and aniline blue, applied sequentially, allow to distinguish nuclei, muscle fibers and erythrocytes, and collagen fibers, respectively (Figure 1). This method is suitable for the assessment of the extent and distribution of fibrosis in a quantifiable manner, and can be successfully applied for the detection/diagnosis of fibrotic diseases.
Figure 1. Masson’s Trichrome staining of multiple organs collected from the WT and Neu1–/– mice.
Fibrotic regions in the Neu1–/– mouse are characterize by massive collagen deposition and therefore appear in blue (asterisks) (Images of WT and Neu1–/– kidney, liver and skeletal muscle are from van de Vlekkert et al., 2019 ). Blue = collagens; Red = erythrocytes and cytoplasm; Dark purple/black = nuclei. Scale bars: kidney and liver = 100 μm, heart, lung and skeletal muscle = 200 μm.
Materials and Reagents
Note: All reagents and materials should be kept at room temperature unless otherwise described. For the shelf life and storage temperature of reagents we refer to the manufacturer’s instructions.
Lab coat and gloves
BD tuberculin syringes (Fisher, BD Biosciences, catalog number: 14-826-88 or BD309626)
Exel International 10 to 12cc syringes (Fisher, catalog number: 14-841-54)
Needles (25 G x 5/8 inch): BD General use and precisionGlide hypodermic needle (Fisher, BD Biosciences, catalog number: 14-826AA or BD305122)
Aluminum dissecting pan with vinyl dissecting pad (Carolina, catalog number: 629004)
Light duty paper wipes (Georgia Pacific, Brawny, catalog number: 29221)
50 ml Falcon conical centrifuge tubes (Fisher Scientific, Corning, catalog number: 14-432-22)
Scientific slide holder (Starplex Scientific, catalog number: V302SH)
Tissue-Tek® base molds, stainless steel (Sakura, catalog number 4217)
Tissue-Tek® Uni-cassette, white (Sakura, catalog number: 4170)
Superfrost Plus Microscope slides, white (Fisher Scientific, Fisherbrand, catalog number: 12-550-15)
Microscope cover glass (24x50-1.5) (Fisherbrand, Fisher Scientific, catalog number: 12-544-E)
Microscope slide box (Fisherbrand, Fisher Scientific, catalog number: 03-448)
-
Adult control and experimental mice (e.g., WT and Neu1-/-)
Notes:
The generation and initial characterization of the Neu1-/- is described in de Geest et al., 2002. WT and Neu1-/- mice are maintained in house.
The gastrocnemius muscle, liver, kidneys, lung and heart from WT and Neu1 - / - mice (FVB/NJ) at 4 months of age are used for the purpose of this protocol. Any background, gender, age or tissue type (paraffin or frozen) can be used for Masson’s staining.
Avertin (tribromoethanol; 12.5 mg/ml); provided by ARC veterinary services at SJCRH
Dulbecco’s Phosphate Buffered Saline (DPBS) (Corning, catalog number: 21-031-CV)
Advantus T-pins (Fisher scientific, catalog number: S174301)
Prefilled container, 10% Neutral Buffered Formalin, 180 ml (Thermo Scientific, catalog number: 591801)
Paraffin (Thermo Fisher Scientific, catalog number: 8337)
Histoprep Xylene (Fisherbrand, catalog number: HC7001GAL)
Ethanol 200 proof (Pharmco by Greenfield Global, catalog number: 111000200)
Ethanol 190 proof (Pharmco by Greenfield Global, catalog number: 111000190)
Ethanol 140 proof (Pharmco by Greenfield Global, catalog number: 111000140)
Bouin’s Fixative (Polysciences, catalog number: 16045-1)
Hematoxylin (Polysciences, catalog number: 02749-25)
29% ferric chloride (Rowley Biochemical, catalog number: SO-125A)
Hydrochloric acid (Fisher Scientific, catalog number: A144S-500)
Biebrich scarlet, C.I.26905 (Polysciences, catalog number: 0336-100)
Acid fuchsin, C.I. 42685 (Polysciences, catalog number: 24991)
Acetic acid, glacial (Fisher Scientific, catalog number: A38S-500)
Phosphotungstic acid (Sigma, catalog number: P4006-25)
Phosphomolybdic acid (Polysciences, catalog number: 01021-25)
Aniline blue, C.I. 42755 (Polysciences, catalog number: 02570-25)
Cytoseal XYL (Thermo Fisher Scientific, catalog number: 8312-4)
-
Weigert’s iron hematoxylin (see Recipes)
Solution A
Solution B
Biebrich scarlet-acid fuchsin solution (see Recipes)
Phosphotungstic/phophomolybdic acid (see Recipes)
Aniline blue (see Recipes)
1% acetic acid (see Recipes)
Equipment
Dissection tools: sterilized scissors and forceps (Roboz, catalog numbers: RS-5983, RS-5877, RS-5358, RS-5135)
Isotemp Incubator (Fisherbrand, Fisher Scientific, catalog number: 15-103-0513)
-
Tissue processor Excelsior AS (Thermo Fisher Scientific, catalog number: A82300001).
