Abstract
Ocean acidification driven by anthropogenic climate change is causing a global decrease in pH, which is projected to be 0.4 units lower in coastal shallow waters by the year 2100. Previous studies have shown that seaweeds grown under such conditions may alter their growth and photosynthetic capacity. It is not clear how such alterations might impact interactions between seaweed and herbivores, e.g. through changes in feeding rates, nutritional value, or defense levels. Changes in seaweeds are particularly important for coastal food webs, as they are key primary producers and often habitat-forming species. We cultured the habitat-forming brown seaweed Fucus vesiculosus for 30 days in projected future pCO2 (1100 μatm) with genetically identical controls in ambient pCO2 (400 μatm). Thereafter the macroalgae were exposed to grazing by Littorina littorea, acclimated to the relevant pCO2-treatment. We found increased growth (measured as surface area increase), decreased tissue strength in a tensile strength test, and decreased chemical defense (phlorotannins) levels in seaweeds exposed to high pCO2-levels. The herbivores exposed to elevated pCO2-levels showed improved condition index, decreased consumption, but no significant change in feeding preference. Fucoid seaweeds such as F. vesiculosus play important ecological roles in coastal habitats and are often foundation species, with a key role for ecosystem structure and function. The change in surface area and associated decrease in breaking force, as demonstrated by our results, indicate that F. vesiculosus grown under elevated levels of pCO2 may acquire an altered morphology and reduced tissue strength. This, together with increased wave energy in coastal ecosystems due to climate change, could have detrimental effects by reducing both habitat and food availability for herbivores.
Introduction
Ocean acidification (OA) is the decrease in pH caused by the absorption of atmospheric CO2 into the surface of the oceans [1]. The majority of dissolved CO2 concentrates above the thermocline, generating an estimated drop in pH to 7.7 [2] or 0.4 units [1,3,4] by year 2100 in open ocean surface waters and the entire water column in the shallow coastal waters [5]. Thus, coastal ecosystems and the organisms that live there are expected to be among the most impacted by OA. Seaweeds are key habitat-forming primary producers that support high biodiversity in coastal areas [6] and therefore their responses to OA may have impacts throughout the ecosystem. Seaweeds primarily use CO2, and most species also use HCO3-, for carbon fixation and growth, and may therefore benefit from the increase in available carbon caused by OA [7]. A growing number of studies have, however, shown that OA can have positive, neutral, or negative direct effects on basic performance traits such as growth and photosynthesis of seaweeds [e.g. 7,8], and these effects may differ between life stages of a species, as well as between closely related species [9–11].
Aside from effects on basic performance traits, OA can also impact both primary and secondary metabolism in seaweeds, sometimes resulting in higher carbon to nitrogen (C:N) and carbon to phosphorous (C:P) ratios [but see 8,12–14], which indicate a change in the nutritional content of the seaweed tissue. Increase in carbon availability for seaweeds generally results in decreased protein content [e.g. 15–19], and either increased [17,18] or decreased [20] levels of fatty acids. Furthermore, the content of secondary metabolites, such as the grazing deterrent dimethylsulfoniopropionate (DMSP) in green seaweeds, has been shown to increase in response to elevated pCO2 levels [15]. In brown seaweeds phlorotannins (polyphenolic compounds) are ubiquitous metabolites that can occur in high concentrations, especially in fucoid species (Fucales). Phlorotannins have multiple functions e.g. as defense against UV-radiation and defense compounds against gastropod grazing [21,22]. To our knowledge, only two studies have investigated the effects of OA on phlorotannin production in brown seaweeds, with mixed results [9,11]. Olischläger et al. [9] found no effect on phlorotannin production in the kelp Laminaria hyperborea when grown under 700 μatm, while Swanson & Fox [11] found increased phlorotannin production in Saccharina latissima but not Nereocystis leutkeana when exposed to 3000 μatm pCO2.
The nutritional and defensive characteristics of seaweeds are critical traits in ecological interactions since they affect the growth and fecundity of herbivores [23,24]. Therefore, apart from direct effects on the physiology and biochemical content, OA may also have indirect effects on macroalgae through interactions with grazers. A decrease in the nutritional value and increase in deterrent defense metabolites under OA may lower the palatability of macroalgae to grazers [e.g. 16,25,26]. This may, however, also lead to an increase in the per capita grazing pressure through compensatory grazing if less nutritious food is available [e.g. 16]. Grazing may also be altered by direct effects of OA on the herbivore, e.g. through changes in respiration or behavior [e.g. 27,28]. Bibby et al. [27] showed that the snail Littorina littorea had noticeable reductions in both metabolic rate and induced defense (shell formation), which increased the avoidance behavior of the snails and could in turn affect their interactions with other species. Additionally, Young et al. [28] found that the grazing rate of a snail (Lacuna vincta) decreased when it was exposed to elevated pCO2, regardless of the effects of pCO2 on the seaweeds (Ulva spp.) that the snail was grazing on.
In temperate coastal ecosystems, fucoids are dominant habitat-forming seaweeds that provide shelter, habitat, and food for other organisms [29]. The presence of fucoids is associated with a local increase in species abundance and diversity [30], but there is no consensus how OA will affect the adult stage of associated species [but see e.g. 31 for effects on early life-stages]. Since many fucoids have an active uptake of bicarbonate [32], which is abundant in seawater (up to 91% [7]), it has been suggested that they should not increase growth in response to increased pCO2 since they may not be carbon limited [33]. We are only aware of two studies that investigate potential indirect effects of OA on fucoids through changes in interactions with herbivores [34,35]. One of these studies found that the herbivore Littorina obtusata consumed more of Ascophyllum nodosum under OA conditions, albeit this difference was not statistically different [35]. In contrast, the other study showed no effect of decreased pH on the interaction between herbivores and F. vesiculosus [34]. This relative lack of literature is surprising, considering the abundance of fucoids. Given the high phlorotannin content found in many fucoids, the effect of OA on phlorotannin production may also alter the interaction between seaweed and herbivore, but this has, to our knowledge, not yet been investigated.
