Abstract
Objective
The MITIGATE study aims to evaluate the real-world clinical effectiveness of pre-treatment with icosapent ethyl (IPE), compared with usual care, on laboratory-confirmed viral upper respiratory infection (URI)-related morbidity and mortality in adults with established atherosclerotic cardiovascular disease (ASCVD).
Background
IPE is a highly purified and stable omega-3 fatty acid prescription medication that is approved for cardiovascular risk reduction in high-risk adults on statin therapy with elevated triglycerides. Preclinical data and clinical observations suggest that IPE may have pleiotropic effects including antiviral and anti-inflammatory properties that may prevent or reduce the downstream sequelae and cardiopulmonary consequences of viral URIs.
Methods
MITIGATE is a virtual, electronic health record-based, open-label, randomized, pragmatic clinical trial enrolling ∼16,500 participants within Kaiser Permanente Northern California – a fully integrated and learning health care delivery system with 21 hospitals and >255 ambulatory clinics serving ∼4.5 million members. Adults ≥50 years with established ASCVD and no prior history of coronavirus disease 2019 (COVID-19) will be prospectively identified and pre-randomized in a 1:10 allocation ratio (∼ 1,500 IPE: ∼15,000 usual care) stratified by age and previous respiratory health status to the intervention (IPE 2 grams by mouth twice daily with meals) vs the control group (usual care) for a minimum follow-up duration of 6 months. The co-primary endpoints are moderate-to-severe laboratory-confirmed viral URI and worst clinical status due to a viral URI at any point in time.
Conclusion
The MITIGATE study will inform clinical practice by providing evidence on the real-world clinical effectiveness of pretreatment with IPE to prevent and/or reduce the sequelae of laboratory-confirmed viral URIs in a high-risk cohort of patients with established ASCVD.
Keywords: Atherosclerotic cardiovascular disease, triglycerides, icosapent ethyl, viral upper respiratory infection, coronavirus disease 2019, seasonal flu
Background
As of January 24, 2021, there are more than 25 million laboratory-confirmed cases of coronavirus disease 2019 (COVID-19) resulting in >415,000 deaths in the United States alone (https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html). Previously published studies have suggested that older patients with comorbid conditions, including established atherosclerotic cardiovascular disease (ASCVD), are at heightened risk for contracting COVID-19 and have a worse prognosis, with a short-term case-fatality rate potentially exceeding 10%.1 , 2 In addition, a retrospective cohort study of >15,000 patients tested for the causative agent of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), between March 1, 2020-March 31, 2020 at Kaiser Permanente Northern California (KPNC), found that among patients with laboratory-confirmed COVID-19, 29.0% were treated as an inpatient and 8.7% in the intensive care unit.3 In addition, of the patients with discharge dispositions at the time of publication, overall in-hospital mortality was 15.6% and 50.0% among those treated in the intensive care unit at any point in time. It is thought that the morbidity and mortality associated with COVID-19 are due both to the direct toxicity of the virus as well as the body's robust inflammatory response leading to a state of ‘cytokine storm.’
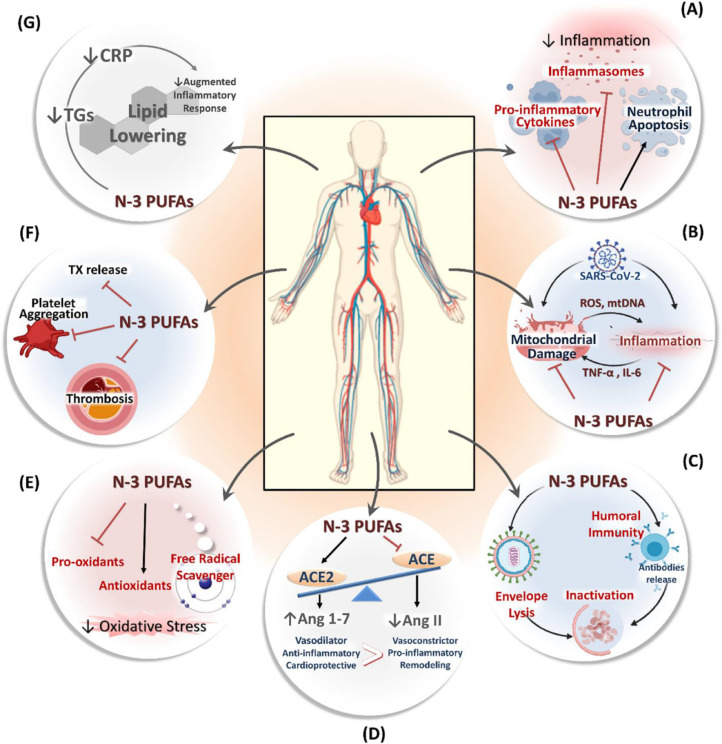
The pivotal REDUCE-IT (REDUction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) study enrolled more than 8,000 patients with diabetes mellitus and other risk factors (ie, primary prevention cohort) or established ASCVD (ie, secondary prevention cohort) who were receiving statin therapy with a well-controlled low density lipoprotein-cholesterol and an elevated fasting triglyceride (TG) level and found that the addition of high-dose icosapent ethyl (IPE) significantly decreased the risk of a variety of nonfatal and fatal ischemic endpoints, including a 20% lower relative risk of cardiovascular death, independent of baseline TG levels and irrespective of the TG level attained at 1 year.4., 5., 6., 7., 8. In addition to IPE's cardioprotective benefits and well-documented safety profile, it has been hypothesized based on preclinical data and clinical observations that bioactive lipids, such as IPE and its metabolites, may have pleiotropic effects (Figure 1 ). These include anti-inflammatory properties, and possibly even direct antiviral activity, that may potentially alleviate the downstream sequelae of COVID-19-related sepsis and acute respiratory distress syndrome, as well as possibly impacting on post-COVID cardiopulmonary complications.9., 10., 11., 12., 13., 14., 15.