Note: For manufacturer instructions go to: https://assets.fishersci.com/TFS-Assets/APD/manuals/A82310100_05%20-%20Operator%20Guide%20-%20Hi%20Res.pdf
Heated paraffin embedding station (HistoCore Arcadia H, Leica Biosystems)
Cold plate (HistoCore Arcadia C, Leica Biosystems)
Shandon Para Trimmer (Thermo Fisher Scientific, catalog number: B3120205)
-
Microtome fully automated rotary (Leica Biosystems, catalog number: RM2255).
Note: For manufacturer instructions go to: https://drp8p5tqcb2p5.cloudfront.net/fileadmin/downloads_lbs/Leica%20RM2255/User%20Manuals/Leica_RM2255_IFU_2v3J_en.pdf
American Painter brush, ½ inch (Loew-Cornell, series 4550 Wash)
Water bath for paraffin sections (Leica Biosystems, model: Leica HI1210)
Bead bath (Precision Scientific, model 83, catalog number: 66551)
Lab Armor Beads (Thermo Fisher Scientific, catalog number: A1254301)
Glass Coplin slide staining jars (Thermo Fisher Scientific, catalog number: E94)
General purpose themometer (Fisherbrand, catalog number: 13-201-644)
Fume hood present in the laboratory
-
Gemini AS automated slide stainer (Thermo Fisher Scientific, catalog number: A81500002).
Note: For manufacturer instructions go to: https://assets.thermofisher.com/TFS-Assets/APD/manuals/Gemini%20AS%20Operator%20Guide.pdf
Light microscope with camera (Leica, catalog numbers: DM2500 and DF450)
Software
Imaging Software. Leica Application Suite (LAS) Version 4.9.0 (Build:129) (Leica Microsystems, CMS GmbH)
Procedure
Notes:
Mice should be cared for and used in accordance with national and institutional policies. All protocols must be approved by the institutional animal committee.
Tissue dissection can be performed outside a biosafety cabinet without need of sterility.
-
Mouse perfusion
Deeply anesthetize the mouse by intraperitoneal injection of Avertin (12.5 mg/ml) at 20 μl/g of body weight.
Pin mouse down in a supine position (ventral side up) to the dissecting pad. Spray the mouse with 70% ethanol.
Open a sterile wrap with scissors and forceps and place a prefilled 10% neutral buffered formalin container in the work area.
Cut open the fur, then cut open the peritoneum along the midline. Cut through the rib cage along the midline.
Cut away part of the rib cage with sternum to expose the heart and lungs.
