Abstract
Background:
People with type 2 diabetes are at greater risk for infections than those without type 2 diabetes. Our objective was to examine the association between type 2 diabetes and the risk of community-acquired pneumonia (CAP).
Methods:
In this systematic review and meta-analysis, we searched MEDLINE, Embase, CINAHL, ProQuest theses and dissertations, Global Health, the Global Index Medicus of the World Health Organization, and Google Scholar. We included observational studies published in English or French between Jan. 1, 1946 (start of MEDLINE) and July 18, 2020. Two independent reviewers extracted data and assessed quality using the ROBINS-I tool. DerSimonian–Laird random-effects models were used to pool estimates of the association between type 2 diabetes and CAP.
Results:
Our systematic review included 15 articles, reporting on 13 cohort studies and 4 case–control studies (14 538 968 patients). All studies reported an increased risk of pneumonia among patients with type 2 diabetes, and all were at serious risk of bias. When estimates were pooled across studies, the pooled relative risk was 1.64 (95% confidence interval [CI] 1.55–1.73); although there was a substantial amount of relative heterogeneity (I2 94.2), the amount of absolute heterogeneity was more modest (T2 0.008). The relative risk was 1.70 (95% CI 1.63–1.77, I2 85.2%, T2 0.002) among cohort studies (n = 13), and the odds ratio was 1.54 (95% CI 1.14–2.09, I2 92.7%, T2 0.07) among case–control studies (n = 4).
Interpretation:
Type 2 diabetes may be associated with an increased risk of CAP; however, the available evidence is from studies at serious risk of bias, and additional, high-quality studies are needed to confirm these findings.
PROSPERO registration:
CRD42018116409
People with type 2 diabetes are at greater risk of infections, including urinary tract and genital infections, than people without diabetes.1 The hyperglycemic environment in these patients, which is conducive to the growth and proliferation of bacteria, can lead to decreased T lymphocyte response and decreased neutrophil and macrophage function.2,3 Patients with diabetes also exhibit worse infection outcomes than patients without diabetes.1 Community-acquired pneumonia (CAP) is a common infection, which often requires hospital admission. In the United States, pneumonia is among the leading causes of hospital admission, especially among older adults.4 Approximately 10% of patients admitted to hospital with a primary diagnosis of pneumonia die in hospital.4
Previous observational studies have examined the association between diabetes and the risk of pneumonia.3,5–10 Although the literature generally suggests an increased risk,1,5–7,9,10 previous studies have produced heterogeneous results, and there is a need to have a better understanding of the potential sources of heterogeneity in this literature. In addition, the literature on the association between type 2 diabetes and CAP has not yet been synthesized. Given the increasing prevalence of type 2 diabetes and the clinical consequences of CAP, it is important to clarify the risk of CAP associated with type 2 diabetes. Our objective was to determine if type 2 diabetes is associated with an increased risk of CAP via a systematic review and meta-analysis of observational studies.
Methods
Study design
We conducted a systematic review and meta-analysis. Our study protocol, which was written following the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 201511 checklist, was registered with the International Prospective Register of Systematic Reviews (PROSPERO no. CRD42018116409). Postregistration protocol changes are specified below. The reporting of this knowledge synthesis follows the PRISMA20 and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.21,22
Data sources and searches
We systematically searched Embase (Ovid; start year: 1974), MEDLINE (Ovid; start year: 1946), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; start year: 1961), ProQuest theses and dissertations (EBSCO host; start year: 1997), Global Health (Ovid; start year: 1973), and the Global Index Medicus of the World Health Organization (start year: 1974) for studies published in English or French on type 2 diabetes (or studies on diabetes in general, because 85% of patients with diabetes have type 2 diabetes23) and pneumonia. We included studies published between Jan. 1, 1946 (the inception date of MEDLINE), and July 18, 2020.
The search strategy, constructed in consultation with a medical librarian, was developed in MEDLINE and then tailored to each database (Appendix 1, Supplemental Table S1, available at www.cmajopen.ca/content/9/1/E62/suppl/DC1). Briefly, we used MeSH terms for MEDLINE and CINAHL and Emtree terms for Embase for the concepts of type 2 diabetes (including terms for diabetes in general) and CAP. Search terms for diabetes in general were added after study registration. We also screened the first 10 pages of Google Scholar for any additional grey literature publications. Finally, we hand-searched references of relevant articles (including previous reviews in this area) for additional studies.