Figure 1.
Potential Cardioprotective Mechanisms of Icosapent Ethyl. This figure has been reproduced with permission from Darwesh et al. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications?Pharmacol Ther. 2020 Oct 5: 107703. PMCID: PMC7534795.
The role of an upfront management strategy incorporating pretreatment with IPE, in addition to usual care, presents an ideal opportunity to leverage a highly efficient and innovative study design to evaluate the effects of a proven cardioprotective medication on viral upper respiratory infection (URI)-related morbidity and mortality given the following observations: (1) the COVID-19 pandemic is rapidly evolving and associated with high rates of attendant morbidity and mortality; (2) the demonstrated urgent need for remotely-administered treatment options focused on prevention in at-risk populations in the outpatient setting; (3) the finding that older adults with pre-existing conditions (ie, ASCVD) are at high-risk for COVID-19 and its complications; and (4) the practical reality that IPE is already approved by the United States Food and Drug Administration (FDA), widely available, orally administered, and has a well-established safety and tolerability profile requiring minimal monitoring and/or routine follow-up. Thus, the objective of the Pragmatic Randomized Trial of Icosapent Ethyl for High Cardiovascular Risk Adults (MITIGATE) is to assess the potential role of IPE to prevent and/or reduce the sequelae of laboratory-confirmed URIs (ie, COVID-19, seasonal flu, and other known viral respiratory pathogens) in a high-risk cohort of ambulatory patients with established ASCVD.
Methods
Design overview
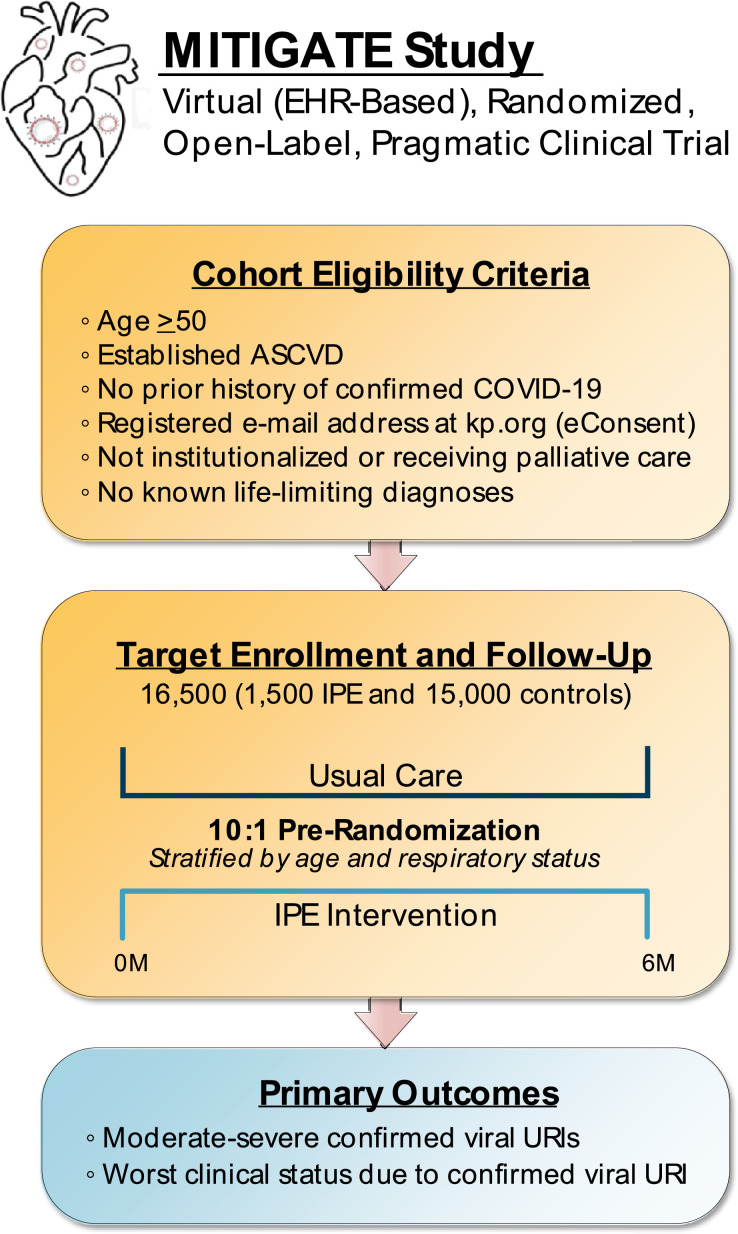
MITIGATE is a prospective, virtual, electronic health record (EHR)-based, open-label, parallel-group, randomized, pragmatic clinical trial (PCT) designed to evaluate the real-world clinical effectiveness of pre-treatment with IPE, compared with usual care, on laboratory-confirmed viral URI-related morbidity and mortality in ambulatory patients with established ASCVD (Figure 2 ). The study is being conducted through the KPNC Division of Research (DOR) based in Oakland, CA and is embedded within KPNC's fully integrated and learning health care delivery system, which includes 21 hospitals and >255 outpatient clinics currently serving approximately (∼)4.5 million members. Essentially all care within KPNC is comprehensively captured through an EHR system that is fully integrated across all practice settings, including KPNC-owned labs and pharmacies. All non-network care in the small proportion of KPNC members that receive it is also systematically captured through associated databases and integrated into KPNC's EHR. Given that KPNC insures and provides care to 35% to 65% of adults in each Northern California county, the KPNC population is highly representative of the local and statewide population with regards to its broad age, sex, and racial/ethnic diversity — enhancing the generalizability of all research findings.16
Figure 2.
Overview of Study Design.
The EHR is prospectively screened in real-time for potentially eligible participants using validated identifying algorithms incorporating demographic data, International Classification of Diseases 9th/10th Edition (ICD-9/10) and Current Procedural Terminology (CPT) codes, pharmacy dispensing information, and relevant laboratory results.17., 18., 19., 20., 21., 22. Eligibility criteria include men and women age ≥50 years, established ASCVD (ie, defined as prior myocardial infarction [MI], percutaneous coronary intervention [PCI], coronary artery bypass graft [CABG], ischemic stroke, and/or peripheral artery disease [PAD]), no prior history of confirmed COVID-19, and a registered e-mail address at kp.org in order to obtain electronic consent (eConsent) (Table 1 ). Exclusion criteria include receipt of IPE within the last 12 months, known hypersensitivity to IPE, fish, and/or shellfish, current use of any omega-3 fatty acid supplements, institutionalized and/or receiving palliative care, and known life-limiting diagnosis.