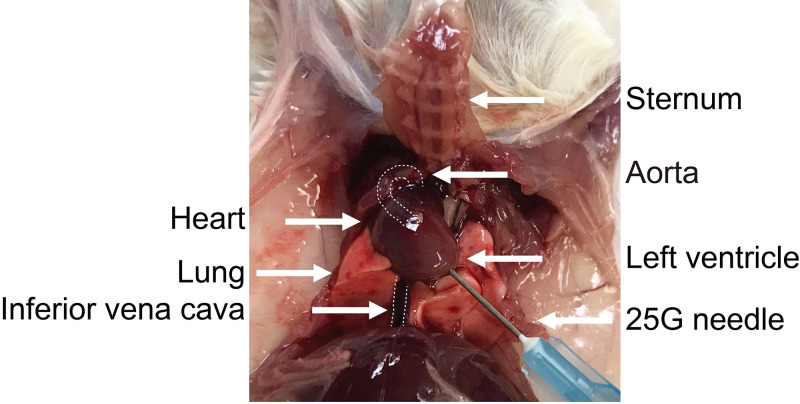
Snip the inferior vena cava (IVC) to exsanguinate the mouse (Figure 2).
-
Take a DPBS filled 10 ml syringe attached to a 25 G x 5/8 inch needle and insert it into the left ventricle in the direction of the aorta (Figure 2). Gently push the plunger of the syringe to perfuse the mouse.
Notes:
The cardiac perfusion is initiated before cessation of the heartbeat.
The perfusion with DPBS is done to clear the tissues from the blood that might interfere with subsequent staining. As an indication of correct perefusion, blood should drain out of the IVC. Organs like the lungs, kidneys and liver should gradually become pale as a sign of good perfusion.
Cardiac perfusion can also be performed using a peristaltic pump.
-
Kidney dissection
Retract the intestine and surrounding tissues to expose the kidneys.
Cut the renal arteries to isolate the kidneys.
Carefully remove the kidneys from the abdominal cavity and place them on the dissecting pad, trim the fat attached to the tissues.
-
Place the kidneys in the pre-filled 10% neutral buffered formalin container. Fix the kidneys for 48 h and proceed directly to Step F1.
Notes:
To better fix the kidneys, they can be cut in 1/2(longitudinal or sagittal cut).
Routinely, for formalin fixed tissues all specimens are placed together in a container large enough to allow a ratio between tissue specimens and neutral buffered formalin (10%) volume of 1 to 15/20x.
Tissue specimens can be stored in containers filled with 10% Neutral Buffered Formalin indefinitely at room temperature without the risk of over-hardening or shrinking of the tissue.
-
Liver dissection
Cut any membranes attaching the liver to the diaphragm, the stomach and small intestine.
Cut the blood vessels which are visible between the liver and diaphragm.
Remove the entire liver from the abdominal cavity.
Place the liver in the pre-filled 10% neutral buffered formalin container. Fix for 48 h and proceed directly to Step F1.
-
Lungs and heart dissection
Cut the esophagus and trachea close to the head.
Using forceps lift the lungs and the heart by the remaining trachea and/or esophagus.
Cut any membranes attached to the diaphragm.
Once removed from the thoracic cavity, place the organs on the dissecting pad and separate the heart from the lungs. Discard the thymus if still attached.
Place the lungs and heart in the pre-filled 10% neutral buffered formalin container. Fix for 48 h and proceed directly to Step F1.
-
Gastrocnemius (GA) muscle dissection
Pin the mouse down in a prone position (dorsal side up) to the dissecting pad. Spray the mouse with 70% ethanol.
-
Remove the skin from both hind limbs to expose the muscles and gently cut away the fascia of both hind limb muscles.
Note: For the isolation of other muscle types, position the mouse in a supine position (ventral side up) onto the dissecting board.
Cut the Achilles tendon of the muscle.
-
Carefully pull the GA muscle upwards towards the knee joint, using scissors to remove the surrounding muscles.
Note: Do not pull too hard as this might affect the tissue integrity and create artifacts in the stained tissue sections.
When the superior tendon close to the knee joint becomes visible, cut it and remove the GA muscle.
Place the muscles in the pre-filled 10% neutral buffered formalin container. Fix for 48 h and proceed directly to Step F1.
-
Tissue processing
Place the tissues in labelled Tissue-Tek Uni-cassettes.
-
Process the tissues overnight using an Excelsior AS tissue processor (Table 1).