Study selection
We defined our inclusion criteria using the population, intervention, comparator and outcome (PICO) format of the Cochrane Handbook for Systematic Reviews of Interventions;24 however, we defined an exposure instead of an intervention (Box 1). Studies were included if they fulfilled the following criteria: the study had an observational design (cohort or case–control study); the study population was aged 18 years and older (population); the study reported data on type 2 diabetes or diabetes with type not specified (i.e., it did not explicitly differentiate between type 2 and type 1 diabetes; exposure); and the study reported data on CAP or unspecified pneumonia (i.e., it did not explicitly differentiate between CAP and nosocomial [hospital-or ventilator-acquired] pneumonia; outcome). We included studies for which the comparator group was patients without type 2 diabetes or without diabetes (comparator). We excluded cross-sectional studies because of their temporal ambiguity. We also excluded letters to the editor, commentaries, editorials, case reports, case series, reviews and meta-analyses, animal studies, basic science studies, conference abstracts (as they typically have insufficient data to adequately assess study quality and because their results are often not final) and studies that evaluated only type 1 diabetes or nosocomial pneumonia.
Box 1. Study question formatted according to the PICO framework.
P: Adults aged 18 years or older
I: Type 2 diabetes (exposure)
C: No diabetes
O: Community-acquired pneumonia
The PICO framework is described in the Cochrane Handbook for Systematic Reviews of Interventions.24 Note: PICO = population, intervention, comparator and outcome.
After removal of duplicates, 2 independent reviewers (V.C.B., H.T.A.) screened the titles and abstracts of the remaining studies for eligibility, with any article deemed potentially eligible by either reviewer carried forward for full-text review. The 2 reviewers conducted their full-text review independently and in duplicate, and they made the final decision about study inclusion by consensus.
Data extraction and quality assessment
The 2 reviewers independently extracted data in duplicate using a pilot-tested data extraction form. The following information was extracted: authors, year and location of study, study design, exposure and outcome definitions, follow-up duration, number of participants, baseline patient characteristics (mean age, sex), study outcomes, number of events by exposure group, crude and adjusted point estimates (odds ratios [ORs], rate ratios or hazard ratios [HRs]) and corresponding 95% confidence intervals [CIs], and variables included in statistical adjustment or matching.
We used an adapted version of the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool (adapted for exposure instead of intervention) to assess study quality. The predefined set of important confounders used to assess the potential level of confounding included age, sex, smoking status, alcohol use, history of asthma and history of chronic obstructive pulmonary disorder (COPD). Study quality was determined by the ROBINS-I domain with the greatest risk of bias. Quality assessment was conducted independently by 2 reviewers (V.C.B., H.T.A.), with disagreements resolved by consensus or by a third reviewer (K.B.F.).
Statistical analysis
Estimates were pooled across studies using DerSimonian–Laird random-effects models with inverse variance weighting.25 We pooled estimates from the model, adjusting for the most covariates reported by each study; unadjusted estimates were used if a study did not report adjusted estimates. If a study reported results from distinct cohorts that were non-overlapping, results from each of these cohorts were analyzed separately. For studies reporting HRs, we assumed that the hazard was proportional over the follow-up time and that the HR therefore approximated the risk ratio;26 if the hazards were constant, they would also have estimated the rate ratio. As pneumonia is a rare outcome,27 we assumed that ORs accurately estimated the risk ratio, and thus we pooled ORs, rate ratios and HRs as relative risks.
We assessed heterogeneity quantitatively using the I2 and T2 statistics and qualitatively by comparing the exposure and outcome definitions of the different studies. We also estimated 95% prediction intervals (PIs) to facilitate the interpretation of results.28 The inclusion of the T2 statistic and 95% PIs were postregistration additions to our study protocol.
We conducted subgroup analyses by study type (cohort v. case–control), exposure definition (type 2 v. unspecified diabetes) and outcome definition (CAP v. unspecified pneumonia). Small-study effects (where smaller studies may show larger treatment effects in meta-analyses)29 were assessed via visual inspection of funnel plots.30 We also conducted 3 sensitivity analyses: a fixed-effects analysis to examine the impact of our model choice, influence analyses to examine the impact of individual studies on the overall measure of association, and an analysis converting reported ORs to risk ratios using the approach described by Cochrane.26,31 All analyses were performed using Stata version 15.
Ethics approval
Ethics approval was not required for this systematic review, as our systematic review used publicly available aggregate data only.
