Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Remdesivir, Favipiravir, Vaccines
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a causal factor of the coronavirus disease 2019 (COVID-19). Drug repurposing, portraying patented drugs as a successful drug development technique, could shorten the period and minimize costs relative to de novo drug exploration. Recently several drugs have been used as anti-SARS-CoV-2 such as Remdesivir, Favipiravir, Hydroxychloroquine, Azithromycin, Lopinavir/Ritonavir, Nafamostat mesylate and so on. Despite such efforts, there is currently no successful broad-spectrum antiviral countermeasures to combat SARS-CoV-2 or possibly potential CoVs pandemic. Therefore it is desperately important to recognize and test widely efficient, reliable anti-CoV therapies now and in the future. Remdesivir and Favipiravir were more promising despite having side effects; it had prominent efficacy and efficiency while still not yet approved as the official anti-viral drug for SARS CoV-2. In this review, we summarizes the current drug and vaccine discovery status against SARS-CoV-2, predicting that these efforts will help create effective drugs and vaccines for SARS-CoV-2.
1. Introduction
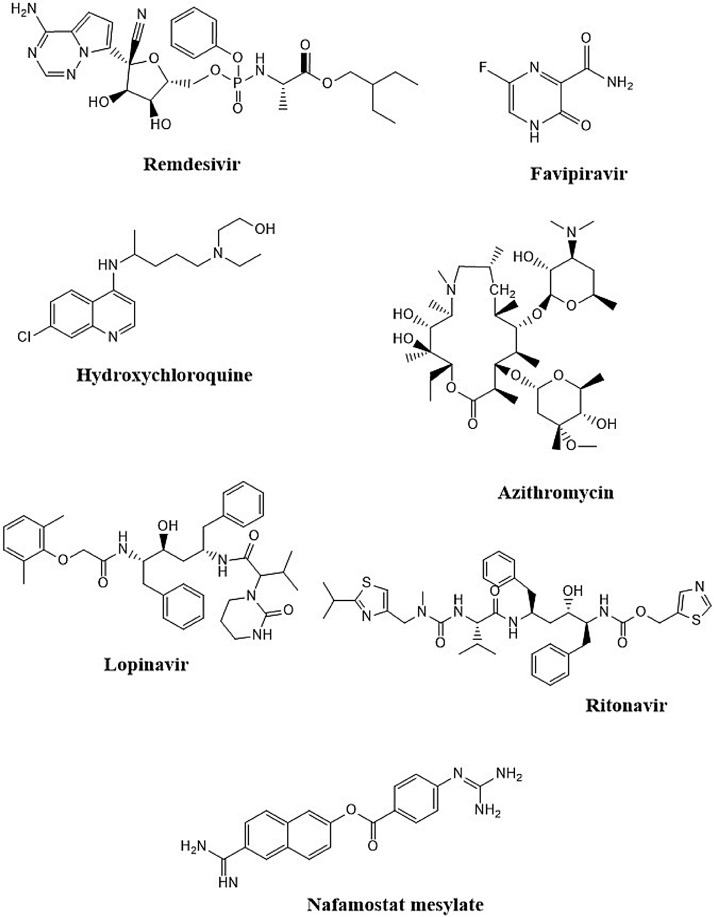
Severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) has arisen as a quickly evolving novel of human pathogen triggering severe respiratory illness and pneumonia-coronavirus disease 2019 (COVID-19) [1,2]. It is the third epidemic triggered by evolving coronaviruses in recent years after SARS-CoV spread in 2002, and Middle East respiratory syndrome (MERS-CoV) spread in 2012. SARS-CoV-2 and SARS-CoV are highly related and have identical genomes and pathways for host cell entry [3,4]. In any case, before we can verify the origin of SARS-CoV-2, future research is needed. In addition to Kenneth Lundstrom et al., the SARS-CoV-2 S protein study compared to other coronaviruses, the SARS-CoV-2 proteins ORF8 and ORF10 were also targeted to shed some light on the origins of SARS-CoV-2, which will likely be published with the scientific society [5]. SARS-CoV-2 infected more than 96,877,399 people globally as of 4:57pm CET, 23 January 2021, leading to more than 2,098,879 deaths, with a fatality rate of 2.14 % [6]. Effective drugs are desperately required to manage SARS CoV-2. While several testing, licensed, and repurposed medications have been proposed, preclinical animal model evidence will direct the quest for successful therapies by removing therapies lacking in vivo efficacy [7]. Remdesivir (GS-5734) is a nucleotide analog medication with potent antiviral activity currently being tested in SARS-CoV-2 clinical trials and previously obtained U.S. Food and Drug Administration (FDA) permission for urgent use (Fig. 1 ) [[8], [9], [10], [11]]. Remdesivir therapy in animal models has been