Notes:
Alternatively, the tissue can be processed manually.
Steps 1-6 (dehydration) are done to replace the water in the tissues/cells by alcohol. The gradual increase in alcohol concentration is done to avoid distortion of the tissue. Steps 7-9 (clearing) are done to remove the alcohol. Alcohol cannot form a homogeneous mixture with paraffin; therefore, xylene is used as an intermediate solvent. Steps 10-12 are done to displace xylene with paraffin to make the tissues ready for embedding.
The incubation time of each step is based on the thickness of the tissues (10-15 mm).
Turn on the cold plate and make sure the paraffin embedding station is on and the paraffin is liquid.
Choose an appropriate size base mold and add some liquid paraffin into it.
-
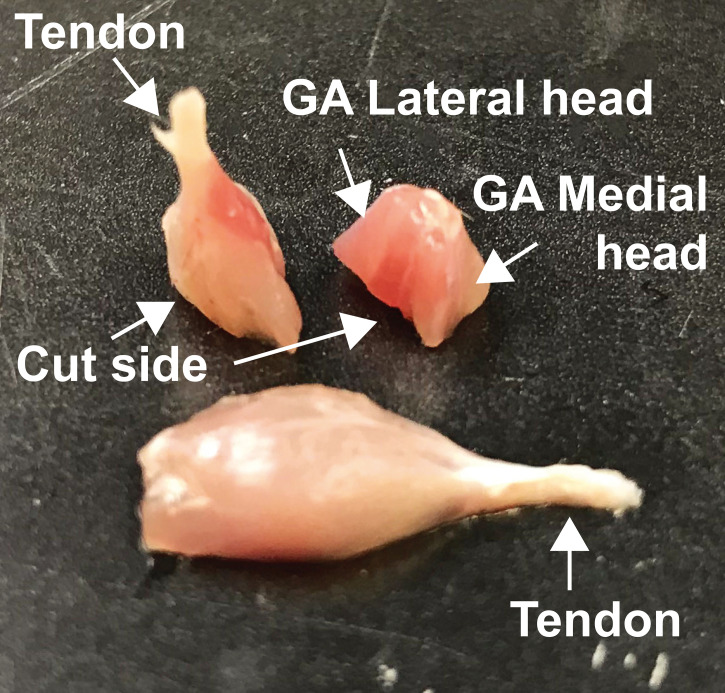
Embedding of the GA muscles for tissue sectioning (Figure 3):
Position the entire muscle from tendon to tendon onto the paraffin base mold for subsequent longitudinal sectioning of the tissue.
Cut the muscle in half transversely and position both halves with with the cut site down onto the paraffin base mold for subsequent coronal (frontal) sectioning of the tissue.
The other tissues can be positioned onto the paraffin base mold according to the type of analyses required.
Place the base mold with the tissue on the cold plate at -10 °C to start solidifying the paraffin, making sure that the tissues are pressed down and fully immersed.
Place the pre-labelled cassette on top of the base mold containing the tissue and fill the mold with additional liquid paraffin.
Let the paraffin to completely solidify before removing the paraffin block from the mold.
Trim excess paraffin from the sides of the cassette with the Para Trimmer.
The blocks are now ready for sectioning and can be stored at room temperature.
-
Sectioning
Prewarm a water bath to 37 °C.
-
Mount the paraffin block on the microtome.
Note: The block can be mounted in a vertical or horizontal position depending on the user’s preference.
-
Cut 6 mm thin sections throughout the block to collect different layers of the tissues.
Note: Skeletal muscles may be difficult to section, maintaining the integrity of the tissue. To improve the quality of the sections, place the blocks on the cold plate before sectioning.
-
Pick up the sections using a small brush and transfer them gently into the water bath.
Note: Leave the sections floating only for a very short time, as prolonged floating may cause separation of the muscle fibers.
Collect the tissue sections by dipping a glass slide into the water bath and allowing the section to adhere to the surface of the slide.
Drain the slide in a slide box.
Dry the slides by placing them in a 45 °C incubator for 1 h or overnight.