Results
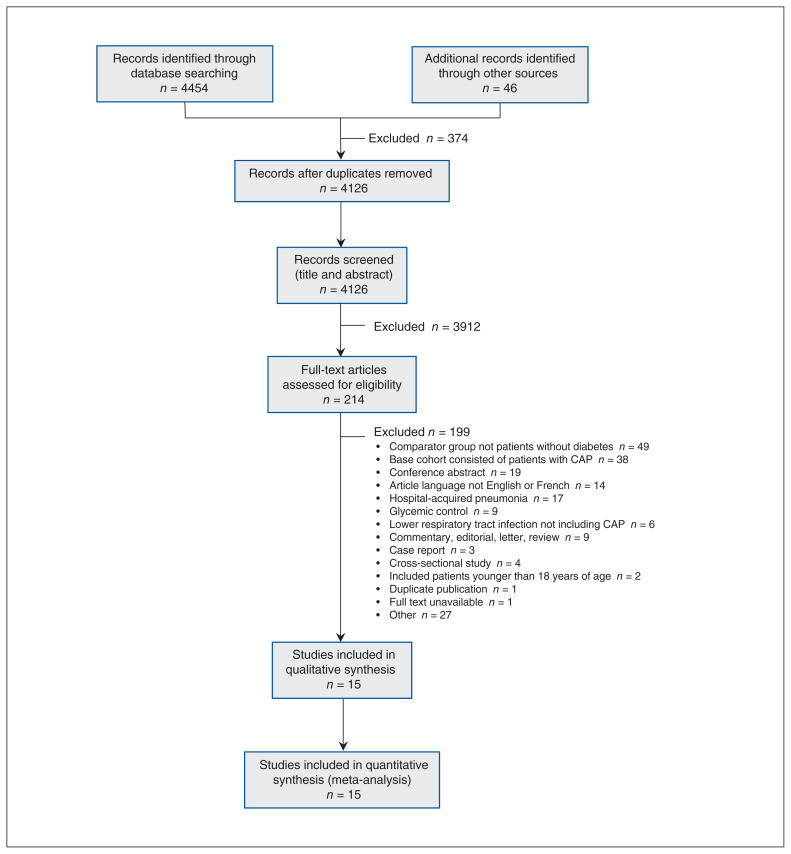
We identified 4454 publications through database searching, and we identified an additional 46 articles through other sources (Figure 1). After removal of duplicates, 4126 publications underwent title and abstract review. Fifteen studies met our inclusion criteria; these studies included a total of 14 538 968 patients.
Figure 1:
Flow diagram, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline, describing the systematic search for studies of type 2 diabetes and the risk of community-acquired pneumonia (CAP). Note: Some of the studies captured in the literature search evaluated the association between change in glycemic control and risk of pneumonia, in patients with or without diabetes. The estimate derived from these studies did not answer the research question posed in the present study, and thus these studies were not included in the meta-analysis.
Characteristics of the included studies are described in Table 1. Fifteen articles3,5,6,8,10,12–16,18 reporting on 13 cohort studies and 4 case–control studies7,9,18,19 were included. The article by Seminog and Goldacre10 reported results from 3 distinct cohorts (Linked English Hospital Episodes Statistics [LHES], Oxford Record Linkage Study 1 [ORLS1] and Oxford Record Linkage Study 2 [ORLS2]) that were non-overlapping, and thus we analyzed results from each of these cohorts separately but considered them collectively when describing study characteristics. The 4 case–control studies were population-based case–control studies. Six studies defined exposure as type 2 diabetes specifically,3,6–9,15 while 9 studies considered diabetes in general.5,10,12,13,16 The exposure and outcome assessment varied among studies (Appendix 1, Supplemental Table S2). Most studies adjusted for age, sex and socioeconomic status in their fully adjusted models. Adjusted estimates were unavailable for 4 studies.6,16,18,19
Table 1:
Characteristics of studies examining the association between type 2 diabetes and the risk of community-acquired pneumonia
| Author, year | Country | Study design | Sample size | Mean age, yr (SD)* | Male, %† | Exposure | Primary outcome | Mean duration of follow-up, yr |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| Jackson et al. 200412 | US | Retrospective cohort | 46 237 | NR | 42.0 | Diabetes | Hospital admission for CAP | 3 |
| Muller et al. 20053 | Netherlands | Prospective cohort | 26 328 | 65.7 (12.7)/63.1 (13.4)‡ | 46.1/39.1‡ | Type 2 diabetes | Pneumonia | 1 |
| O’Meara et al. 200513 | US | Prospective cohort | 5888 | 75.0/72.6§ | 42.3 | Diabetes | Hospital admission for pneumonia | 10.7¶ |
| Benfield et al. 20075 | Denmark | Retrospective cohort | 10 063 | 67.8/60.7‡ | NR | Diabetes | Hospital admission for pneumonia | 7 |
| Ehrlich et al. 201014 | US | Retrospective cohort | 121 866§ | 57.2¶ | 50.1 | Diabetes | Hospital admission for pneumonia | NR |
| Hamilton et al. 20136 | Australia | Prospective cohort | 6450 | 63.6/66.1** | 48.8/NR‡ | Type 2 diabetes | Hospital admission for pneumonia | 12.06 |
| Seminog and Goldacre 201310 (LHES) | UK | Retrospective cohort | 11 220 545 | 64 | NR | Diabetes | Pneumonia | 4 |
