Abstract
Background
Effective halting of outbreaks in skilled nursing facilities (SNFs) depends on the earliest recognition of cases. We assessed confirmed COVID-19 cases at an SNF impacted by COVID-19 in the United States to identify early indications of COVID-19 infection.
Methods
We performed retrospective reviews of electronic health records for residents with laboratory-confirmed SARS-CoV-2 during February 28–March 16, 2020. Records were abstracted for comorbidities, signs and symptoms, and illness outcomes during the 2 weeks before and after the date of positive specimen collection. Relative risks (RRs) of hospitalization and death were calculated.
Results
Of the 118 residents tested among approximately 130 residents from Facility A during February 28–March 16, 2020, 101 (86%) were found to test positive for SARS-CoV-2. At initial presentation, about two-thirds of SARS-CoV-2–positive residents had an abnormal vital sign or change in oxygen status. Most (90.2%) symptomatic residents had elevated temperature, change in mental status, lethargy, change in oxygen status, or cough; 9 (11.0%) did not have fever, cough, or shortness of breath during their clinical course. Those with change in oxygen status had an increased relative risk (RR) of 30-day mortality [51.1% vs 29.7%, RR 1.7, 95% confidence interval (CI) 1.0–3.0]. RR of hospitalization was higher for residents with underlying hepatic disease (1.6, 95% CI 1.1–2.2) or obesity (1.5, 95% CI 1.1–2.1); RR of death was not statistically significant.
Conclusions and Implications
Our findings reinforce the critical role that monitoring of signs and symptoms can have in identifying COVID-19 cases early. SNFs should ensure they have a systematic approach for responding to abnormal vital signs and oxygen saturation and consider ensuring common signs and symptoms identified in Facility A are among those they monitor.
Keywords: Skilled nursing facility, SARS-CoV-2, COVID-19, virus, signs and symptoms
Skilled nursing facilities (SNFs) have been a focal point for COVID-19 outbreaks, with rapid transmission noted soon after the first cases are recognized.1 , 2 Early recognition of persons with COVID-19 in SNFs will remain critical to halting infection transmission and ensuring appropriate clinical care even after residents and staff are vaccinated.3 The sum total of clinical signs and symptoms experienced by SNF residents has been previously reported from larger data sets.4 , 5 However, many of these may not occur until days after illness onset. For practical purposes, the earliest signs and symptoms are the most meaningful ones for caregivers to know so that cases are recognized at the earliest point in illness and action subsequently taken.
This report describes the earliest signs and symptoms experienced by residents of a severely impacted SNF, Facility A, during March 2020.1 , 2 The Centers for Disease Control and Prevention (CDC) has frequently updated its list of signs and symptoms known to be associated with COVID-19.6 This study informed CDC recommendations about the signs and symptoms that may most effectively identify affected SNF residents, including residents with dementia who may be unable to reliably convey symptoms to caregivers. Facility A is a 190-bed SNF in King County, Washington, that provides skilled nursing care, inpatient rehabilitation, and long-term residential care and was severely impacted by COVID-19, with 101 (86%) of 118 tested residents confirmed positive for SARS-CoV-2 and 35 deaths.2 We assessed the clinical characteristics documented for Facility A residents immediately preceding positive test results to determine early warning signs for COVID-19 and inform monitoring and response strategies in SNFs in the United States.
Methods
During February 28–March 16, 2020, testing for SARS-CoV-2 in Facility A residents occurred because of suspicion for COVID-19 during acute care hospitalizations or at death, or during a facility-wide point prevalence survey conducted on March 7–8. Specimen collection and diagnostic testing were conducted in accordance with CDC guidelines.7 Diagnostic testing was done using the CDC's SARS-CoV-2 real-time reverse transcription polymerase chain reaction (rRT-PCR) panel for detection of SARS-CoV-2.8
We defined a case as a patient with laboratory-confirmed SARS-CoV-2 among residents of Facility A during February 28–March 16, 2020. We performed a retrospective review of electronic health records for the 2 weeks before and the 2 weeks after the date of positive specimen collection to capture signs and symptoms associated with COVID-19. The electronic health records were reviewed to obtain the needed information, including admission and daily clinical progress notes, recorded vital signs and oxygen saturation, medication orders, and active medical diagnoses. Demographics, comorbidities, body mass index (BMI), objective signs and symptoms, and scheduled medications while at Facility A were abstracted; BMI ≥30 was defined as obese and medications abstracted included β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, narcotics, and immunosuppressive medications such as chemotherapy and corticosteroids.