-
Masson’s Trichrome staining for formalin fixed paraffin embedded (FFPE) tissue
Note: Perform all steps in a fume hood wearing protective gloves throughout the staining procedure.
Prepare all reagents in advance, prior to starting the staining procedure.
Place the bead bath in the fume hood and set it to 60 °C.
-
Prewarm Bouin’s solution in a slide holder to 60 °C in the bead bath.
Notes:
The Bouin's solution (inorganic oxide) rapidly penetrates the tissues preserving glycogen and enhancing the binding of the dyes.
Bouin’s solution can be prepared by combining 75 ml Picric acid (saturated), 25 ml formaldehyde (37-40%) and 5 ml glacial acetic acid.
-
Deparaffinize the slides using Gemini AS automated slide stainer (Table 2).
Note: This step can also be conducted manually.
Place the slides into the slide holder containing the Bouin’s solution and close it tightly.
Incubate the slides in Bouin’s solution for 1 h at 60 °C.
-
Discard the solution in a properly labeled waste bottle.
Notes:
Since Bouin’s solution contains formaldehyde, picric acid and acetic acid it is a hazardous solution and correct safety and disposable measures should be taken. The picric acid, in less than 10% water, is explosive.
The solution should be discarded after use in a proper waste container following institutional guidelines for the disposal of hazardous solutions.
Wash the slides in running tap water for 5 min to ensure complete removal of the Bouin’s solution.
Transfer the slides into a coplin jar.
-
Stain tissue sections with Weigert’s iron hematoxylin for 10 min (Recipe 1).
Notes:
Weigert’s iron hematoxylin solution will stain nuclei dark purple/black. See Note b of Step H7 for disposal guidelines.
Weigert’s iron hematoxylin binds to chromatin and nuclear structures. The oxidation product of hematoxylin, hematin, is anionic and therefore does not have good affinity for DNA. However, the combination of an iron salt with hematin results in a positively charged dye complex that efficiently binds to anionic chromatin.
Wash in running tap water for 5 min and rinse twice in distilled water.
-
Incubate the slides in Biebrich scarlet-acid fuchsin solution for 5 min (Recipe 2).
Notes:
Biebrich scarlet-acid fuchsin solution will stain the cytoplasm and muscle tissues red. Decreased red staining indicates that the staining solution is old or overused. See Note b of Step H7 for disposal guidelines.
The chemical basis and exact mechanism of Biebrich Scarlet, like other experimental stains, is still unclear. However, its specificity to low pH cytoplasm and muscle fibers is obtained by additional treatment with the combination of phosphotungstic/phosphomolybdic acids, that enables the less permeable structures to retain the red, while the dye is removed from the collagen fibers.
Rinse the slides three times in distilled water.
-
Place the slides in phosphotungstic/phosphomolybdic acid solution for 10 min (Recipe 3).
Note: The phosphotungstic/phosphomolybdic acid solution is used as a decolorizer causing Biebrich scarlet-acid fuchsin to diffuse out of the collagen fibers, while leaving the muscle fibers stained in red. See Note b of Step H7 for disposal guidelines.
-
Drain the slides and transfer them directly to aniline blue for 5 min (Recipe 4).
Notes:
Aniline blue will stain collagens blue. See Note b of Step H7 for disposal guidelines.
As a consequence of the previous reaction (H14) with the combined mixture of phosphotungstic/phosphomolybdic acids, the amphionic aniline blue dye can now stain collagen fibers.
Rinse the slides three times in distilled water.
-
Differentiate the colors of the dyed tissue structures by incubating the slides in 1% acetic acid for 1 min (Recipe 5).
Notes:
If the blue staining of connective tissue appears faded, the incubation time in the acetic acid solution should be shortened.
The acetic acid solution can be discarded in the sink with running water.
Rinse the slides in distilled water.
-
Dehydrate in 95% ethanol, and then 100% ethanol for 2 min each.
Note: Depending on the tissue, the time of dehydration may be reduced to avoid fading of the red color.