| Seminog and Goldacre 201310 (ORLS1) | UK | Retrospective cohort | 640 549 | 64 | NR | Diabetes | Pneumonia | 35 |
| Seminog and Goldacre 201310 (ORLS2) | UK | Retrospective cohort | 508 965 | 62 | NR | Diabetes | Pneumonia | 3 |
| Hine et al. 20178 | UK | Retrospective cohort | 647 330 | 67.0/46.0†† | 49.1 | Type 2 diabetes | Pneumonia | 1 |
| López-de-Andrés et al. 201715 | Spain | Retrospective cohort | 901 136 | 77.1 (10.5) | 60.1 | Type 2 diabetes | Hospital admission for CAP | 9 |
| Ray et al. 201716 | US | Retrospective cohort | 411 | 47.0 (16.3) | 73.8 | Diabetes | Pneumonia | NR |
| Williams et al. 201717 | UK | Retrospective cohort | 14 513 | 70.3 (10.8) | 53.6 | Diabetes | CAP | 5 |
| Case–control studies | ||||||||
| Farr et al. 200018 | UK | Population-based case–control | 555 | 45.2 | 46.1 | Diabetes | Pneumonia | NR |
| Thomsen et al. 20049 | Denmark | Population-based case–control | 6578 | 67 (18–94)/67 (17–94)‡‡ | 47.3 | Type 2 diabetes | CAP | 9 |
| van de Garde et al. 200619 | Netherlands | Population-based case–control | 4925 | 67 | 55.0 | Diabetes | Hospital admission for CAP | NR |
| Kornum et al. 20087 | Denmark | Population-based case–control | 376 629 | 74 (61–82)/74 (61–82)§§ | 52.9 | Type 2 diabetes | Hospital admission for pneumonia | NR |
Note: CAP = community-acquired pneumonia, LHES = Linked English Hospital Episodes Statistics, NR = not reported, ORLS = Oxford Record Linkage Study, SD = standard deviation, UK = United Kingdom, US = United States.
For entire population, unless otherwise specified.
For entire population, unless otherwise specified.
Diabetes/no diabetes.
Subcohort of patients for whom diabetes status was evaluated.
Median.
Admitted to hospital/not admitted to hospital.
Median: diabetes/no diabetes.
Median (full range): cases/controls.
Median (interquartile range): cases/controls.
Quality assessment
The combined risk of bias for all studies was serious, as all studies presented a serious risk of bias in at least 1 of the ROBINS-I domains (Appendix 1, Supplemental Table S3). Four, 7 and 4 studies were at low,7,12,13,15 moderate5,8,10,14,16,18,19 and serious risk3,6,9,17 of selection bias, respectively. All studies were either at low3,5,7–10,12,15–17,19 or moderate6,13,14,18 risk of exposure misclassification (classification of intervention in ROBINS-I). All included studies were at serious risk of bias for confounding because of inadequate control of important confounders; only 3 of the included studies controlled for COPD,12,13,17 only 5 controlled for smoking5,8,12–14 and only 4 controlled for asthma3,12 or other markers of pulmonary function.5,13 As all studies presented a serious risk of bias, it was not possible to perform stratified analyses by study quality.
Diabetes and pneumonia
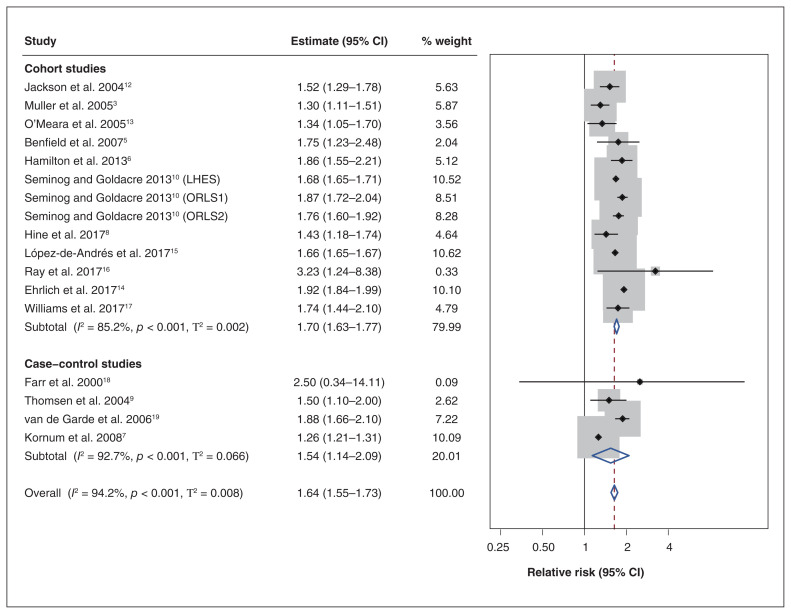
All included studies reported an increased risk of pneumonia among patients with diabetes (Table 2). When estimates were pooled across all studies, the pooled relative risk was 1.64 (95% CI 1.55–1.73) (Figure 2). Although the amount of relative heterogeneity that was present was high (I2 94.2%), the amount of absolute heterogeneity that was present was more modest (T2 0.008). The 95% PI was 1.30 to 2.06.