We defined asymptomatic infected patients as those residents with a positive SARS-CoV-2 test for whom we did not find documented evidence of signs and symptoms consistent with COVID-19 in the 2 weeks before or after the positive test was performed, and we defined presymptomatic infected patients as residents without symptoms at the time of specimen collection who developed symptoms in the 2 weeks after. Asymptomatic residents and those with unknown illness onset date were excluded from analysis of signs and symptoms. We defined illness onset date as the first day of any of the following: elevated temperature, change in oxygen status, or any new symptom in the 2 weeks before or after testing positive for COVID-19 while at Facility A. Change in oxygen status was defined as a decrease of 3% or more from baseline oxygen saturation, a new supplemental oxygen requirement, or a decrease in oxygen saturation to lower than 90%. Tachycardia was defined as heart rate higher than 90 beats per minute, tachypnea as respiratory rate higher than 20 breaths per minute, elevated temperature as any temperature greater than 99°F, low-grade fever as a temperature between 99° and 100°F, and a fever as above 100°F. Signs and symptoms assessed throughout the clinical course included fever, cough, sputum production, shortness of breath, change in mental status, lethargy, fatigue, nasal congestion or rhinorrhea, chills, acute diarrhea, headache, conjunctivitis, myalgias, nausea or vomiting, or sore throat. Any signs and symptoms other than cough, fever, or shortness of breath were classified as atypical for COVID-19 because at the time of this investigation, these were less known.
Outcomes assessed included hospitalization and death; deaths were obtained from epidemiologic data collected by Public Health–Seattle & King County (PHSKC) of all confirmed cases of COVID-19 and were included in the study if they occurred within 30 days of illness onset. Unadjusted relative risk was calculated for hospitalization and death by age group, sex, selected comorbidities, and scheduled medications. Pearson chi-square tests were used to calculate P values, which were not adjusted for multiple comparisons because of the exploratory nature of the study. Risk ratios were calculated as the ratio of the proportion exposed with outcome to proportion unexposed with outcome, with 95% confidence intervals (CIs) being calculated using the Taylor Series method. Risk ratios with CIs that did not cross 1.0 and P values ≤.05 were considered statistically significant. Analysis was performed using SAS 9.4. This activity was conducted as part of a public health response and was therefore deemed nonhuman subjects research by CDC.