Clear the slides in xylene for 2 min.
Mount with Cytoseal XYL mounting medium by placing 2 drops on the sections and covering them with a cover slip.
Drain excess Cytoseal and dry the slides.
-
Image sections by using a light microscope.
Notes:
A 4x or 10x objective can be used for an overview of the sections, for more detailed pictures 20x-100x objectives can be used.
Analyses of the extent of tissue fibrosis can be done manually by a pathologist or performed quantitatively using imaging/processing software, such as ImageJ, Photoshop and Aperio.
Figure 2. Thoracic cavity.
Image of the mouse thoracic cavity showing the anatomical structures in reference to the position of the perfusion needle. The aorta and inferior vena cava are marked with dashed lines.
Table 1. Stepwise procedure of tissue processing in the Excelsior AS.
| Steps | Reagent | Time (min) | Temperature |
|---|---|---|---|
| Dehydration | |||
| 1 | 70% Ethanol | 45 | |
| 2 | 85% Ethanol | 45 | |
| 3 | 95% Ethanol | 45 | All steps at RT |
| 4 | 100% Ethanol | 45 | |
| 5 | 100% Ethanol | 45 | |
| 6 | 100% Ethanol | 45 | |
| Clearing | |||
| 7 | Xylene | 45 | |
| 8 | Xylene | 45 | All steps at RT |
| 9 | Xylene | 45 | |
| Wax infiltration | |||
| 10 | Paraffin | 45 | |
| 11 | Paraffin | 45 | All steps at 60 °C |
| 12 | Paraffin | 45 |
Figure 3. Embedding positions of the GA muscles.
Table 2. Deparaffinization and rehydration steps.
| Steps | Reagent | Time (min) |
|---|---|---|
| 1 | Xylene | 3 |
| 2 | Xylene | 3 |
| 3 | Xylene | 3 |
| 4 | 100% Ethanol | 3 |
| 5 | 100% Ethanol | 3 |
| 6 | 95% Ethanol | 3 |
| 7 | 70% Ethanol | 3 |
| 8 | Distilled water | 1 |
Data analysis
To visualize the collagen deposition/distribution in different affected organs/tissues and compare it to the corresponding healthy tissues it is necessary to section throughout the organs and image similar area of both affected and non-affected tissues.
The extent of connective tissue/collagen (blue) present in the tissue’s sections can be quantified using an image analysis approach (e.g., ImageJ, Photoshop and Aperio). Using the Aperio Scanscope XT (Leica), whole slide images can be scanned and the Aperio color deconvolution algorithms used to quantify the collagen extent. For example, the fibrotic areas in the GA muscle, stained with Masson’s Trichrome, may be expressed as percentage of connective tissue area (blue staining) divided by the total area scanned. Comparison between a WT and affected mouse muscle at different ages will allow to assess the progressive increase of connective tissue/collagen area. This progression is directly correlated with the incrased invasion of the muscle fibers by expanding connective tissue, which leads to the disruption of muscle fiber architecture and ensuing loss of function, characterizing fibrosis ( Zanoteli et al., 2010 ; van de Vlekkert et al., 2019 ). A similar analyses can be applied to all tissues stained with Masson’s Trichrome.
-
For more information on the Aperion Scanscope and software please visit the website and user’s guide: https://aperiosoftware.com/
https://tmalab.jhmi.edu/aperiou/userguides/Image_Analysis_UG.pdf
Notes
The procedure for the Masson’s trichrome staining was optimized for use in our lab; some procedural steps and reagent composition were adapted from published protocols (Ruegg and Meinen, 2015; Carson et al., 1990 ).
Recipes
-
Weigert’s iron hematoxylin
Mix one part of solution A with one part of solution B. The working solution is stable at room temperature for 10 days.
Solution A
Stable at room temperature for 1 year.
1.0 g hematoxylin
500.0 ml ethanol 190 proof
Solution B
Stable at room temperature for 1 year.
20.0 ml of 29% ferric chloride
5.0 ml hydrochloric acid
475.0 ml distilled water
-
Biebrich scarlet-acid fuchsin solution
Stable at room temperature for 6 months.