positive against infection with MERS-CoV and SARS-CoV [9,12,13]. In vitro, SARS-CoV-2 replication had been inhibited by Remdesivir [14,15]. As well as one of the most promising medications currently effective against SARS-CoV-2 is Favipiravir, an inhibitor of RNA-dependent polymerase (RdRp) prescribed to Ebola virus-infected patients, authorized for evolving influenza in Japan and SARS-CoV-2 therapy in China [[16], [17], [18]]. Preliminary tests on 80 patients in China have shown that Favipiravir exerts an antiviral activity more effective in the treatment of SARS-CoV-2 than Lopinavir/Ritonavir and that no significant adverse reactions have been recorded for this medication (Fig. 1) [16,18].
Fig. 1.
Chemical structures of drugs used to fight SARS-CoV-2 infection.
The antimalarial and immunomodulatory medication hydroxychloroquine has attracted considerable interest in the multitude of drugs proposed as possible repurposing candidates for SARS-CoV-2 [19]. Following the launch of hydroxychloroquine's preliminary positive findings in suppressing SARS-CoV-2 involvement in vitro [20], and a brief open-label, non-randomized, single treatment center research recording hydroxychloroquine effectiveness and possible synergistic interaction with macrolide antibiotic azithromycin in enhancing viral clearance in SARS-CoV-2 patients (Fig. 1) [21]. The US (FDA) has utilized its leading authority to enable the usage of hydroxychloroquine for SARS-CoV-2 in situations where clinical trials are impractical or unfeasible [22]. Nonetheless, subsequent tests could not find a significant advantage of hydroxychloroquine in SARS-CoV-2, although some showed possibly severe toxicity correlated with its use [[23], [24], [25], [26], [27]]. Nafamostat mesylate, licensed by the (FDA) for indications unrelated to coronavirus disease, blocked SARS-CoV-2 S-mediated penetration into host cells with an approximately 15-fold higher efficacy than Camostat mesylate, with an (Half maximal effective concentration) EC50 in the low nanomolar range (Fig. 1) [28]. In addition, Nafamostat mesylate prevented SARS-CoV-2 infection of human lung cells with slightly higher effectiveness than Camostat mesylate [28]. Taking into consideration the global effect of SARS-CoV-2 on public health, the proven efficacy of Nafamostat mesylate and its improved antiviral activity in contrast with Camostat mesylate, Markus Hoffmann et al. suggest that this compound will be tested as SARS-CoV-2 therapy in clinical trials [28]. To date, given the intensive research initiative demonstrated by more than 600 currently underway clinical trials, no appropriate medications have been licensed to combat or cure SARS-CoV-2, and the only routine therapy is compassionate care [29]. Hence, immediate inquiries are needed based on the fast and global dissemination of the virus to establish effective drugs.