Table 1.
Eligibility criteria
| Inclusion criteria |
|---|
| • Men and women age ≥50 years |
| • Able to provide informed consent |
| • No prior history of confirmed COVID-19 (ie, based on a positive PCR or other FDA-approved assay for SARS-CoV-2 and no documented serological (FDA-approved) test results for SARS-CoV-2 antibodies |
| • Established ASCVD (ie, defined as prior MI, PCI, CABG, ischemic stroke, and/or PAD) |
| • At least 12 months of continuous KPNC membership and prescription drug benefit prior to enrollment |
| • A registered e-mail address at kp.org in order to obtain eConsent for study participation |
| Exclusion criteria |
| • Receipt of IPE on or within 12 months before the day of enrollment |
| • Known hypersensitivity to IPE, fish and/or shellfish |
| • Ongoing use of any omega-3 fatty acid medications or dietary supplements containing omega-3 fatty acids |
| • Women who are pregnant or planning to become pregnant |
| • Hospitalization for MI and/or elective PCI within the past 1 month. |
| • Currently receiving triple therapy (ie, defined as aspirin + a second antiplatelet agent + warfarin or a direct acting oral anticoagulant) |
| • Stage D HF (ie, defined as inotrope-dependent, prior/planned left ventricular assist device, and/or prior/current listing for cardiac transplant) |
| • Severe liver disease (ie, defined as documented compensated and/or decompensated cirrhosis) |
| • ESRD requiring chronic dialysis or eGFR <15 mL/min/1.73 m2 |
| • Metastatic cancer and/or receiving active systemic chemotherapy |
| • Institutionalized and/or receiving palliative care |
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; CABG = coronary artery bypass graft; COVID-19 = Coronavirus Disease 2019; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; FDA = Food and Drug Administration; HF = heart failure; IPE = icosapent ethyl; KPNC = Kaiser Permanente Northern California; MI = myocardial infarction; PCR = Polymerase Chain Reaction; PCI = percutaneous coronary intervention; PAD = peripheral artery disease; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Study procedures
A targeted ∼16,500 participants will be pre-randomized in a 1:10 allocation ratio (ie, ∼1,500 IPE vs. ∼15,000 usual care) stratified by age (ie, <75 years vs. ≥75 years) and previous respiratory health status (ie, presence of chronic obstructive pulmonary disease [COPD] and/or interstitial lung disease [ILD]) to the intervention arm (ie, IPE) or the control arm (ie, usual care). Participants randomly assigned to the control arm are not directly contacted by any member of the study team. The KPNC Institutional Review Board has granted a waiver of informed consent for subjects randomly assigned to the control group and these patients are passively followed for outcome ascertainment only. Participants randomly assigned to the intervention group are contacted by the study team to explain the study, obtain informed consent to receive IPE, and provide the patient with IPE using existing health plan pharmacies and mail order system. Patients who do not provide informed consent to receive study drug will be offered passive follow-up for outcome ascertainment only. There are no in-person study visits, although a member of the study team contacts the patient on a monthly basis to reinforce and/or confirm adherence with study drug, provide updates on study progress, address any questions and/or concerns, and connect the patient with a study physician as necessary (Table 2 ).