Table 2:
Measures of association of included studies examining the association between type 2 diabetes and the risk of community-acquired pneumonia
| Study | No. events/ no. exposed | No. events/ no. unexposed | Measure of association | Unadjusted estimate (95% CI) | Adjusted estimate (95% CI) | Covariates (adjusted for or matched) |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Jackson et al. 200412 | – | – | HR | – | 1.52 (1.29–1.78) | Age, sex, smoking status, CHF, ischemic heart disease, cancer, dementia, stroke, COPD, asthma, renal disease, use of prednisone or other immunosuppressive medication, no. of outpatient visits in the previous year, hospital admission for pneumonia in in the previous year, home oxygen therapy, receipt of home health care |
| Muller et al. 20053 | – | – | OR | 1.31 (1.15–1.50) | 1.30 (1.11–1.52) | Age, sex, asthma, pulmonary disease (including tuberculosis, acute bronchitis and asthma), insurance type, cardiovascular disease, peripheral neuropathy, neurologic disease |
| O’Meara et al. 200513 | – | – | Risk ratio | – | 1.34 (1.05–1.70) | Age, race, education level, smoking status, prior vaccination for pneumonia, vaccination for influenza in the previous year, FEV1, FVC, maximal inspiratory pressure, 3MSE score, history of: MI, angina pectoris, CAD, claudication, CHF, CVA, COPD, pneumonia |
| Benfield et al. 20075 | 90/353 | 1104/9710 | HR | 2.55 (1.86–3.29) | 1.75 (1.23–2.48) | Age, sex, smoking status, SES (education, income), cholesterol, triacylglycerol, hypertension, physical activity, lung function |
| Ehrlich et al. 201014 | – | – | HR | – | 1.92 (1.84–1.99) | Age, sex, smoking status, race or ethnicity, education, alcohol, BMI, no. of outpatient visits occurring in the 12 mo before baseline |
| Hamilton et al. 20136 | 181/1294 | 435/5156 | Rate ratio | 1.86 (1.55–2.21) | – | – |
| Seminog and Goldacre 201310 (LHES) | – | – | Rate ratio | – | 1.68 (1.65–1.71) | Age, sex, the time period in single calendar years, SES (region of residence deprivation score) |
| Seminog and Goldacre 201310 (ORLS1) | – | – | Rate ratio | – | 1.87 (1.72–2.04) | Age, sex, the time period in single calendar years, SES (district of residence) |
| Seminog and Goldacre 201310 (ORLS2) | – | – | Rate ratio | – | 1.76 (1.60–1.92) | Age, sex, the time period in single calendar years, SES (district of residence) |
| Hine et al. 20178 | 34 278* | 613 052† | OR | – | 1.43 (1.18–1.74) | Age, sex, smoking status, SES, comorbidities, general practice |
| López-de-Andrés et al. 201715 | NR/223 715 | NR/677 621 | Rate ratio | 1.66 (1.65–1.67) | Age, sex, year of discharge | |
| Ray et al. 201716 | 7/47 | 15/292 | OR | 3.23 (1.24–8.38) | – | – |
| Williams et al. 201717 | – | – | OR | – | 1.74 (1.44–2.10) | Age, sex, smoking status, BMI, prior diagnosis of pneumonia, exacerbation frequency, pharmacotherapy, comorbidities, GOLD stage |
| Case–control studies | ||||||
| Farr et al. 200018 | – | – | OR | 2.50 (0.34–14.11) | – | – |
| Thomsen et al. 20049 | 53/351 | 545/6227 | OR | 1.9 (1.4–2.6) | 1.5 (1.1–2.0) | Age (matched), sex (matched), Charlson Comorbidity Index score, alcohol-related disease |
| van de Garde et al. 200619 | 134/393 | 974/4532 | OR | 1.88 (1.66–2.10) | – | – |
| Kornum et al. 20087 | 4489/32 975 | 29 750/ 343 654 | OR | 1.68 (1.62–1.74) | 1.26 (1.21–1.31) | Age (matched), sex (matched), SES (marital status, degree of urbanization) |
Note: BMI = body mass index, CAD = coronary artery disease, CHF = congestive heart failure, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CVA = cerebrovascular accident, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, GOLD = Global Initiative on Obstructive Lung Disease, HR = hazard ratio, LHES = Linked English Hospital Episodes Statistics, MI = myocardial infarction, NR = not reported, OR = odds ratio, ORLS = Oxford Record Linkage Study, SES = socioeconomic status, 3MSE = Modified Mini-Mental State Examination.
Total no. of exposed patients.
Total no. of unexposed patients.