The overall aim of the present study was to examine potential direct effects of OA on the fucoid F. vesiculosus, as well as indirect effects on the seaweed through changes in its interactions with the gastropod grazer L. littorea, both common species along coasts in the North Atlantic. We conducted manipulative experiments to determine how the growth rate, photosynthesis, carbon and nitrogen content, as well as chemical defense (phlorotannin content), and breaking strength of F. vesiculosus will be affected by increasing pCO2 levels in the future. Furthermore, we also tested the effect of elevated pCO2 on consumption, feeding preference, and condition index of L. littorea.
Materials and methods
Experimental design
Sixty individuals of F. vesiculosus were collected from the west coast of Sweden, in July 2018 and kept at Tjärnö Marine Laboratory (TML, 58°52’36.4”N 11°6’42.84”E) under ambient conditions (Table 1) for 7 days to acclimatize. Due to the small tidal range in the area, F. vesiculosus in western Sweden can be submerged for long time periods depending on prevailing weather conditions (personal observations, A. Kinnby), hence the algae were kept under water throughout the experiment. The experiment was performed in a greenhouse with natural lighting (natural light cycle 18:6 h, L:D). After the acclimation period, each seaweed was split into one experimental thallus and one control thallus, placed in separate 1L aquaria (a total of 120 aquaria, i.e. n = 60) with constant seawater flow from header tanks (4 per treatment, n = 15). Control thalli were maintained at ambient pCO2 (400 μatm) while experimental thalli were exposed to gradually increasing (~100 μatm/day) CO2 until a pCO2 of 1100 μatm was reached 7 days later (corresponding to the projected value at the end of this century [36]). 360 individuals of similar sized L. littorea were also collected and exposed to the same conditions as the seaweed, i.e. 180 snails were exposed to ambient water and 180 snails were exposed to treatment water in separate tanks from the seaweed thalli (these snails were used in a grazing experiment described below). The header tanks were aerated with either ambient atmospheric air (pCO2 of 400 ppm) or CO2-enriched air controlled by solenoid valves and pH-computers (Aqua Medic) to provide a final pCO2 of 1100 μatm. The pCO2 was monitored daily with LI-850 CO2/H2O Gas Analyzer (Li-COR). The CO2 analyzer was calibrated with custom mixed gas, 970 ppm (Linde Gas AB, Sweden). Filtered seawater (5 μm) flow was constant at 0.3 L/min in each aquarium throughout the experiment. Salinity, temperature, pCO2, and pHNBS were measured in the 1L aquaria. pH was recorded using HANNA instruments pH electrode HALO probe (HI-1102) calibrated with NBS pH 4.01, 7.01, and 10.01 standards (HANNA instruments) before each measurement. Total alkalinity was estimated from salinity using long-term salinity:alkalinity relationship data for this location (r = 0.94; data obtained from SMHI https://www.smhi.se/data/oceanografi/datavardskap-oceanografi-och-marinbiologi/sharkweb) [37] and pHT was calculated from the temperature, salinity, pCO2, and total alkalinity using CO2calc [38; Table 1].
Table 1. Seawater chemistry of experimental treatments; partial pressure of CO2 (pCO2), pHNBS, salinity, and temperature were measured twice a week.
| pCO2 (μatm) | pHNBS | pHT | AT (μmolkg-1) | Salinity (PSU) | Temperature (°C) | |
|---|---|---|---|---|---|---|
| Ambient | 400 ± 47 | 8.04 ± 0.03 | 8.05 | 2258 | 32 ± 0.8 | 15 ± 1 |
| Treatment | 1100 ± 61 | 7.64 ± 0.04 | 7.66 | 2258 | 32 ± 0.8 | 15 ± 1 |
Total alkalinity was estimated from salinity using long-term salinity:alkalinity relationship data for this location (r = 0.94) and pHT was calculated from the temperature, salinity, pCO2, and total alkalinity using CO2calc. Data are averages (SD), n = 8.
All seaweed thalli were weighed fresh (n = 60) and photographed (for area (n = 60) measurements using Image J [39]) at the beginning and end of the 30-day experiment. At the end of the experiment the efficiency of photosystems II (Fv/Fm and P- index, n = 60 for both measurements) were measured in the new tissue formed during the experiment. The tissue was dark-adapted for 10 minutes, the fiber optics were held at a fixed 10 mm distance from the algae, and measurements were taken with a PAM (pulse amplitude-modulated fluorometer; Walz, Effeltrich, Germany). Breaking strength was measured by securing the seaweed to a dynamometer (Lutron FG-5020; Taiwan) such that only one apical tip was being strained, and increasing the strain until the thallus broke (n = 10). Thus, measuring breaking strength on tissue that was formed during the experiment. Following this, apical tissue samples were frozen (-60°C) for further elemental and phlorotannin analysis, the remaining tips were used in the consumption and preference experiment with L. littorea (see below).
Phlorotannin analysis
For phlorotannin analysis, the frozen samples (n = 60) were freeze-dried, homogenized to a fine powder, and 10 mg of each sample was extracted in 60% acetone. Total phlorotannin content was quantified colorimetrically using the Folin-Ciocalteu method [40], with phloroglucinol (1,3,5-trihydroxybenzene, art. 7069; Merck, Darmstadt, Germany) as a standard. Results are presented as % dw (dry weight).