Table 2.
Data collection and schedule of assessments
| Screening | Consent | Medication confirmation | Follow-up | Closeout | |
|---|---|---|---|---|---|
| Visit number | 0 | 1 | 2 | 3…X | X+1 |
| Day | 0 | 0±14 | Within 7 days | Every 30±7 | End of Follow-up |
| Informed Consent | X | ||||
| Randomization | X | ||||
| Demographics | X | X | |||
| Medical History | X | X | |||
| Medications | X | X | |||
| Vital Signs | X | X | |||
| Laboratory Values | X | X | |||
| Study Drug Dispensed | X | X | X | ||
| Study Close Out | X | ||||
| Endpoints | X |
The daily dose of IPE is 2 grams by mouth twice daily with food. Patients are advised to swallow the capsules whole and not to open, crush, dissolve, or chew IPE. This is the only dose that has been tested and found to be efficacious in a prior landmark randomized clinical trial.4., 5., 6., 7., 8. In contrast to other federally-funded23 , 24 and industry-sponsored trials of omega-3 fatty acid supplements,25 , 26 which have not been found to be efficacious, IPE undergoes a proprietary purification process, which has been approved and validated by the U.S. FDA, leaving behind highly purified and stable ethyl ester of eicosapentaenoic acid (EPA). In addition, it is given at a higher dose, compared with other commercially-available preparations, and the benefits of IPE have been found to be strongly correlated with on-treatment EPA levels achieved (Unpublished Data Presented by Bhatt DL et al at ACC 2020 Virtual Meeting).
Outcomes and follow-up
The first of the co-primary endpoints is the incidence of moderate-to-severe confirmed viral URIs (ie, COVID-19, seasonal flu, and other known viral respiratory pathogens) based on laboratory testing leading to an urgent care appointment, emergency department (ED) visit, or hospitalization with an SpO2 ≤94% on room air (RA) and/or requiring any form of supplemental O2 (Table 3 ). There are 2 main PCR-based laboratory panels for viral URIs available at KPNC, a general respiratory viral panel (ie, COVID-19, Influenza A, Influenza B, and Respiratory Syncytial Virus) and an extended respiratory viral panel (ie, Influenza A, Influenza B, Respiratory Syncytial Virus , Parainfluenza, Enterovirus and/or Rhinovirus, Adenovirus, and Human Metapneumovirus). The second co-primary endpoint is the worst clinical status at any point in time during follow-up due to a laboratory-confirmed viral URI based on a 7-point ordinal scale (1 = death, 2 = mechanically ventilated/ Extracorporeal Membrane Oxygenation (ECMO), 3 = high-flow supplemental O2, 4 = low-flow supplemental O2, 5 = hospitalized with no supplemental O2 requirements, 6 = urgent care or ED visit not leading to hospitalization, and 7 = no relevant clinical encounters) (Figure 3 ). Safety endpoints of interest include the incidence of new-onset atrial fibrillation, hospitalizations for atrial fibrillation, and bleeding events requiring hospitalization. Exploratory endpoints include all-cause death; major adverse cardiovascular event (MACE) (3-point) including death due to any cause, acute MI, or ischemic stroke; expanded MACE (5-point) including MACE, hospitalization for acute coronary syndrome (ACS), and coronary revascularization (ie, PCI and/or CABG), hospitalizations for worsening heart failure (HF); and the composite of hospitalizations and ED visits due to any cause.
Table 3.
Study Objectives and endpoints
| Primary objective(s) | Endpoint(s) for primary objective(s) |
|---|---|
| • To evaluate the clinical effectiveness of IPE vs. usual care on the rate of occurrence and morbidity of laboratory-confirmed viral URIs in patients with established ASCVD. | • Moderate-to-severe laboratory-confirmed viral URIs • Worst clinical status due to a laboratory-confirmed viral URI at any point in time |
| Exploratory objective(s) | Endpoint(s) for exploratory objective(s) |
| • To assess the impact of IPE vs. usual care on morbidity and mortality in patients with established ASCVD. | • All-cause mortality • MACE (3-point) including death due to any cause, MI, or ischemic stroke • Expanded MACE (5-point) including MACE, hospitalization for ACS, and coronary revascularization (ie, PCI and/or CABG) • Hospitalization for worsening HF (ie, defined as ≥1 symptom, ≥2 objective findings including ≥1 sign, and a change in HF-related therapy) • All-cause hospitalizations + ED visits |
Abbreviations: ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CABG = coronary artery bypass graft; ED = emergency department; HF = heart failure; IPE = icosapent ethyl; MACE = major adverse cardiovascular events; PCI = percutaneous coronary intervention; URI = upper respiratory infection.