Figure 2:
Forest plot of association between type 2 diabetes and risk of community-acquired pneumonia by study design. Note: CI = confidence interval, LHES = Linked English Hospital Episodes Statistics, ORLS = Oxford Record Linkage Study. DerSimonian–Laird random-effects models were used to pool estimates across studies. The shaded areas represent the weight of the study in the overall estimate. The 95% prediction intervals were 1.51–1.92 for cohort studies, 0.74–3.21 for case–control studies and 1.30–2.06 overall.
The pooled relative risk was 1.70 (95% CI 1.63–1.77, I2 85.2%, T2 0.002, 95% PI 1.51–1.92) among cohort studies (n = 13) and the pooled OR was 1.54 (95% CI 1.14–2.09, I2 92.7%, T2 0.07, 95% PI 0.74–3.21) among case–control studies (n = 4). In subgroup analyses that included both cohort and case–control studies, the pooled estimate for studies where exposure was restricted to type 2 diabetes was 1.48 (95% CI 1.26–1.74; I2 97.4%, T2 0.033, 95% PI 0.90–2.42) and 1.76 (95% CI 1.64–1.88; I2 80.5%, T2 0.006, 95% PI 1.43–2.17) for studies of diabetes in general (Appendix 1, Supplemental Figure S1).
Estimates were similar by outcome definition; the pooled relative risk was 1.65 (95% CI 1.45–1.87; I2 97.6%, T2 0.023, 95% PI 1.10–2.47) for hospital admission for pneumonia and 1.64 (95% CI 1.53–1.76, I2 63.3%, T2 0.005, 95% PI 1.34–2.00) for studies with the outcome defined by a pneumonia diagnosis (Appendix 1, Supplemental Figure S2).
In sensitivity analyses, fixed-effects models produced results that were consistent with those of our primary analysis (Appendix 1, Supplemental Figure S3). Influence analyses, conducted using random-effects models, suggested that the study by Kornum and colleagues (2008)7 had the greatest impact on the overall estimate and amount of heterogeneity (Appendix 1, Supplemental Figures S4 and S5; overall estimate excluding that study: 1.71, 95% CI 1.64–1.78, I2 82.6%, T2 0.002, 95% PI 1.51–1.93). Asymmetry of our funnel plot showed some evidence of small-study effects (Appendix 1, Supplemental Figure S6), although they were mainly due to 2 small studies. Finally, results were similar for our repeated primary analysis when ORs were converted to risk ratios (Appendix 1, Supplemental Table S4 and Figure S7).
Interpretation
Our systematic review and meta-analysis was designed to assess the association between type 2 diabetes and CAP. All included studies reported an increased risk of pneumonia in patients with type 2 diabetes. When data were pooled across all studies, the pooled relative risk was 1.64 (95% CI 1.55–1.73). The corresponding 95% PI ranged from 1.30 to 2.06, suggesting that the increased risk would be found in this range in 95% of future clinical settings.33 Quality assessment revealed that the included studies had a serious risk of bias, and thus our results should be interpreted with caution.
Pneumococcal and influenza vaccination are recommended by most guidelines23,33 and are suggested as a cost-effective strategy to prevent CAP in patients with type 2 diabetes.34 Although the included evidence has important limitations, our results are compatible with current clinical treatment guidelines.23,33 The increased risk of CAP in patients with type 2 diabetes should be taken into consideration in clinical practice, and prevention of pneumonia should be discussed by physicians.
The specific biological mechanism behind the increased risk of CAP in patients with type 2 diabetes has not been established. The increased risk may be due to the impaired function of neutrophils and monocytes caused by hyperglycemia.2,3,34 Patients with type 2 diabetes may be at greater risk of pneumococcal pneumonia because of increased susceptibility to certain organisms probably caused by their hyperglycemic environment.35–39 It is also possible that complications associated with diabetes, including disordered sleep patterns and impaired lung function, may be involved in this mechanism.40,41 Patients with type 2 diabetes also appear to have worse pneumonia outcomes,34,42 as certain microorganisms may become more virulent in a hyperglycemic environment.43 Thus, attaining glycemic control may improve outcomes in these patients.44
A previous meta-analysis on diabetes and the risk of all infections revealed an increased risk of lower respiratory tract infections in patients with diabetes (cohort studies: OR 1.35, 95% CI 1.28–1.43, I2 79.4%; case–control studies: OR 1.60, 95% CI 1.35–1.89, I2 86.7%).1 However, this study did not differentiate between diabetes types nor between nosocomial and community-acquired respiratory infections.1 Although the notion that type 2 diabetes is a risk factor for CAP is well known and accepted in a clinical setting, the literature on this topic is surprisingly sparse and consists of studies at serious risk of bias. We found that the main limitation of the included studies was inadequate control for confounding. Future research examining the biological mechanism behind the increased risk of CAP in patients with type 2 diabetes is needed to understand this association fully and to develop appropriate preventive strategies.