Results
Demographic Characteristics and Association With Clinical Outcome
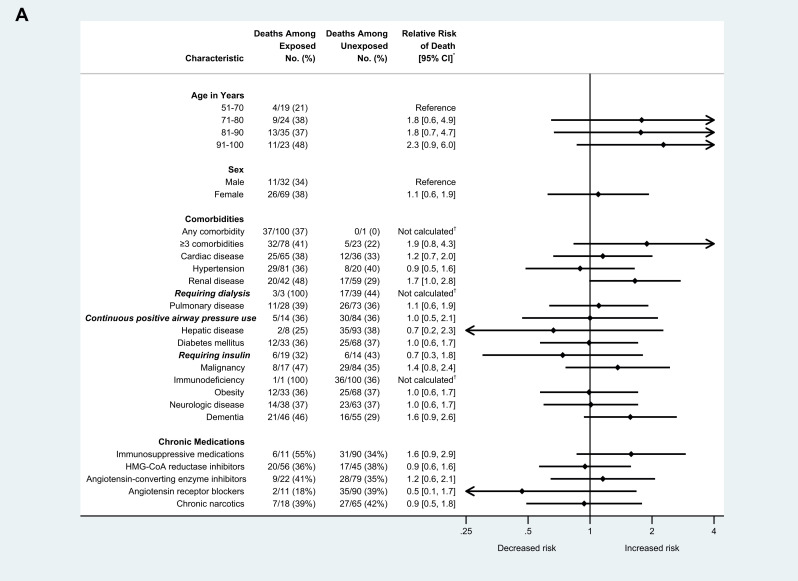
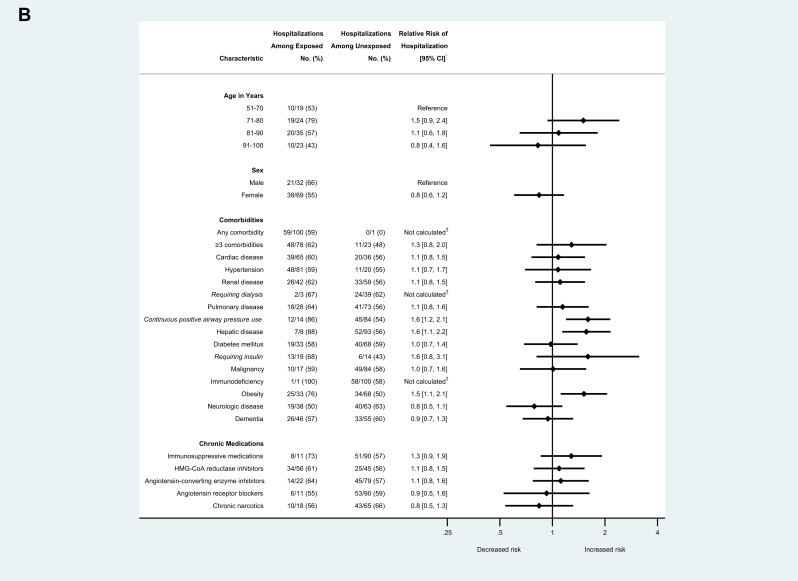
Of approximately 130 residents at Facility A, 118 were tested during February 28–March 16, 2020, and 101 (85.6%) tested positive for SARS-CoV-2, and 35 (34.7%) died. Among positive case-patients, median age was 83 years (range: 51–100), and 69 (68.3%) were female. One hundred (99.0%) residents with COVID-19 had at least 1 comorbidity, and 70 (69.3%) had at least 3 comorbidities; hypertension, cardiac disease, and dementia were the most common comorbidities documented. Relative risk (RR) of hospitalization was higher for residents with COVID-19 who had underlying hepatic disease (RR 1.6, 95% CI 1.1–2.2) or obesity (RR 1.5, 95% CI 1.1–2.1), compared to residents without these conditions. RR of hospitalization was also higher for residents who were on continuous positive airway pressure (RR 1.6, 95% CI 1.2–2.1) compared with those who were not (Figure 1 A.). No age, sex, or comorbidities were associated with death (Figure 1B). Fifty-six (55.5%) residents with COVID-19 were prescribed HMG-CoA reductase inhibitors, 33 (32.7%) were prescribed either an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, and 11 (10.9%) were prescribed immunosuppressive medications. Scheduled chronic narcotics were prescribed for 18 (21.7%) residents with COVID-19. None of these medications were associated with increased risk of, or protective effect for, hospitalization or death. Age, sex, comorbidities, and the medications abstracted from health records were not associated with change in oxygen status.
Fig. 1.
(A) Risk of hospitalization among skilled nursing facility residents with COVID-19 and select demographic and clinical characteristics (n = 101), King County, Washington, February-March 2020. (B) Risk of death among skilled nursing facility residents with COVID-19 and select demographic and clinical characteristics (n = 101), King County, Washington, February-March 2020. Arrows indicate CIs that extend beyond the x-axis range displayed. ∗Relative risks were calculated as the ratio of the proportion of residents with each characteristic who experienced the outcome (hospitalization or death) divided by the proportion of residents without that characteristic who experienced the outcome. Next, 95% CIs were calculated using the Taylor Series method. †Relative risks were not calculated for these characteristics because of the very small number of residents in either the numerator or denominator, which make relative risk estimates unreliable.