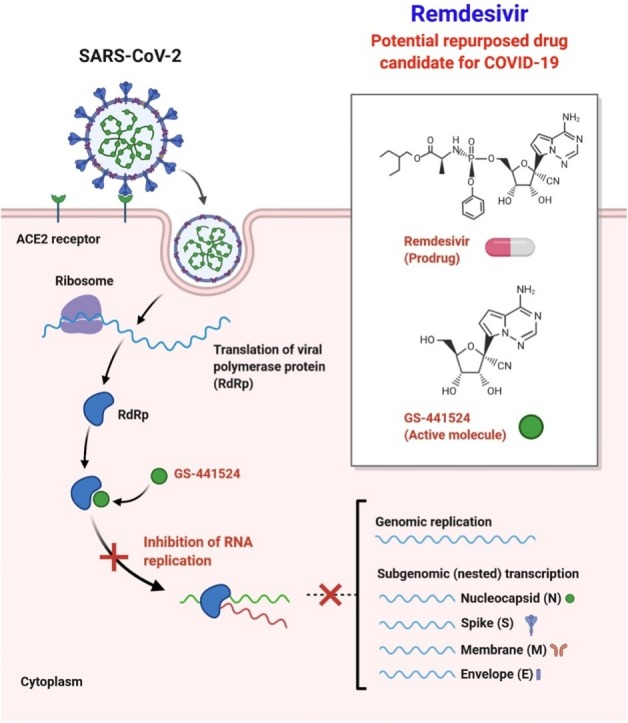
2. Remdesivir
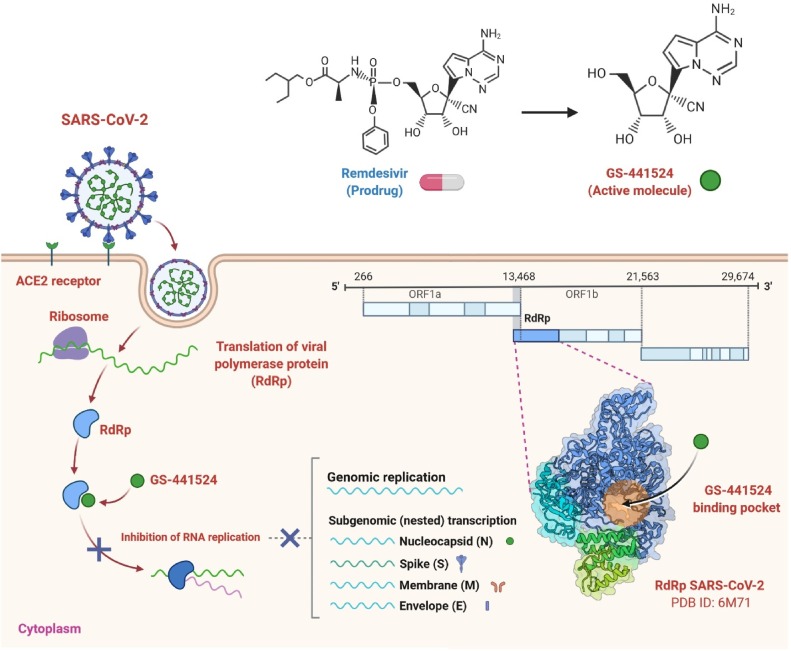
Remdesivir displays potent efficacy against several genetically based CoVs as well as unknown new viruses such as Ebola [9,[30], [31], [32]]. Andrea J. Pruijssers et al. prove that Remdesivir and its parent nucleoside GS-441524 are effective in the physiologically important Calu3 2B4 cell line against SARS-CoV-2 and that Remdesivir has significant antiviral efficacy in primary human airway cultures (Fig. 2 ) [33]. Remdesivir's efficacy was explicitly linked to the intracellular concentration of the pharmacologically active triphosphate (TP) metabolite, that was substantially greater in primary human airway epithelial (HAE) cultures relative to human lung cells (Calu3 2B4) and monkey kidney cells (Vero E6) [33]. Their study is compatible with recent findings indicating substantial contributions to natural variability in trends to host- and tissue-specific gene expression and microbiome-specific contributions to drug metabolism, stability and pharmacokinetics in various tissues [34,35]. Remdesivir-TP modelling on the SARS-CoV-2 RdRp showed that the location of Remdesivir-TP in the active site strongly matched that of the cognate natural substrate ATP, associated with effective RNA integration throughout viral genome replication [33]. Remdesivir reduced viral loads and increased lung function when administered at 1 dpi in mice infected with the chimeric virus SARS/SARS2-RdRp [33]. This is the first rigorous demonstration of potent inhibition of SARS-CoV-2 in continuous and primary human lung cultures and the first study suggesting efficacy of Remdesivir against SARS-CoV-2 in mice [33].
Fig. 2.
Remdesivir's possible mechanism of action against the replication of coronavirus. It also indicates the proposed binding pocket of RdRp polymerase on the right in a 3D structure. SARS-CoV-2 genomic organization, showing the coding regions for proteins that are possible targets for drugs [46]. This mechanism of action could be used for Favipiravir as well.