This study has several strengths. Our search strategy, which was developed with an experienced librarian, allowed us to assess the available literature comprehensively. Our study was conducted according to a prespecified protocol registered at PROSPERO. It included a detailed assessment of study quality and included subgroup and sensitivity analyses to gain a better understanding of sources of clinical and statistical heterogeneity in this literature. Finally, the inclusion of 95% PIs strengthens the clinical interpretation of our results.
Limitations
Our study has potential limitations. We found some evidence of small-study effects. Although this appears to be due to 2 small studies, publication bias and small-study effects are inherent limitations of all knowledge syntheses. The estimated I2 statistics suggest the presence of substantial statistical heterogeneity; however, this statistic is a relative measure, and the T2 statistics suggest a modest amount of absolute heterogeneity. Some subgroup analyses also had important heterogeneity (by exposure and outcome definition); subsequent analyses determined that it was largely driven by 1 study,7 the exclusion of which greatly reduced the I2 statistic. All of the included studies had a serious risk of bias, which prevented us from conducting subgroup analyses by study quality, and systematic reviews are inherently affected by the limitations of the included studies. We applied the ROBINS-I tool to case–control studies. However, our adaptation of the ROBINS-I tool for exposure instead of intervention allowed us to use it for the case–control studies as they were part of a well-defined cohort.
Some of the included studies examined diabetes in general rather than being restricted to type 2 diabetes, which may introduce exposure misclassification. However, 85% of patients with diabetes have type 2 diabetes;23 thus, we deemed it appropriate to include such studies. We presented the I2 for all subgroups for transparency, although it may be biased in small samples.45 We included only studies published in English or French, which may have introduced language bias, a form of selection bias. Because of insufficient data and data variability, we were unable to examine how the distributions of characteristics such as age and sex affected study-specific measures of association. Finally, our search of the grey literature may have been incomplete as our search strategy was not tailored to the grey literature.
Conclusion
The available evidence suggests that type 2 diabetes is associated with an increased risk of CAP. However, this evidence is from studies at serious risk of bias, and additional high-quality studies are needed to confirm these findings. While awaiting such studies, health care providers should inform patients to seek medical attention promptly if they develop symptoms of CAP to facilitate early detection and treatment, given the morbidity and mortality associated with CAP. Physicians and patients should be aware of the importance of attaining glycemic control to prevent resulting infections in this patient population.
Supplementary Material
Acknowledgements
We would like to thank Andrea Quaiattini, medical librarian at McGill University, for her help in developing the search strategy. Vanessa Brunetti is supported by a doctoral training scholarship from the Fonds de recherche du Québec — Santé (FRQS); the Drug Safety and Effectiveness Cross-Disciplinary Training (DSECT) program award, funded by the Canadian Institutes of Health Research (CIHR); and the David G. Guthrie Fellowship from the Faculty of Medicine at McGill University. Henok Tadesse Ayele is supported by a postdoctoral training scholarship from the FRQS. Oriana Hoi Yun Yu holds a Chercheur-boursier clinicien junior I salary support award from the FRQS. Kristian Filion holds a Chercher-boursier senior salary support award from the FRQS and a William Dawson Scholar award from McGill University.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Kristian Filion conceived the study idea and supervised the study. Vanessa Brunetti developed the search strategy, performed the statistical analyses and drafted the manuscript. Vanessa Brunetti and Henok Ayele performed the screening of relevant articles, data extraction and quality assessment. Pierre Ernst and Oriana Yu provided substantive knowledge expertise. All authors were involved in designing the study, interpreting the data and critically reviewing the manuscript for intellectual content. All authors agreed to be accountable for all aspects of the work.
Data sharing: The present study used aggregate, published data that are publicly available. No additional data are available for sharing.
Previous presentation: Results from this study were presented in poster form at the 2019 Society for Epidemiologic Research (SER) Annual Meeting, Minneapolis, Minn., June 18, 2019.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/1/E62/suppl/DC1.