Figure 3.
Schematic of Ordinal Scale.
All endpoints are automatically assessed via the EHR using previously derived and validated approaches relying on a combination of ICD-9/10 and CPT codes, and other structured data elements (ie, vital signs and laboratory values).17., 18., 19., 20., 21., 22. Enrollment will occur over a projected 6-month timeframe and all patients will complete a minimum follow-up duration of 6 months from the index date. The index date for patients in the IPE arm will be defined as the medication start date. To account for potential bias induced by the temporal lag between the pre-randomization date and the medication start date in the IPE arm, but not the usual care arm, index dates for the usual care arm will be randomly assigned to match the distribution of time from pre-randomization to medication start date for participants in the IPE arm. The proposed timeline for the MITIGATE study will extend follow-up through the projected end of the 2020 to 2021 flu season and allow a systematic evaluation of the impact of pretreatment with IPE vs. usual care on laboratory-confirmed viral URIs.
Statistical considerations
This study has been designed and powered based on the co-primary outcome of the rate of moderate-to-severe laboratory-confirmed viral URI requiring an urgent care appointment, ED visit, and/or hospitalization with documented hypoxemia (ie, SpO2 ≤94% on RA) and/or requiring administration of supplemental O2. Based on the relatively high initial reproduction number (R0) (ie, the number of secondary cases directly attributable to each index case) of ∼2-3, likely due to a prolonged latency phase and/or asymptomatic and/or minimally symptomatic transmission, it has been previously estimated that upwards of 50% of the United States population may eventually become infected with SARS-CoV-2 before the pandemic has subsided and/or effective vaccines have been developed, manufactured, distributed, and administered.27 , 28 In addition, estimates for COVID-19 cases requiring inpatient treatment have ranged from 20% to 30% depending on the patient population under investigation. Thus, including all urgent and/or emergent clinical encounters (ie, urgent care appointments, ED visits, and hospitalizations) for all laboratory-confirmed viral URIs (ie, COVID-19, seasonal flu, etc.) and allowing for recurrent infections (ie, the same or different viral pathogen as immunity may be transient), the anticipated health care utilization rate is ∼30 events per 100 person-years among those infected. Based on these assumptions for the percent infected (ie, ∼50%) and health care utilization rate (ie, ∼30 events per 100 person-years), this would provide an overall event rate (ie, infection rate multiplied by the health care utilization rate) of ∼15 events per 100 person-years. A total enrollment of 16,500 patients pre-randomized in a 1 (IPE):10 (usual care) allocation ratio with an overall event rate of 15 per 100 person-years, would result in 80% power to detect a RR = 0.82 (ie, 18% relative rate reduction, 2.7% absolute rate reduction) given a 2-sided α = 0.05.
The intention-to-treat (ITT) population will consist of all patients pre-randomized to usual care and those patients pre-randomized to the intervention arm and consented to IPE or passive follow-up. For all analyses using the ITT population, patients will be analyzed as pre-randomized. The per-protocol population is a subset of the ITT population including data from participants in the intervention arm who received ≥1 dose of study drug. All primary and exploratory endpoints will be analyzed using recurrent event models and/or proportional odds models, as appropriate, to estimate the rate ratio and odds ratio of IPE on outcomes of interest. Interaction analyses will be performed in pre-specified subgroups based on age and pre-existing respiratory disease status (ie, COPD and/or ILD) to assess for heterogeneity with respect to treatment effect on all endpoints. Finally, analyses will be adjusted for any observed differences in baseline clinical characteristics including sociodemographic factors, past medical history, medications, vital signs, and laboratory values known to be associated with viral URI-related and ASCVD-related morbidity and mortality. The threshold for statistical significance will be pre-specified as a 2-sided P-value of <.05.
A single planned interim analysis will be performed after 50% of patients have been enrolled and accrued a minimum follow-up duration of 3 months. This timeframe has specifically been chosen to coincide with the projected peak of the 2020-2021 flu season and a potential resurgence of COVID-19 cases. Given the public health implications, the co-Principal Investigators (PIs) (APA and ASG) will specifically review unblinded data comparing IPE vs. usual care for the co-primary outcomes. Based on the findings, the PIs reserve the right to publish the results of all interim analyses, but there will be no formal stopping rule in the event of suspected superiority given that this observation would be based on a smaller than planned number of events and potentially underpowered. Thus, the MITIGATE study will complete recruitment as planned, acknowledging that interim findings may influence the subsequent management of patients pre-randomized to the usual care arm (ie, passive control). Given the pragmatic nature of this research, there will be no adjustment of the overall α for interim analyses. In addition, there is also a strong probability that the co-primary endpoints are highly positively correlated, greatly diminishing the risk of multiple testing leading to an inflated type I error rate (ie, false positives) and therefore adjustments for multiplicity with respect to the co-primary outcomes will also not be performed.