Signs and Symptoms and Association With Clinical Outcome
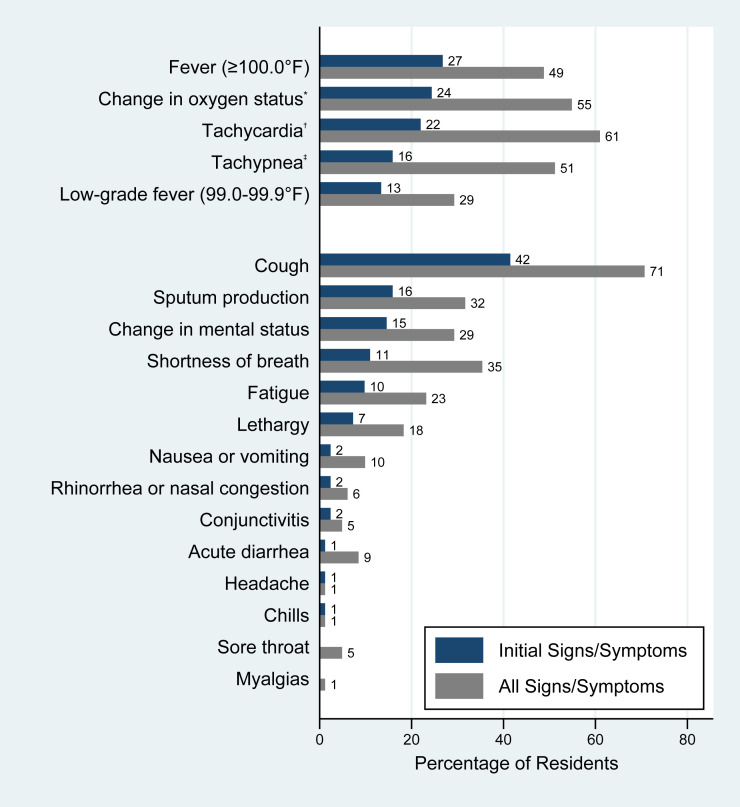
Of those who tested positive, 4 residents were defined as asymptomatic, 12 were presymptomatic, and 15 others had an unknown illness onset date. For the remaining 82 residents with positive test results and known illness onset date, the most common signs and symptoms on date of illness onset included cough (41.5%), fever (26.8%), tachycardia (22.0%), and change in oxygen status (24.4%) (Figure 2 ). Most (74 of the 82 residents; 90.2%) presented with one of the following signs or symptoms at illness onset: elevated temperature, change in mental status, lethargy, change in oxygen status, or cough. The most common signs and symptoms at any point during the clinical course were cough (70.7%), tachycardia (61%), change in oxygen status (54.9%), tachypnea (51.2%), and fever (48.8%). At initial presentation, about two-thirds (63.4%) of SARS-CoV 2–positive residents had an abnormal vital sign (ie, elevated temperature, tachycardia, or tachypnea) or change in oxygen status, and 76 (92.7%) had at least 1 of these objective signs during the clinical course documented at Facility A.
Fig. 2.
Signs and symptoms of skilled nursing facility residents with COVID-19 (n = 82), King County, Washington, February-March 2020. ∗A decrease of 3% or more from baseline oxygen saturation, a new supplemental oxygen requirement, or a decrease in oxygen saturation to lower than 90%. †Heart rate greater than 90 beats per minute. ‡Respiratory rate greater than 20 breaths per minute.
Of the 45 residents with a documented change in oxygen status, 20 (24.4%) developed this on the date of illness onset. For 6 (7.3%) residents, change in oxygen status was the only sign documented at Facility A. The median time from illness onset to change in oxygen status was 1 day (range: 0–18 days). Among those who died, median time from change in oxygen status to death was 5 days (range: 0–44 days). Those with change in oxygen status had an increased relative risk of 30-day mortality compared to those without (51.1% vs 29.7%, RR 1.7, 95% CI 1.0–3.0). However, among those who had a change in oxygen status and were transferred to a hospital, there was no difference in risk of death among those who were transferred the day of the change in oxygen status compared to those who were transferred later in their clinical course (64.3% vs 58.3%, RR 0.9, 95% CI 0.5–1.7).
Sixty-three percent of COVID-19–positive residents had at least 1 atypical symptom during the clinical course documented at Facility A. Among the 82 residents with symptomatic COVID-19, 9 (11.0%) did not have fever, cough, or shortness of breath at any time during their clinical course. These residents had low-grade fever, change in mental status, lethargy, conjunctivitis, nausea or vomiting, diarrhea, or fatigue; 5 (6.1%) had change in oxygen status in addition to these signs and symptoms. Four (4.9%) residents with COVID-19 had documented conjunctivitis during their clinical course. Overall, resident-reported symptoms were documented less frequently than signs or symptoms that can be directly observed by health care personnel; sore throat was reported by 4 (4.9%) residents, headache by 1 (1.2%) resident, and myalgias by 1 (1.2%) resident. Residents with dementia (n = 37) had similar signs and symptoms compared to residents without dementia (n = 45) other than fatigue (13.5% vs 31.1%, P = .06); no findings were statistically significant.
Median times from illness onset to transfer to an acute care hospital and from illness onset to death were 4 days (range: 0–18) and 10.5 days (range: 0–24), respectively. Seven residents were transferred on the day of illness onset, and 1 died on the day of illness onset. Of the 37 residents who died, 25 (67.6%) had a do-not-resuscitate order documented in Facility A, and 7 of those were transitioned to comfort care in the same facility before they died.