Elemental analysis
For the determination of carbon (C) and nitrogen (N) content the frozen seaweed tissue was freeze-dried and homogenized to a fine powder and weighed to the nearest 0.01 mg. The total tissue C and N content, as well as δ13C and δ15N of the samples (n = 60) were analyzed with an elemental analyzer (ANCA-GSL, Sercon Ltd., Crewe, UK) coupled to an isotope ratio mass spectrometer (20–22, Sercon Ltd., Crewe, UK).
Consumption and feeding preference of Littorina littorea
The palatability of the F. vesiculosus thalli grown in ambient and elevated pCO2 during 30 days was measured in two-choice feeding trials using starved L. littorea as the grazer. The feeding experiment was performed using a total of 120 containers (200 mL) with constant seawater flow of ambient pCO2 (400 μatm). In each container two similarly sized apical pieces of F. vesiculosus were placed (0.50 ± 0.019 g mean ± SD), one piece from the ambient pCO2 treatment and one from the elevated pCO2 treatment; as both pieces came from the same thallus they were genetically identical. Six individuals of L. littorea, exposed to either ambient or elevated pCO2 were placed in half of the containers (n = 30, i.e. a total of 60 containers with herbivores). To control for autogenic changes in mass (i.e. growth) during the experiment that was not caused by the grazing of the snails, each container with seaweed pieces and herbivores were paired with an identical control container without herbivores containing similarly sized apical pieces from the same genetic individual of seaweed. The wet weight of all seaweed pieces was determined at the start and at the end of the 24-hour experiment by using a standard blotting procedure, and the wet-weight change of each seaweed piece was calculated by subtracting the weight at the end of the experiment from the starting weight. The consumption of the snails exposed to different pCO2-levels was determined by calculating the total change in weight between initial and post-grazing weights for each container and subtracting the weight change in the autogenic control containers. To study feeding preference of herbivores exposed to different pCO2-levels, the difference between weight changes of the two seaweed pieces in each container was calculated by subtracting the wet weight change of the seaweed exposed to elevated pCO2 from the weight change of the seaweed piece exposed to ambient pCO2 [41].
Condition index for Littorina littorea
Following the feeding preference experiment all snails were euthanized by freezing at -20°C. To assess whether the elevated pCO2 treatment had affected the physiological status of the snails a condition index was calculated, where a higher condition index is a sign of a healthier individual [35,42]. Snails were thawed and weighed whole; following this the shell was weighed alone. The dry weight of the soft tissue was obtained by weighing the body after drying for 48 hours at 50°C. The condition index was derived from the weights according to the following formula:
Data analysis
The seaweed response variables, i.e. growth (% increase in area and weight), breaking strength, efficiency of photosystem II (Fv/Fm and P-index), as well as phlorotannin, and nutritional content were all statistically analyzed with mixed model ANOVAs with pCO2 treatment as a fixed factor and header tank as a random factor nested within pCO2 treatment. However, since header tank was non-significant (p > 0.40 for all variables, the mean square for this factor was pooled with the residual mean square and paired t-tests were used to determine if there was a significant difference between the seaweed in ambient and elevated levels of pCO2. Paired t-tests were used because every treatment thallus was paired with a genetically identical control thallus. Before analysis the data for each response variable was checked and found to meet the assumptions of normality. To investigate if there was a difference in condition index between the snails exposed to ambient and treatment water a t-test was performed. The condition index data was not normally distributed, hence a Mann Whitney U-test was run. The consumption of herbivores exposed to ambient and elevated pCO2 was analyzed with a t-test. Preference for seaweed grown under the different pCO2 conditions was evaluated by comparing the difference in weight change between the seaweed pieces kept with the herbivores and their respective autogenic controls with two separate paired t-tests; one each for herbivores exposed to ambient and elevated pCO2. A significantly lower difference in wet weight change for seaweed pieces kept with herbivores compared to autogenic controls will indicate a preference for feeding on the control seaweed [41]. All analyses were performed in RStudio (version 1.0.136).
Results
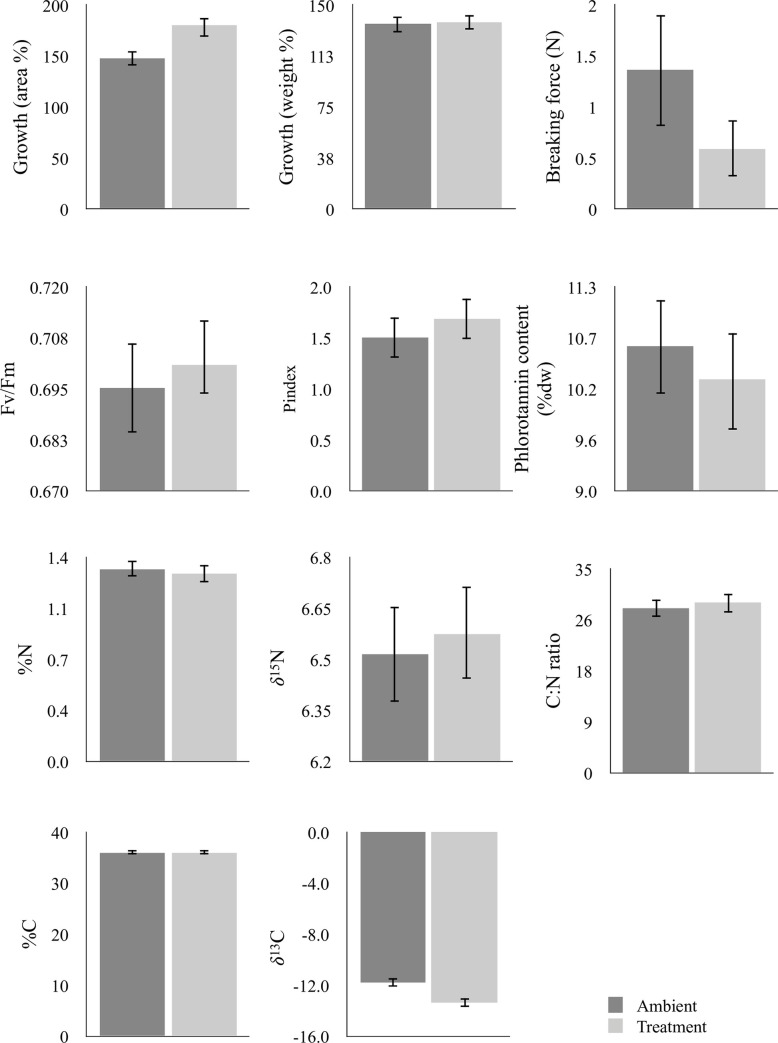
The seaweed thalli exposed to elevated levels of pCO2 grew significantly more than the thalli exposed to ambient pCO2 when growth was measured as increase in surface area of the seaweed (Fig 1A; Table 2). On average, growth rates under elevated pCO2 were 34% higher than growth under ambient conditions. However, thallus weight did not differ significantly between the two treatments (Fig 1B; Table 2). We found a significant difference in the force needed to break the seaweed tissue in the control group compared to the seaweeds exposed to elevated pCO2; thalli from the treatment group were 57% weaker than those in the control group (Fig 1C; Table 2). We found no statistically significant differences in the efficiency of photosystem II measured as Fv/Fm and P-index (Fig 1D and 1E; Table 2).