Funding and study organization
This is an investigator-initiated trial (IIT) funded by Amarin Pharma, Inc. (Bridgewater, NJ) with study coordination provided by the KPNC DOR (Oakland, CA). Overall responsibility for the oversight and management of the MITIGATE study lies with the Operations Committee. The Data and Safety Monitoring Board (DSMB) includes 2 experienced physicians with content and methodologic expertise and a senior biostatistician responsible for active surveillance of safety data including all adverse and serious adverse events. The DSMB includes 2 experienced physicians with content and methodologic expertise and a senior biostatistician responsible for active surveillance of safety data including all adverse and serious adverse events. The DSMB will meet after the first 500 patients have been enrolled in the intervention arm and at a minimum of every 6 months thereafter for the duration of the study. All DSMB meetings include both an open session (ie, with the MITIGATE study team) to review blinded aggregate outcome data and a closed session (ie, DSMB only) to review unblinded efficacy, safety, and exploratory endpoints. The DSMB has been authorized to request additional data inquiries and/or safety reviews, recommend protocol amendments, and/or temporarily pause/permanently stop the study based on their findings. Members of the Operations Committee and DSMB are listed in Appendix A. The authors are solely responsible for design and conduct of this study, all study analyses, the drafting and editing of this paper, and its final contents.
Ethical considerations
This clinical study has been designed and implemented and all findings will be reported in accordance with the International Conference on Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), with applicable local regulations, and with the ethical principles that originate from the Declaration of Helsinki. The Institutional Review Board independently approved the protocol and eConsent will be obtained from all study participants prior to enrollment. The MITIGATE study is registered at clinicaltrials.gov (NCT04505098).
Discussion
The MITIGATE study is a virtual, EHR-based, open-label, randomized, PCT designed to evaluate the real-world clinical effectiveness of pre-treatment with IPE, compared with usual care, on laboratory-confirmed viral URI-related morbidity and mortality in ambulatory patients with ASCVD. COVID-19 has served as a catalyst for research broadly aimed at developing and expanding access to diagnostic testing for active infection and serologic evidence of prior infection, investigating novel therapeutic agents with antimicrobial and/or anti-inflammatory properties, and testing vaccine candidates aimed at slowing the transmission of SARS-CoV-2.29 , 30 However, there is growing recognition in the scientific community that ongoing research should also focus efforts on enrolling at-risk and vulnerable populations in sufficient numbers, preventing and/or reducing the severity of COVID-19 (and other relevant viral infections) in the outpatient setting, and investigating oral pharmacotherapies that are safe, well-tolerated, and widely available. Thus, the MITIGATE study addresses several unmet clinical needs while leveraging a highly efficient and innovative design.
Notably, this is the first large-scale study to focus on COVID-19 prevention and/or risk reduction in an ambulatory cohort of older adults with a pre-existing condition. The eligibility criteria, which primarily reflect key safety considerations, have been streamlined in order to facilitate recruitment and enhance the overall generalizability of the findings. As previously alluded to, the majority of the clinical trials completed to date have focused almost exclusively on treating patients hospitalized for moderate or severe COVID-19 with hypoxemia and/or supplemental oxygen requirements relatively late in the natural history of the disease.31., 32., 33., 34. In contrast, the MITIGATE study will evaluate the potential role of pre-treatment with IPE vs. usual care in patients without a known prior history of laboratory-confirmed COVID-19. Based on the prolonged latency phase and protracted clinical course of COVID-19, it can be rationalized that upstream treatment with a safe and effective therapy may further improve clinical outcomes in lower acuity patients with less severe symptoms (NCT04501952). IPE has several competitive advantages as a potential therapy for viral URIs managed in the outpatient setting including an oral route of administration, a well-established safety and tolerability profile, few significant drug-drug interactions, and no requirements for routine lab monitoring (https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf).35
The proposed general mechanisms of action for IPE include direct antiviral activity by promoting removal and inactivation of enveloped viruses in addition to known anti-inflammatory properties as evidenced by the early and sustained decrease in high-sensitivity C-reactive protein and other acute phase reagents observed in prior studies.5 , 36., 37., 38. Notably, the VASCEPA COVID-19 CardioLink-9 Randomized Trial (Presented as a Late Breaking Clinical Trial at the National Lipid Association Scientific Sessions on December 14, 2020) found that a loading dose of 8 grams/day for 3 days followed by 4 grams/day for 11 days led to a 25% reduction in high-sensitivity C-reactive protein and an improvement in global and domain-specific symptoms based on the influenza patient-reported outcome (FLU-PRO) score.