References
- 1.Abu-Ashour W, Twells L, Valcour J, et al. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res Care. 2017;5:e000336. doi: 10.1136/bmjdrc-2016-000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Weerarathna T, McCarthy JS, et al. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 3.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–8. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 4.Fry AM, Shay DK, Holman RC, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–9. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 5.Benfield T, Jensen J, Nordestgaard B. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–54. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton EJ, Martin N, Makepeace A, et al. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the Fremantle Diabetes Study. PLoS One. 2013;8:e60502. doi: 10.1371/journal.pone.0060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornum JB, Thomsen RW, Riis A, et al. Diabetes, glycemic control and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31:1541–5. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hine JL, de Lusignan S, Burleigh D, et al. Association between glycaemic control and common infections in people with type 2 diabetes: a cohort study. Diabet Med. 2017;34:551–7. doi: 10.1111/dme.13205. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen RW, Hundborg HH, Lervang H-H, et al. Risk of community-acquired pneumococcal bacteremia in patients with diabetes: a population-based case-control study. Diabetes Care. 2004;27:1143–7. doi: 10.2337/diacare.27.5.1143. [DOI] [PubMed] [Google Scholar]
- 10.Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people hospitalized with diabetes mellitus: English record-linkage studies. Diabet Med. 2013;30:1412–9. doi: 10.1111/dme.12260. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–50. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Meara ES, White M, Siscovick DS, et al. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005;53:1108–16. doi: 10.1111/j.1532-5415.2005.53352.x. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich SF, Quesenberry CP, Jr, Van Den Eeden SK, et al. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-de-Andrés A, de Miguel-Díez J, Jiménez-Trujillo I, et al. Hospitalisation with community-acquired pneumonia among patients with type 2 diabetes: an observational population-based study in Spain from 2004 to 2013. BMJ Open. 2017;7:e013097. doi: 10.1136/bmjopen-2016-013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray JJ, Meizoso JP, Allen CJ, et al. Admission hyperglycemia predicts infectious complications after burns. J Burn Care Res. 2017;38:85–9. doi: 10.1097/BCR.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 17.Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis. 2017;12:313–22. doi: 10.2147/COPD.S121389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farr BM, Woodhead MA, MacFarlane JT, et al. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med. 2000;94:422–7. doi: 10.1053/rmed.1999.0743. [DOI] [PubMed] [Google Scholar]
- 19.van de Garde EMW, Souverein PC, van den Bosch JMM, et al. Angiotensin-converting enzyme inhibitor use and pneumonia risk in a general population. Eur Respir J. 2006;27:1217–22. doi: 10.1183/09031936.06.00110005. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. PRISMA-P Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. American Diabetes Association standards of medical care in diabetes: 2019. Diabetes Care. 2019;42(Suppl 1):S1–187. [Google Scholar]
- 24.Thomas J, Kneale D, McKenzie JE, et al. Chapter 2: Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Oxford (UK): Cochrane Collaboration; 2019. [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Deeks JJ, Higgins JPT, Altman DG. Cochrane Statistical Methods Group Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 60. 6th ed. Oxford (UK): Cochrane Collaboration; 2019. [Google Scholar]
- 27.Chalmers J, Campling J, Ellsbury G, et al. Community-acquired pneumonia in the United Kingdom: a call to action. Pneumonia (Nathan) 2017;9:15. doi: 10.1186/s41479-017-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 30.Light R, Pillemer D. Summing up: the science of reviewing research. Cambridge (MA): Harvard University Press; 1984. [Google Scholar]
- 31.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 32.IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1):S1–325. [Google Scholar]
- 34.Joshi N, Caputo GM, Weitekamp MR, et al. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 35.Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am. 1995;9:65–96. [PubMed] [Google Scholar]
- 36.Ahluwalia A, Sood A, Sood A, et al. Nasal colonization with Staphylococcus aureus in patients with diabetes mellitus. Diabet Med. 2000;17:487–8. doi: 10.1046/j.1464-5491.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyko EJ, Lipsky BA, Sandoval R, et al. NIDDM and prevalence of nasal Staphylococcus aureus colonization: San Luis Valley diabetes study. Diabetes Care. 1989;12:189–92. doi: 10.2337/diacare.12.3.189. [DOI] [PubMed] [Google Scholar]
- 38.Marrie TJ. Bacteraemic pneumococcal pneumonia: a continuously evolving disease. J Infect. 1992;24:247–55. doi: 10.1016/s0163-4453(05)80029-5. [DOI] [PubMed] [Google Scholar]
- 39.Bouter KP, Diepersloot RJ, van Romunde LK, et al. Effect of epidemic influenza on ketoacidosis, pneumonia and death in diabetes mellitus: a hospital register survey of 1976–1979 in The Netherlands. Diabetes Res Clin Pract. 1991;12:61–8. doi: 10.1016/0168-8227(91)90131-v. [DOI] [PubMed] [Google Scholar]
- 40.Incalzi RA, Fuso L, Giordano A, et al. Neuroadrenergic denervation of the lung in type I diabetes mellitus complicated by autonomic neuropathy. Chest. 2002;121:443–51. doi: 10.1378/chest.121.2.443. [DOI] [PubMed] [Google Scholar]
- 41.Sandler M, Bunn AE, Stewart RI. Cross-section study of pulmonary function in patients with insulin-dependent diabetes mellitus. Am Rev Respir Dis. 1987;135:223–9. doi: 10.1164/arrd.1987.135.1.223. [DOI] [PubMed] [Google Scholar]
- 42.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–41. [PubMed] [Google Scholar]
- 43.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, Koirala J, Khardori R, et al. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21:617–38. vii. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 45.von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.