Fig 1. Effects on response variables of Fucus vesiculosus grown under ambient (400 μatm) and elevated (1100 μatm) pCO2 for 30 days.
Values are means ± 95% CI, n = 60 for a-j and n = 10 for k. Response variables measured as a) Growth measured % increase in area (n = 60), b) growth measured as % increase in weight (n = 60), c) breaking force (N) (n = 10), d) efficiency of photosystem II (Fv/Fm) (n = 60), e) efficiency of photosystem II (P index) (n = 60), f) Phlorotannin content (%dw) (n = 60), g) %Nitrogen (n = 60), h) δ15N (n = 60), i) C:N ratio (n = 60), j) %Carbon (n = 60), and k) δ13C (n = 60).
Table 2. Summary of effects of ambient (400 μatm) and elevated (1100 μatm) levels of pCO2 on Fucus vesiculosus measured as ten responses.
| Response variable | p-value | t-value | Df | Mean (400ppm) | 95%CI (400ppm) | Mean (1100ppm) | 95%CI (1100ppm) |
|---|---|---|---|---|---|---|---|
| Growth: area (%) | 9.2e-07 | -5.48 | 59 | 147.5 | 6.54 | 180.1 | 11.47 |
| Growth: weight (%) | 0.767 | -0.30 | 59 | 136.0 | 5.91 | 136.7 | 4.68 |
| Breaking force (N) | 0.032 | 2.33 | 18 | 1.36 | 0.54 | 0.59 | 0.27 |
| Photosystem II (Fv/Fm) | 0.407 | -0.83 | 59 | 0.695 | 0.011 | 0.701 | 0.007 |
| Photosystem II (P index) | 0.147 | -1.47 | 59 | 1.5 | 0.20 | 1.7 | 0.19 |
| Phlorotannin content (% dw) | 0.030 | 2.22 | 59 | 10.6 | 0.53 | 10.3 | 0.57 |
| % Nitrogen | 0.379 | 0.89 | 59 | 1.32 | 0.055 | 1.29 | 0.066 |
| % Carbon | 0.865 | 0.17 | 59 | 36.04 | 0.43 | 36.00 | 0.37 |
| C:N | 0.236 | -1.20 | 59 | 28.2 | 1.44 | 29.3 | 1.80 |
| δ13C | 4.762e-09 | 6.85 | 59 | -11.77 | 0.32 | -13.33 | 0.41 |
| δ15N | 0.315 | -1.01 | 59 | 6.51 | 0.14 | 6.57 | 0.13 |
P-values and corresponding t-values and degrees of freedom of paired t-tests are reported for the analyses of all response variables as well as means and 95% confidence intervals. Values in bold denote statistically significant values.
The results of chemical analyses showed that there was a statistically significant decrease (3%) in phlorotannin content between the tips of thalli exposed to elevated levels of pCO2 and those being exposed to ambient levels (Fig 1F; Table 2). There were no statistically significant differences in C or N tissue content, nor in the C:N ratio. However, the stable carbon isotope (δ13C) content of F. vesiculosus was significantly reduced when exposed to elevated pCO2; the δ13C values decreased to -13% under elevated pCO2 levels. Tissue δ15N was not significantly changed when exposed to elevated levels of pCO2 (Fig 1G–1K; Table 2).
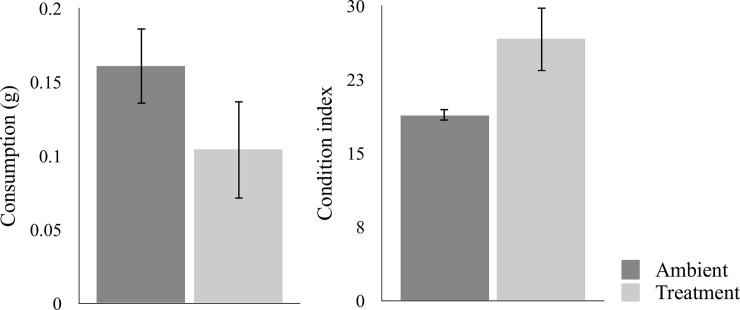
The mean condition index of L. littorea was 28.9% higher in snails that had been exposed to high pCO2-levels than those exposed to ambient pCO2-levels (U = 13155, z-score = -2.60, p = 0.0093; Fig 2). Despite having a higher condition index, the herbivores exposed to high pCO2 levels consumed 37.5% less than the snails exposed to ambient pCO2 (t-test, t58 = 2.67, p = 0.0098, Fig 2). However, the herbivores did not show a statistically significant difference in preference based on the experimental treatment of the seaweed, regardless if the herbivores had been exposed to ambient pCO2 (paired t-test, t29 = -0.517, p = 0.609) or elevated pCO2 (paired t-test, t29 = -0.584, p = 0.564).