There are several unique aspects of the design of the MITIGATE study that merit further mention. First, the study is entirely virtual and requires no in-person visits allowing it to be conducted while simultaneously adhering to local and regional guidelines for social distancing. This study leverages the KPNC DOR's robust health informational technology infrastructure and KPNC's state-of-the-art EHR to perform all study-related activities remotely and in an automated fashion from initial screening to final outcome ascertainment. Second, the study will employ pre-randomization (ie, occurring before obtaining informed consent), which will allow all subjects assigned to the control group to be passively followed for the outcomes of interest. This also allows IPE, which is FDA-approved to reduce the risk of recurrent fatal and nonfatal ischemic events in adults with established ASCVD and elevated TG levels, to be offered to all patients directly contacted by a member of the study team. Hence, the MITIGATE study should be properly viewed as testing an initial strategy of care (ie, consent and pre-treatment with IPE vs. standard of care), and the goal will be to maintain a consent rate ≥70% for the duration of the study. This is consistent with the initial recruitment experience and is likely attributable to the favorable safety profile and tolerability of IPE and its innate familiarity to potential participants.
The co-primary outcomes of the MITIGATE study are complementary and have been purposefully selected to capture both the frequency and the severity of clinically significant COVID-19 infections. Although early COVID-19 treatment trials distinguished between moderate and severe cases solely on the basis of documented hypoxemia (ie, O2 saturation ≤94%) and/or supplemental oxygen requirements, practice patterns have continued to progress, and it is now common within integrated health care delivery systems for patients with mild hypoxemia to be discharged directly home from the ED with supplemental O2.31., 32., 33., 34. As a result, the co-primary endpoints will incorporate clinical encounters including urgent care and ED visits not requiring hospital admission. Given the uncertainties around the underlying assumptions for the power calculation and uncontrollable extrinsic factors (ie, public health measures, testing capabilities, therapeutic advances, vaccine development, etc.), it is notable that the MITIGATE study is adequately powered (ie, β ≥0.80) to detect a clinically meaningful difference between groups for actual event rates as low as 10 events per 100 person-years. In addition, because KPNC is a fully integrated and learning health care delivery system, there is the built-in flexibility to amend the protocol to either modify the co-primary endpoints and/or include additional secondary efficacy endpoints for viral URI-related morbidity as new data emerge from other COVID-19-related studies. In contrast, although the exploratory outcomes will be underpowered and hypothesis-generating only, this study is the first to further probe the results of a pivotal approval randomized clinical trial 4., 5., 6., 7., 8. (ie, efficacy) through a real-world PCT (ie, effectiveness). Beyond the primary hypotheses, MITIGATE will for the first time also provide an assessment of the safety and tolerability of IPE in a population not specifically enriched with elevated TG levels.
There are several limitations of the data that should be fully acknowledged. First, the MITIGATE study will utilize pre-randomization, which will allow all subjects assigned to the control group to be passively followed for the outcomes of interest with no direct contact with any members of the study team. A potential downside is that if a lower than anticipated number of patients in the intervention arm consent to participate in this ITT protocol, the results may be biased towards the null hypothesis but will still provide a more accurate estimate of therapeutic effectiveness when applied to the target population. As previously discussed, the initial experience with recruitment has been promising and additional safeguards are in place including a prespecified plan to perform a per-protocol sensitivity analysis adjusting for differences in baseline clinical characteristics. In addition, pre-randomizing potential participants in a 1:10 allocation ratio of intervention to control patients allows us to more rapidly and efficiently recruit all patients meeting eligibility criteria, thereby enhancing the generalizability and maximizing the statistical power of this study. Second, the study is open-label, which has the potential to introduce bias, but this is a practical necessity given the growing incidence and high morbidity and mortality associated with COVID-19, particularly among older adults with established ASCVD. Despite this potential limitation, the co-primary and exploratory endpoints for this study are objective outcomes (ie, hospitalizations requiring specific diagnostic codes and patients will be blinded to outcome ascertainment). Third, there will be no face-to-face clinical encounters which will preclude the performance of direct “pill counts” and other in-person approaches for assessing compliance and monitoring medication adherence,39 , 40 although patients will be contacted monthly to reinforce/confirm study medication adherence and automatic refills will be centrally managed. Finally, the MITIGATE study will examine the role of pre-treatment with IPE in ambulatory patients with established ASCVD but is not generalizable to prevention in other at-risk groups nor the use of IPE as an active treatment for symptomatic COVID-19. However, other clinical trials will address the safety and potential efficacy of IPE or EPA in subjects at high-risk of exposure to SARS-CoV-2 such as essential workers (NCT04460651) or in outpatients (NCT04412018) or hospitalized patients with COVID-19 (NCT04335032).
In conclusion, the MITIGATE study addresses several unmet clinical needs and will leverage an innovative virtual and EHR-based design to clarify the real-world comparative effectiveness and safety of pre-treatment with IPE, an effective therapy for ASCVD risk reduction with putative antiviral effects and known anti-inflammatory properties, in a diverse and representative population of high-risk patients with established ASCVD.