Prior research of Remdesivir anti-SARS-CoV-2 activity recorded EC50 values of 0.77 μM as calculated by the copy number of the genome [15], 23.2 μM as calculated by 50 % tissue culture infectious dose (TCID50), 26.9 μM as calculated by the copy number of the RNA [14], and 0.65 μM as calculated by cytopathic effect (CPE) [36], all in the Vero E6 cells. The efficacy of Remdesivir in Vero E6 cells (EC50, 1.65 μM) found in their analysis is consistent with values recorded by Wang et al., but higher than stated by Choy et al. [33]. Sequence analysis of the nsp12 from the Seattle, Washington isolates used during their research with SARS-CoV-2 isolates used in the previously described studies evaluating Remdesivir potency did not show consensus variations in the nsp12 sequence, indicating that any isolate-specific variability in Remdesivir susceptibility is not likely to be attributable to discrepancies in the Remdesivir-TP interaction with the RdRp [33]. Thus, the variations in EC50 values may be partly clarified by intrinsic discrepancies in SARS-CoV-2 virus isolates, quantitative processes, Vero cell lineages, and test parameters, including such incubation time and virus entry.
Remdesivir is the first antiviral therapy with confirmed effectiveness against SARS-CoV-2 in an animal model of SARS-CoV-2 [7]. Remdesivir therapy of rhesus macaques diagnosed with SARS-CoV-2 minimized acute illness and injury to the lungs [7]. The Remdesivir dosages utilized in rhesus macaques is similar to that used in humans; nevertheless, owing to the severe complexity of the disorder in rhesus macaques, it is difficult to precisely relate the pacing of therapy used to related disease periods in humans [7]. In the research of Brandi N. Williamson et al., therapy was given before the height of virus replication in the lungs as demonstrated by viral loads in bronchoalveolar lavages and the first results of treatment on clinical signs and virus replication were detected in 12 h [7]. The effectiveness of direct-acting antivirals against acute viral respiratory tract infections usually declines with delays of medication initiation [37]. Therefore, Remdesivir therapy in SARS-CoV-2 patients should be started as soon as possible to obtain the maximum therapeutic benefit. Notwithstanding the limited of apparent respiratory symptoms and decreased proliferation of viruses in the lungs of animals treated with Remdesivir, there has been no improvement in the shedding of viruses. Such a result is of particular significance regarding health care where a therapeutic development is not to be viewed as a loss of infectivity [7].
Although the previous study indicates the existence of Remdesivir metabolites in the lower respiratory tract, the drug rates in the upper respiratory tract have not been described, and novel formulations with alternate drug delivery route should be considered to boost dissemination to the upper respiratory tract, thus minimizing shedding and the possible risk of transmission [7]. Nevertheless, since severe SARS-CoV-2 infection is a consequence of viral lung disease, this organ is the primary focus of therapy with Remdesivir. Remdesivir's bioavailability and therapeutic role in the lungs of infected rhesus macaques facilitate Remdesivir’s treatment of SARS-CoV-2 patients. Even in severe cases of SARS-CoV-2 the treatment with Remdesivir did not occur in a therapeutic outcome in one clinical trial [38]; however, a shorter period for recovery was observed in another clinical trial that recruited more patients [39]. The previous study of Brandi N. Williamson et al. in rhesus macaques showed that therapy with Remdesivir would be treated appropriately as quickly as possible in order to avoid relapse to pneumonia in SARS-CoV-2 patients. With the continued proliferation of zoonotic relatives of SARS-CoV, SARS-CoV-2, and MERS-CoV in bat species, further outbreaks of novel CoVs are predicted [40,41].
The European Medicines Agency (EMA) released a professional opinion from the Committee for Medicinal Products for Human Use (CHMP) on the compassionate usage of Remdesivir on 3 April 2020 [42]. This assessment was mainly focused on effectiveness in the SARS-CoV-2 and MERS-CoV animal models because no clinical efficacy data were accessible, and the safety profile of Remdesivir was inadequately described [42]. EMA-CHMP launched a rotating analysis of Remdesivir on 30 April 2020 with a purpose of recommending, or not recommending, a Remdesivir Marketing Authorization. The required legal framework was a Conditional Marketing Authorization (CMA), given the scarcity of usable but positive data at that time and the high likelihood of acquiring more data [43]. Although the CHMP rotating analysis was underway, the opinion on compassionate use has been updated on 11 May 2020 to extend the reach of the indication to accommodate patients who, according to data from the U.S. National Institute of Allergy and Infectious Diseases (NIAID) trial, get a SpO2 ≤ 94 % or need additional oxygen [43].