Discussion
This study evaluated early clinical signs and symptoms associated with COVID-19 in residents of an SNF in King County, Washington. In Facility A, nearly 3 in 5 residents with COVID-19 required hospitalization, and more than one-third died. These results are consistent with previous global scientific literature illustrating higher rates of hospitalization and death in nursing home residents with COVID-19.9 , 10 Asymptomatic infection has occurred in SNFs nationwide rendering symptom-based screening alone inadequate for identifying COVID-19 cases.5 , 11 , 12 Serial point prevalence surveys, that is, repeated testing of residents and health care personnel regardless of symptoms, is, however, not currently feasible at every SNF. Multiple logistical challenges must be overcome to obtain specimens from a large number of persons, some of whom may be unable to cooperate because of dementia, and test results may not be available for several days from the date of collection rendering them unhelpful in the interim when transmission may be occurring.13 For these SNFs but also for time intervals between serial point prevalence survey in facilities where serial testing is feasible, symptom-based screening remains critical.3 Fever and cough, typical signs and symptoms of COVID-19 in the general population and previous nursing home studies,4 , 14, 15, 16 were common in infected Facility A residents as well. However, the majority (62.2%) of the infected residents also presented with atypical signs and symptoms during the course of illness indicating that limiting monitoring to these typical signs and symptoms could miss cases. Additionally, 11.0% presented only with atypical symptoms, similar to findings reported from other SNF residents with COVID-19.11 The identification of atypical symptoms, in addition to more recognized signs and symptoms of fever and cough, should prompt placement in transmission-based precautions and testing for SARS-CoV-2.17 These adjustments will be especially important for residents with dementia or cognitive decline who may not be able to identify subjective symptoms reliably and whose caregivers could identify alternative symptoms such as change in mental status.
The results of our study support the current CDC recommendations for monitoring persons with signs or symptoms consistent with COVID-19. Consistent with our findings, CDC guidance indicates that changes in vital signs and pulse oximetry are important tools for screening and measuring severity of illness for SNF residents with known or suspected COVID-19.3 Only a small number of residents (n = 4) were found to be asymptomatic in our study compared to other studies in nursing homes; however, we identified signs frequently in residents.4 , 12 We found that abnormal vital signs, including change in oxygen status, were present at illness onset in 63.4% of infected residents, and change in oxygen status was associated with greater 30-day mortality. In addition, 90.2% of residents had at least 1 sign, such as elevated temperature, lethargy, change in mental status, change in oxygen status, or new cough, at illness onset that could be observed by a health care personnel without relying on a resident to report a complaint. SNFs should consider a systematic process for routine screening of COVID-19 among residents and include the 5 signs and symptoms that occurred during date of illness onset for most patients at Facility A. Closely monitoring these objective signs among all SNF residents in a facility with 1 or more cases of COVID-19, coupled with early testing, should facilitate rapid detection of any additional symptomatic cases soon after illness onset. Residents with multiple symptoms at the time of testing have had increased mortality.4 For early detection of serious COVID-19 infections, CDC recommends monitoring all residents daily for fever and symptoms and ill residents, which includes evaluating vital signs, symptoms, and respiratory examination including oxygen saturation, at least 3 times a day.3 Some older adult residents may not experience the typical symptoms of COVID-19 such as fever, shortness of breath, or cough and may not exhibit any respiratory symptoms at all.11 , 12 Persons with dementia pose an additional challenge to SNFs because these residents may be unable to reliably convey symptoms to caregivers.11
We found that only chronic hepatic disease, obesity, and receiving treatment with continuous positive airway pressure use were associated with statistically significant increased risk for hospitalization; each of these increased risk of hospitalization between 50% and 60%. Other studies specific to hospitalized patients have shown that older age, hypertension, diabetes, cardiovascular disease, and respiratory disease are risk factors for poor outcomes; similarly, having multiple comorbidities, chronic hepatic disease, or obesity have been associated with in-hospital mortality, and obesity is one of the most common comorbidities found in hospitalized patients with COVID-19 in the United States.16 , 18, 19, 20, 21, 22, 23 Some other comorbidities and chronic medication use (eg, insulin use) had similar magnitudes of increased risk of hospitalization but had insufficient sample sizes to determine significance. Similarly, chronic renal disease, dementia, having at least 3 comorbidities, and use of immunosuppressive medication had similar effect sizes (RRs: 1.5–1.9) that could be clinically relevant but were not statistically significant in our sample. Studies with larger sample sizes of the SNF population suggest coronary artery disease, heart failure, peripheral vascular disease, anemia, diabetes, end-stage kidney disease, and depression are associated with increased risk of hospitalization, and chronic obstructive pulmonary disease is associated with mortality.4 Our study did not find an association between taking angiotensin receptor blockers or angiotensin-converting enzyme inhibitors and hospitalization or death.24