Fig 2.
a) Consumption of the alga Fucus vesiculosus by the gastropod Littorina littorea, exposed to ambient (400 μatm) and elevated (1100 μatm) pCO2 for 30 days. b) Condition index for individuals of L. littorea following the grazing experiment. Values are means ± 95% CI.
Discussion
Seaweeds play important ecological roles in coastal habitats and are often foundation species, with a key role for ecosystem structure and function. Hence, it is important to understand how seaweeds will be directly affected by changes in their environment, and also if these changes will alter seaweed interactions with other species. Here, we show that elevated pCO2-levels increased the thallus area, decreased the phlorotannin content, and reduced the breaking strength of F. vesiculosus. This may result in that the seaweeds become less robust in field conditions. This could lead to an overall loss of seaweed coverage which in turn is likely to affect all the organisms that live in, or consume, this seaweed. The condition index of the snails increased under exposure to elevated levels of pCO2, but the consumption decreased and we saw no significant effect of treatment on the palatability of F. vesiculosus thalli.
Effects on growth
Increased pCO2 had no significant effect on the weight change of F. vesiculosus, but significantly increased the thallus surface area. Previous studies with F. vesiculosus have reported unaltered [43,44] or reduced [45] growth, measured as wet weight, under elevated pCO2-levels. Graiff et al. [46], however, reported a tendency (not statistically significant) of higher growth of F. vesiculosus, measured both as wet weight and length in apical tips, at elevated pCO2 levels. These different experimental results suggest that other factors interact with pCO2 to determine growth, e.g. seasonality or the life-cycle stage (age) of the seaweed, or genetic differences due to local adaptation among the populations used in the different studies. Such genetic differences in phlorotannin production and growth were recently demonstrated among F. vesiculosus populations at distances less than 100 km [47].
Effects on breaking strength
The combination of a significantly larger thallus with no effect on the weight of F. vesiculosus under enhanced CO2 conditions found in the present study strongly indicates a decrease in tissue density, which is corroborated by the drastic (57%) decrease in breaking strength of the thallus. To our knowledge, this is the first time such an effect of increased pCO2 levels is reported for seaweeds, and it parallels findings in developing seaweed spores and terrestrial plants. For example, Guenther et al. [48] found that reduced pH delayed spore attachment in two different red algae, while Pretzsch et al. [49] documented an increase in growth rate, attributed to increasing CO2 levels, among tree species in central Europe between 1960 and 2014. They also showed that this increase in growth was coupled with a decrease in tissue density and/or strength. This leaves forests more vulnerable to the increased weather variability that is also associated with climate change [49,50]. Our results suggest that similar effects may also be present in coastal marine systems. The reduced breaking strength could make seaweeds more vulnerable to storms and wave action, which are projected to become more frequent as the climate changes [51,52]. Increased vulnerability will likely reduce the role F.vesiculosus plays in the nearshore ecosystem, with negative impacts on species that rely on this seaweed for food or habitat.
Effects on photosynthesis
We observed no significant differences in the Fv/Fm ratio or P-index in our experiment, suggesting that F. vesiculosus does not increase the maximum quantum efficiency of photosystem II or sample vitality in response to elevated pCO2. This follows the findings of Fernández and colleagues [53], who demonstrated that the increased carbon availability from increased pCO2-levels had no effect on photosynthesis. Seaweeds in general acquire carbon by passive diffusion of CO2 and active transportation of bicarbonate; as the concentration of CO2 rises the amount of passively diffusing CO2 potentially also rises, reducing the seaweed’s reliance on active transport proteins, and potentially allowing more energy to be allocated for growth [54]. Increased uptake of CO2 coincides with a decrease in tissue δ13C [8]. In this study we found that the δ13C decreased from -11.77% to -13.33% when seaweeds were exposed to increased levels of CO2, which indicates a transition away from active intake of bicarbonate towards passive uptake of CO2. A similar change was previously documented in both Gracillaria sp. and Ulva sp.,[54], as well as in Lomentaria australis where an increase in growth and decrease in δ13C were hypothesized to indicate a transition away from a more costly CCM (carbon dioxide-concentrating mechanism) [8]. In our study, however, this did not translate to an increase in biomass as we did not find any significant differences in the weight gain of the seaweeds exposed to different levels of CO2. Fucus vesiculosus can use two parallel CO2 pathways for photosynthesis, both directly taking up carbon from their environment and also storing it as an organic intermediate for use when other carbon in less available [55], suggesting that under normal circumstances F. vesiculosus plants are most likely not carbon-limited. This, together with the fact that F. vesiculosus used in our experiment were constantly submerged (which is common due to the low tidal range along the Swedish west coast) may explain the lack of effect from increased pCO2 on growth measured as weight gain.
Effects on elemental and phlorotannin content
In terrestrial plants, increased atmospheric CO2 has been shown to increase C:N ratios as well as lead to an accumulation of phenolic compounds, such as tannins, affecting the consumption and growth rates of grazers [56,57]. However, we found no significant differences in C or N tissue content, nor in the C:N ratio. In contrast, Gutow et al., [45] showed that elevated levels of pCO2 (700 μatm) decreased the C:N ratio of F. vesiculosus. Studies on the effects of increased CO2 on phenolic compounds in marine macrophytes are few and only one previous study on kelp species found that elevated CO2 leads to increased levels of phlorotannins [11]. By contrast, marine vascular plants have been shown to reduce phenolic acid production under increased CO2 conditions [58], which aligns with our results on F. vesiculosus showing slightly lower phlorotannin content in apical tips exposed to elevated pCO2-levels.