2.7 g biebrich scarlet
0.3 g acid fuchine
3.0 ml glacial acetic acid
300.0 ml distilled water
-
Phosphotungstic/phophomolybdic acid
Stable at room temperature for 6 months.
25.0 g phosphotungstic acid
25.0 g phosphomolybdic acid
1.0 L distilled water
-
Aniline blue
Stable at room temperature for 6 months.
2.5 g aniline blue
1.0 ml glacial acetic acid
100.0 ml distilled water
-
1% acetic acid
Stable at room temperature for 2 years.
1.0 ml glacial acetic acid
99.0 ml distilled water
Acknowledgments
Funding: This work was supported in part by NIH grants R01GM104981, RO1DK095169, and CA021764, the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities. The protocol was adapted from van de Vlekkert et al., 2019 .
Competing interests
The authors have no conflict of interest or competing interests to declare.
Ethics
Animals were housed in a fully AAALAC (Assessment and Accreditation of Laboratory Animal Care)–accredited animal facility with controlled temperature (22 °C), humidity, and lighting (alternating 12-hour light/dark cycles). Food and water were provided ad libitum. All procedures in mice were performed according to animal protocols approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
The St Jude Children’s Research Hospitals Environmental Health and Safety Office procedures are implemented to ensure all hazardous materials are disposed properly in accordance with state and federal regulations.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Carson F.(1990). Histotechnology A Self-Instructional Text. 1990, 1st Ed, pp142-143, ASCP, Ill [Google Scholar]
- 2. de Geest N., Bonten E., Mann L., de Sousa-Hitzler J., Hahn C. and d'Azzo A.(2002). Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum Mol Genet 11(12): 1455-1464. [DOI] [PubMed] [Google Scholar]
- 3. Gattazzo F., Urciuolo A. and Bonaldo P.(2014). Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840(8): 2506-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinderer S. and Schenke-Layland K.(2019). Cardiac fibrosis- A short review of causes and therapeutic strategies. Adv Drug Deliv Rev 146: 77-82. [DOI] [PubMed] [Google Scholar]
- 5. Kendall R. T. and Feghali-Bostwick C. A.(2014). Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lederer D. J. and Martinez F. J.(2018). Idiopathic Pulmonary Fibrosis. N Engl J Med 379(8): 797-798. [DOI] [PubMed] [Google Scholar]
- 7. Mahdy M. A. A.(2019). Skeletal muscle fibrosis: an overview. Cell Tissue Res 375(3): 575-588. [DOI] [PubMed] [Google Scholar]
- 8. Majo J., Klinkhammer B. M., Boor P. and Tiniakos D.(2019). Pathology and natural history of organ fibrosis. Curr Opin Pharmacol 49: 82-89. [DOI] [PubMed] [Google Scholar]
- 9. Rockey D. C., Bell P. D. and Hill J. A.(2015). Fibrosis--a common pathway to organ injury and failure. N Engl J Med 372(12): 1138-1149. [DOI] [PubMed] [Google Scholar]
- 10. Ruegg M. and Meinen S.(2015). Histopathology in masson trichrome stained sections. SOP. 2015 V 1.0, MDC1A_M.1.2.003. Basel, Switzerland . [Google Scholar]
- 11. van de Vlekkert D., Demmers J., Nguyen X. X., Campos Y., Machado E., Annunziata I., Hu H., Gomero E., Qiu X., Bongiovanni A., Feghali-Bostwick C. A. and d'Azzo A.(2019). Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci Adv 5(7): eaav3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanoteli E., van de Vlekkert D., Bonten E. J., Hu H., Mann L., Gomero E. M., Harris A. J., Ghersi G. and d'Azzo A.(2010). Muscle degeneration in neuraminidase 1-deficient mice results from infiltration of the muscle fibers by expanded connective tissue. Biochim Biophys Acta 1802(7-8): 659-672. [DOI] [PMC free article] [PubMed] [Google Scholar]