Limitations
Our study has several limitations. This was a retrospective study using data abstracted from one SNF's electronic medical records; the findings are dependent on the quality and completeness of the documentation and may not be representative of all residents of SNFs nationwide. Additionally, changes in the facility's care practices following recognition of the first COVID-19 case in the facility may have changed detection and documentation of signs and symptoms, biased providers to screen for known symptoms of COVID-19 at the time of the study, or resulted in a change in the threshold for transfer to an acute care facility. Further, we used medication orders rather than medication administrations as a proxy for medication use; thus, it is unknown if or when residents were given each medication, including chronic medications and antipyretics, which may mask fever. Given the limited sample size, we were unable to adjust for potential confounding when examining the relationship between comorbidities and poor outcomes. Lastly, nursing home residents have many comorbidities associated with poor clinical outcomes, including age, which reduces the generalizability of this clinical experience outside of this SNF population.
Conclusions and Implications
Our study shows that monitoring of SNF residents for abnormal vital signs and both typical and atypical symptoms of COVID-19 might facilitate early detection of infection among residents. Inclusion of the signs and symptoms observed at date of illness onset among Facility A residents should be included among any facility's systematic monitoring; however, because these data are limited to findings from 1 SNF, the approach should not be limited to those signs and symptoms. Early detection is critical for rapid implementation of infection prevention and control measures, diagnostic evaluation, and treatment of residents with suspected COVID-19. CDC guidance recommends the following: (1) active monitoring for all residents on admission to an SNF at least daily for fever and symptoms consistent with COVID-19; (2) increased monitoring of ill residents at least 3 times daily for symptoms, vital signs, oxygen saturation assessed by pulse oximetry, and respiratory exam; and (3) if COVID-19 cases are suspected in the facility, consideration of more frequent monitoring of asymptomatic residents to enhance identification of COVID-19 illness.3 Implementation of frequent daily resident monitoring will require increased staff training, access to appropriate equipment, and increased access to clinical providers.
Footnotes
Farrell A. Tobolowsky and Ana C. Bardossy contributed equally.
Disclaimer: The findings and conclusions in the manuscript are those of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
References
- 1.McMichael T.M., Clark S., Pogosjans S. COVID-19 in a long-term care facility—King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . US Department of Health and Human Services; Atlanta, GA: 2020. Preparing for COVID-19: Long-term Care Facilities, Nursing Homes. [Google Scholar]
- 4.Tang O., Bigelow B.F., Sheikh F. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: Results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21:1767–1773.e1. doi: 10.1016/j.jamda.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S.M., Bakaev I., Chen H. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21:1378–1383.e1. doi: 10.1016/j.jamda.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fabout%2Fsymptoms.html 2020. Available at:
- 7.Centers for Disease Control and Prevention Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fguidelines-clinical-specimens.html 2020. Available at:
- 8.Centers for Disease Control and Prevention CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. https://www.fda.gov/media/134922/download 2020. Available at:
- 9.Garcia Rada A. Covid-19: The precarious position of Spain's nursing homes. BMJ. 2020;369:m1554. doi: 10.1136/bmj.m1554. [DOI] [PubMed] [Google Scholar]
- 10.Trabucchi M., De Leo D. Nursing homes or besieged castles: COVID-19 in northern Italy. Lancet Psychiatry. 2020;7:387–388. doi: 10.1016/S2215-0366(20)30149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball A., Hatfield K.M., Arons M. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Testing guidance for nursing homes. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html 2020. Available at:
- 14.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Criteria to guide evaluation and laboratory testing for COVID-19. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html 2020. Available at:
- 18.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagg S., Jylhava J., Wang Y. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559.e2. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes A., Serratrice C., Herrmann F.R. Predictors of in-hospital mortality in older patients with COVID-19: The COVIDAge study. J Am Med Dir Assoc. 2020;21:1546–1554.e3. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Wang X., Chen J. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]