Funding
The MITIGATE study is an IIT funded by Amarin Pharma, Inc. (Bridgewater, NJ).
Disclosures
APA is supported by a Mentored Patient-Oriented Research Career Development Award (K23HL150159) through the National Heart, Lung, and Blood Institute, has received relevant research support through grants to his institution from Amarin Pharma, Inc., Abbott, and Novartis, and modest reimbursement for travel from Novartis. SP and CG are employees of Amarin Pharma, Inc. (Bridgewater, NJ). DLB discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. ASG has received relevant research support through grants to his institution from the National Heart, Lung and Blood Institute, National Institute of Diabetes, Digestive and Kidney Diseases, National Institute on Aging, Amarin Pharma, Inc., and Novartis. All other authors have no relevant conflicts of interest to declare.
Acknowledgments
We would like to thank the study nurse and research coordinators for their assistance with trial operations and the patients for their participation in the trial.
Appendix A
Operations Committee:
Andrew P. Ambrosy, MD (Principal Investigator)
Rachel C. Thomas, RN (Research Nurse)
Rishi V. Parikh, MPH (Data Consultant)
Thida C. Tan, MPH (Project Manager)
Alan S. Go, MD (Study Chair)
Data and Safety Monitoring Board:
Edward J. McNulty, MD (Medical Director, Regional Cardiac Services)
Catherine Lee, PhD (Senior Biostatistician)
Douglas A. Corley, MD, PhD (DSMB Chair)
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 3.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020 doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Mille, et al. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Jiao L, et al. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol. 2019;74:1159–1161. doi: 10.1016/j.jacc.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg PG, Ketchum SB, et al. REDUCE-IT USA: results from the 3146 patients randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das UN. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch Med Res. 2020 doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020 doi: 10.1161/ATVBAHA.119.313286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada H, Yoshida M, Nakano Y, Suganami T, Satoh N, Mita T, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28:2173–2179. doi: 10.1161/ATVBAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 13.Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35:2631–2639. doi: 10.2337/dc12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darwesh AM, Bassiouni W, Sosnowski DK, Seubert JM. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol Ther. 2020 doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger AA, Sherburne R, Urits I, Patel H, Eskander J. Icosapent ethyl - a successful treatment for symptomatic COVID-19 infection. Cureus. 2020;12:e10211. doi: 10.7759/cureus.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon N, Lin T. The Kaiser Permanente Northern California Adult Member Health Survey. Perm J. 2016;20:15–225. doi: 10.7812/TPP/15-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 21.Smith DH, Johnson ES, Boudreau DM, Cassidy-Bushrow AE, Fortmann SP, Greenlee RT, et al. Comparative effectiveness of statin therapy in chronic kidney disease and acute myocardial infarction: a retrospective cohort study. Am J Med. 2015;128 doi: 10.1016/j.amjmed.2015.06.030. 1252 e1-1252 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circulation Cardiovascular quality and outcomes. 2013;6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parodi SM, Liu VX. From containment to mitigation of covid-19 in the US. JAMA. 2020 doi: 10.1001/jama.2020.3882. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slaoui M, Hepburn M. Developing safe and effective covid vaccines - operation warp speed's strategy and approach. N Engl J Med. 2020 doi: 10.1056/NEJMp2027405. [DOI] [PubMed] [Google Scholar]
- 30.Gaba P, Bhatt DL. The COVID-19 pandemic: a catalyst to improve clinical trials. Nat Rev Cardiol. 2020;17:673–675. doi: 10.1038/s41569-020-00439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinner CD, Gottlieb RL, Criner GJ, Arribas Lopez JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 [Google Scholar]
- 35.Bhatt DL, Hull MA, Song M, Van Hulle C, Carlsson C, Chapman MJ, et al. Beyond cardiovascular medicine: potential future uses of icosapent ethyl. Eur Heart J Suppl. 2020;22:J54–J64. doi: 10.1093/eurheartj/suaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinton EA, Ballantyne CM, Bays HE, Kastelein JJ, Braeckman RA, Soni PN. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200-500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12:100. doi: 10.1186/1475-2840-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13:37–46. doi: 10.1007/s40256-012-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Doyle RT, Jr., Philip S, et al. Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester): effects upon high-sensitivity c-reactive protein and lipid parameters in patients with metabolic syndrome. Metab Syndr Relat Disord. 2015;13:239–247. doi: 10.1089/met.2014.0137. [DOI] [PubMed] [Google Scholar]
- 39.Rudd P, Byyny RL, Zachary V, LoVerde ME, Titus C, Mitchell WD, et al. The natural history of medication compliance in a drug trial: limitations of pill counts. Clin Pharmacol Ther. 1989;46:169–176. doi: 10.1038/clpt.1989.122. [DOI] [PubMed] [Google Scholar]
- 40.Rudd P, Byyny RL, Zachary V, LoVerde ME, Mitchell WD, Titus C, et al. Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens. 1988;1:309–312. doi: 10.1093/ajh/1.3.309. [DOI] [PubMed] [Google Scholar]