Effects on interactions with a grazer
Despite finding a somewhat lower phlorotannin content in seaweeds exposed to elevated pCO2, we did not find a difference in grazing preference of the gastropod L. littorea between seaweeds from the different treatments. However, snails exposed to elevated pCO2 generally consumed less than those exposed to ambient conditions, regardless of which food type they were offered. Reduced consumption by snails at increased pCO2 levels could indicate easier ingestion and digestion of food, or decreased activity of the grazer (and therefore decreased caloric requirements), in line with previous research on L. littorea [27] and other marine invertebrates [59] showing reduced metabolic rates at increased pCO2 levels. The snails exposed to elevated levels of pCO2 had a higher condition index than snails exposed to ambient conditions. This combination of results is surprising, as a decreased consumption would be expected to result in a drop in condition index. Increased condition index, i.e. a higher dry to wet weight ratio of the soft tissue, could indicate more accumulation of tissue, i.e. increased growth, but also possibly failure to osmoregulate or other associated physiological problems. In summary, the results from the feeding experiment in the present study suggest that there are direct effects of increased pCO2 on herbivores and their consumption of seaweeds, but any indirect effects mediated by changes the palatability of the seaweeds are harder to discern.
Conclusion
In conclusion, our study shows that under OA conditions the habitat forming seaweed F. vesiculosus increases growth by thallus area, reduces reliance on active carbon uptake, shows a slight decrease in phlorotannin content and a drastic reduction in breaking strength. At the same time the herbivore L. littorea seems to tolerate increased pCO2 with an increased condition index even as they reduce their consumption of seaweeds. Reduced consumption for the herbivore suggests that the seaweed could gain some ecological benefits under OA. However, our most unanticipated finding–that the seaweed could become more vulnerable to physical forces under OA because of a significantly reduced breaking strength–could result in loss of seaweed biomass due to increased storm events that are associated with climate change. This might in turn have implications for the future community structure of shallow coastal areas under OA.
Supporting information
(XLSX)
Acknowledgments
We are grateful to Gunnar Cervin (University of Gothenburg) for help with the experiments and Kerstin Johannesson (University of Gothenburg) for valuable comments on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by the Swedish Research Council VR and Formas through a Linnaeus grant to the Centre for Marine Evolutionary Biology (CeMEB; http://cemeb.science.gu.se 217-2008-1719 awarded to Henrik Pavia, and by Rådman och Fru Ernst Collianders stiftelse för välgörande ändamål awarded to Alexandra Kinnby. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.K. C, E. WM. Anthropogenic carbon and ocean pH. Nature. 2003;425: 2003. [DOI] [PubMed] [Google Scholar]
- 2.Stocker TF, Qin D, Plattner GK, Tignor MMB, Allen SK, Boschung J, et al. Climate change 2013 the physical science basis: Working Group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Clim Chang 2013 Phys Sci Basis Work Gr I Contrib to Fifth Assess Rep Intergov Panel Clim Chang. 2013;9781107057: 1–1535. 10.1017/CBO9781107415324 [DOI] [Google Scholar]
- 3.Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science (80-). 2004;305: 362–366. 10.1126/science.1097329 [DOI] [PubMed] [Google Scholar]
- 4.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437: 681–686. 10.1038/nature04095 [DOI] [PubMed] [Google Scholar]
- 5.Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, et al. The Oceanic Sink for Anthropogenic CO 2. Science (80-). 2004;305: 367–371. [DOI] [PubMed] [Google Scholar]
- 6.Wernberg T, Krumhansl K, Filbee-Dexter K, Pedersen MF. Status and trends for the world’s kelp forests. Second Edi World Seas: An Environmental Evaluation Volume III: Ecological Issues and Environmental Impacts. Elsevier Ltd.; 2018. [DOI] [Google Scholar]
- 7.Koch M, Bowes G, Ross C, Zhang XH. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol. 2013;19: 103–132. 10.1111/j.1365-2486.2012.02791.x [DOI] [PubMed] [Google Scholar]
- 8.van der Loos LM, Schmid M, Leal PP, McGraw CM, Britton D, Revill AT, et al. Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol Evol. 2019;9: 125–140. 10.1002/ece3.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olischläger M, Bartsch I, Gutow L, Wiencke C. Effects of ocean acidification on different life-cycle stages of the kelp Laminaria hyperborea (Phaeophyceae). Bot Mar. 2012;55: 511–525. 10.1515/bot-2012-0163 [DOI] [Google Scholar]
- 10.Langer G, Nehrke G, Probert I, Ly J, Ziveri P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences. 2009;6: 2637–2646. 10.5194/bg-6-2637-2009 [DOI] [Google Scholar]
- 11.Swanson AK, Fox CH. Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs. Glob Chang Biol. 2007;13: 1696–1709. 10.1111/j.1365-2486.2007.01384.x [DOI] [Google Scholar]
- 12.Urabe J, Togari J, Elser JJ. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Glob Chang Biol. 2003;9: 818–825. 10.1046/j.1365-2486.2003.00634.x [DOI] [Google Scholar]
- 13.Van De Waal DB, Verschoor AM, Verspagen JMH, Van Donk E, Huisman J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front Ecol Environ. 2010;8: 145–152. 10.1890/080178 [DOI] [Google Scholar]
- 14.Leung JYS, Nagelkerken I, Russell BD, Ferreira CM, Connell SD. Boosted nutritional quality of food by CO2 enrichment fails to offset energy demand of herbivores under ocean warming, causing energy depletion and mortality. Sci Total Environ. 2018;639: 360–366. 10.1016/j.scitotenv.2018.05.161 [DOI] [PubMed] [Google Scholar]
- 15.Borell EM, Steinke M, Fine M. Direct and indirect effects of high pCO2 on algal grazing by coral reef herbivores from the Gulf of Aqaba (Red Sea). Coral Reefs. 2013;32: 937–947. 10.1007/s00338-013-1066-5 [DOI] [Google Scholar]
- 16.Duarte C, López J, Benítez S, Manríquez PH, Navarro JM, Bonta CC, et al. Ocean acidification induces changes in algal palatability and herbivore feeding behavior and performance. Oecologia. 2016;180: 453–462. 10.1007/s00442-015-3459-3 [DOI] [PubMed] [Google Scholar]
- 17.Gao G, Clare AS, Chatzidimitriou E, Rose C, Caldwell G. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem. 2018;258: 71–78. 10.1016/j.foodchem.2018.03.040 [DOI] [PubMed] [Google Scholar]
- 18.Gao G, Clare AS, Rose C, Caldwell GS. Ulva rigida in the future ocean: potential for carbon capture, bioremediation and biomethane production. GCB Bioenergy. 2018;10: 39–51. 10.1111/gcbb.12465 [DOI] [Google Scholar]
- 19.Chen B, Lin L, Ma Z, Zhang T, Chen W, Zou D. Carbon and nitrogen accumulation and interspecific competition in two algae species, Pyropia haitanensis and Ulva lactuca, under ocean acidification conditions. Aquac Int. 2019; 721–733. 10.1007/s10499-019-00360-y [DOI] [Google Scholar]
- 20.Liu C, Zou D. Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Mar Biol Res. 2015;11: 1043–1052. 10.1080/17451000.2015.1062520 [DOI] [Google Scholar]
- 21.Pavia H, Cervin G, Lindgren A, Åberg P. Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodos. Mar Ecol Prog Ser. 1997;157: 139–146. [Google Scholar]
- 22.Pavia H, Toth GB. Inducible Chemical Resistance to Herbivory in the Brown Seaweed Ascophyllum nodosum. Ecology. 2000;81: 3212–3225. [Google Scholar]
- 23.Cruz-Rivera E, Hay ME. Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology. 2000;81: 201–219. 10.1890/0012-9658(2000)081[0201:CQRQFC]2.0.CO;2 [DOI] [Google Scholar]
- 24.Toth GB, Langhamer O, Pavia H. Inducible and constitutive defenses of valuable seaweed tissues: Consequences for herbivore fitness. Ecology. 2005;86: 612–618. 10.1890/04-0484 [DOI] [Google Scholar]
- 25.Poore AGB, Graba-Landry A, Favret M, Sheppard Brennand H, Byrne M, Dworjanyn SA. Direct and indirect effects of ocean acidification and warming on a marine plant-herbivore interaction. Oecologia. 2013;173: 1113–1124. 10.1007/s00442-013-2683-y [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Rivera E, Hay ME. Macroalgal traits and the feeding and fitness of an herbivorous amphipod: The roles of selectivity, mixing, and compensation. Mar Ecol Prog Ser. 2001;218: 249–266. 10.3354/meps218249 [DOI] [Google Scholar]
- 27.Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J. Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett. 2007;3: 699–701. 10.1098/rsbl.2007.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young CS, Lowell A, Peterson B, Gobler CJ. Ocean acidification and food limitation combine to suppress herbivory by the gastropod Lacuna vincta. Mar Ecol Prog Ser. 2019;627: 83–94. 10.3354/meps13087 [DOI] [Google Scholar]
- 29.Schiel DR, Wood SA, Dunmore RA, Taylor DI. Sediment on rocky intertidal reefs: Effects on early post-settlement stages of habitat-forming seaweeds. J Exp Mar Bio Ecol. 2006;331: 158–172. 10.1016/j.jembe.2005.10.015 [DOI] [Google Scholar]
- 30.Watt CA, Scrosati RA. Regional consistency of intertidal elevation as a mediator of seaweed canopy effects on benthic species richness, diversity, and composition. Mar Ecol Prog Ser. 2013;491: 91–99. 10.3354/meps10521 [DOI] [Google Scholar]
- 31.Al-Janabi B, Kruse I, Graiff A, Winde V, Lenz M, Wahl M. Buffering and amplifying interactions among OAW (ocean acidification & warming) and nutrient enrichment on early life-stage Fucus vesiculosus L. (phaeophyceae) and their carry over effects to hypoxia impact. PLoS One. 2016;11: 1–18. 10.1371/journal.pone.0152948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surif MB, Raven JA. Exogenous inorganic carbon sources for photosynthesis in seawater by members of the Fucales and the Laminariales (Phaeophyta): ecological and taxonomic implications. Oecologia. 1989;78: 97–105. 10.1007/BF00377203 [DOI] [PubMed] [Google Scholar]
- 33.Hepburn CD, Pritchard DW, Cornwall CE, Mcleod RJ, Beardall J, Raven JA, et al. Diversity of carbon use strategies in a kelp forest community: Implications for a high CO2 ocean. Glob Chang Biol. 2011;17: 2488–2497. 10.1111/j.1365-2486.2011.02411.x [DOI] [Google Scholar]
- 34.Werner FJ, Graiff A, Matthiessen B. Temperature effects on seaweed-sustaining top-down control vary with season. Oecologia. 2016;180: 889–901. 10.1007/s00442-015-3489-x [DOI] [PubMed] [Google Scholar]
- 35.Cardoso PG, Grilo TF, Dionísio G, Aurélio M, Lopes AR, Pereira R, et al. Short-term effects of increased temperature and lowered pH on a temperate grazer-seaweed interaction (Littorina obtusata/Ascophyllum nodosum). Estuar Coast Shelf Sci. 2017;197: 35–44. 10.1016/j.ecss.2017.08.007 [DOI] [Google Scholar]
- 36.Hammill E, Johnson E, Atwood TB, Harianto J, Hinchliffe C, Calosi P, et al. Ocean acidification alters zooplankton communities and increases top-down pressure of a cubozoan predator. Glob Chang Biol. 2018;24: e128–e138. 10.1111/gcb.13849 [DOI] [PubMed] [Google Scholar]
- 37.Eriander L., Wrange A-L., Havenhand JN. Simulated diurnal pH fluctuations radically increase variance in—but not the mean og—growth in the barnacle Balanus improvisus. ICES J. Mar. Sci. 2016;79:596–603. [Google Scholar]
- 38.Robbins L., Hansen ME, Kleypas JA, Meylan SC. CO2calc- A user-friendly carbon calculator for Windows, Mac OS X, and iOS (iPhone) Florida Shelf Ecosystems Response to Climate Change Project CO2calc: A User-Friendly Seawater Carbon Calculator for Windows, Mac OS X, and iOS (iPhone). 2010.
- 39.Schneider CA., Rasband WS., Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–5. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Alstyne KL. Comparison of three methods for quantifying brown algal polyphenolic compounds. J Chem Ecol. 1995;21: 45–58. 10.1007/BF02033661 [DOI] [PubMed] [Google Scholar]
- 41.Peterson CH, Renaud PE. Analysis of feeding preference experiments. Oecologia. 1989;80: 82–86. 10.1007/BF00789935 [DOI] [PubMed] [Google Scholar]
- 42.Hickman RW, Illingworth J. Condition cycle of the green-lipped mussel Perna canaliculus in New Zealand. Mar Biol. 1980;60: 27–38. 10.1007/BF00395603 [DOI] [Google Scholar]
- 43.Ober GT, Thornber CS. Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2 and nutrients. Mar Environ Res. 2017;131: 69–79. 10.1016/j.marenvres.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 44.Takolander A, Cabeza M, Leskinen E. Seasonal interactive effects of pCO2 and irradiance on the ecophysiology of brown macroalga Fucus vesiculosus L. Eur J Phycol. 2019;54: 380–392. 10.1080/09670262.2019.1572226 [DOI] [Google Scholar]
- 45.Gutow L, Rahman MM, Bartl K, Saborowski R, Bartsch I, Wiencke C. Ocean acidification affects growth but not nutritional quality of the seaweed Fucus vesiculosus (Phaeophyceae, Fucales). J Exp Mar Bio Ecol. 2014. 10.1016/j.jembe.2014.01.005 [DOI] [Google Scholar]
- 46.Graiff A, Bartsch I, Ruth W, Wahl M, Karsten U. Season exerts differential effects of ocean acidification and warming on growth and carbon metabolism of the seaweed fucus vesiculosus in the Western Baltic Sea. Front Mar Sci. 2015;2 10.3389/fmars.2015.00112 [DOI] [Google Scholar]
- 47.Kinnby A, Jonsson PR, Ortega-martinez O, Töpel M, Pavia H, Pereyra RT, et al. Combining an Ecological Experiment and a Genome Scan Show Idiosyncratic Responses to Salinity Stress in Local Populations of a Seaweed Projection of Future Salinity. Front. Mar. Sci. 2020;7: 1–12. 10.3389/fmars.2020.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guenther R, Miklasz K, Carrington E, Martone PT. Macroalgal spore dysfunction: Ocean acidification delays and weakens adhesion. J. Phycol. 2017;54:153–158. 10.1111/jpy.12614 [DOI] [PubMed] [Google Scholar]
- 49.Pretzsch H, Biber P, Schütze G, Uhl E, Rötzer T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun. 2014;5: 1–10. 10.1038/ncomms5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pretzsch H, Biber P, Schütze G, Kemmerer J, Uhl E. Wood density reduced while wood volume growth accelerated in Central European forests since 1870. For Ecol Manage. 2018;429: 589–616. 10.1016/j.foreco.2018.07.045 [DOI] [Google Scholar]
- 51.Bindoff, N.L., J. Willebrand, V. Artale, A, Cazenave, J. Gregory, S. Gulev, et al. Shum LDT and AU. Observations: Oceanic Climate Change and Sea Level. Changes.
- 52.Hawkins SJ, Sugden HE, Mieszkowska N, Moore PJ, Poloczanska E, Leaper R, et al. Consequences of climate-driven biodiversity changes for ecosystem functioning of north European rocky shores. Mar Ecol Prog Ser. 2009;396: 245–259. 10.3354/meps08378 [DOI] [Google Scholar]
- 53.Fernández PA, Roleda MY, Hurd CL. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynth Res. 2015;124: 293–304. 10.1007/s11120-015-0138-5 [DOI] [PubMed] [Google Scholar]
- 54.Young CS, Gobler CJ. Ocean acidification accelerates the growth of two bloom-forming macroalgae. PLoS One. 2016;11: 1–21. 10.1371/journal.pone.0155152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamitsu Y, Boyer JS. Photosynthesis and carbon storage between tides in a brown alga, Fucus vesiculosus. Mar Biol. 1999;133: 361–369. 10.1007/s002270050475 [DOI] [Google Scholar]
- 56.Robinson EA, Ryan GD, Newman JA. A meta-analytical review of the effects of elevated CO 2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012;194: 321–336. 10.1111/j.1469-8137.2012.04074.x [DOI] [PubMed] [Google Scholar]
- 57.Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front Plant Sci. 2018;9: 1–10. 10.3389/fpls.2018.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnold T, Mealey C, Leahey H, Miller AW, Hall-Spencer JM, Milazzo M, et al. Ocean acidification and the loss of phenolic substances in marine plants. PLoS One. 2012;7: 1–10. 10.1371/journal.pone.0035107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pörtner HO., Langenbuch M., Reipschläger A. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 2004;60:705–718. [Google Scholar]