Taiwan previously approved the use of Remdesivir for patients with severe COVID-19 in late May 2020. This was accompanied in different countries/regions, including the EU and Canada, by a fast progression of contingent approvals [44]. An emergency use permission for Remdesivir was issued in the USA before these contingent approvals on 1 May 2020 and a special emergency use authorization was issued in Japan on 7 May 2020 [44]. A CMA application was submitted to the EMA on 5 June 2020, and a positive risk-benefit decision was released on 25 June 2020 by the CHMP [45]. That decision accompanied the Marketing Authorization decision provided by the European Commission on 3 July 2020. Safety management is described in the Risk Management Plan released at the same time [45]. As of 25 June 2020, there has been no official regulatory authorization in Canada or Switzerland for Remdesivir, however compassionate use of the Remdesivir was available [43]. Therefore, the discovery and assessment of widely efficient, reliable anti-CoV therapies are desperately required now and in the future.
3. Favipiravir
Favipiravir is an analog nucleoside capable of inhibiting RNA-dependent polymerase and was licensed for sale in Japan in 2014 [[47], [48], [49]]. It's being used to combat influenza A and B antiviral drugs, which can successfully prevent Ebola virus, yellow fever virus, and so on [49,50]. In vitro studies have demonstrated that for SARS-CoV-2, Favipiravir is potent, and its EC50 is 61.88 μM [15]. To date, several clinical trials of Favipiravir have been performed in China for the therapy of SARS-CoV-2 (Fig. 2). The latest clinical trials have shown that Favipiravir's therapeutic benefit is quite significant compared to the antiviral medication arbidol. Positive-to-negative nucleic acid, mean antipyretic duration, and period for the cessation of cough were higher than those of the arbidol group [51]. In random clinical trials, patients with SARS-CoV-2 are enrolled to assess the effectiveness of Favipiravir plus interferon-α (ChiCTR2000029600) and Favipiravir plus baloxavir marboxil (an authorized influenza inhibition affecting the cap-dependent endonuclease) (ChiCTR2000029544) [52]. An empirical analysis performed in Shenzhen, China in February 2020, found that Favipiravir had a slightly quicker mean period for viral clearance than Lopinavir/Ritonavir [4 days vs 11 days (P < 0.001)] [53]. Such findings were followed by an early Chinese randomized clinical trial (RCT), in which therapy with Favipiravir contributed to a slightly higher rate of survival in non-critical SARS-CoV-2 patients than with Umefenovir (71.4 % vs 55.9 % [P < 0.05]) [54]. While neither of these early findings has proven successful in improving the survival rate for chronically sick patients in this same trial [5.6 % vs 0.0 % (P = n.s.)] [54], additional trials have been conducted in China and Italy as a consequence.
Despite the likely for Favipiravir usage in SARS-CoV-2 therapy, the safety process is essential to advise both current clinical studies and broader application in the future. Current Favipiravir safety data for SARS-CoV-2, however, and previous warnings, are restricted and challenging to obtain. Victoria Pilkington et al. examined current clinical data suggesting that Favipiravir is relatively healthy regarding overall adverse events (AEs) and serious AEs and less extreme gastrointestinal AEs. Nevertheless, changes in uric acid in the blood remain a health risk, based on a combined review of broader trials, with some proof of a growing dose-dependence rate. Further health issues, such as teratogenicity risk and prolonged QTc (QT interval corrected for heart rate), have not yet been adequately tested [53]. There is data to endorse the short-term usage of Favipiravir in terms of safety and tolerability. Further data, however, is required to determine the treatment's long-term impact [53]. Considering the shortcomings of the proof and the underlying real safety consequences, Favipiravir's broad usage against pandemic SARS-CoV-2 merits caution.
4. Hydroxychloroquine and azithromycin
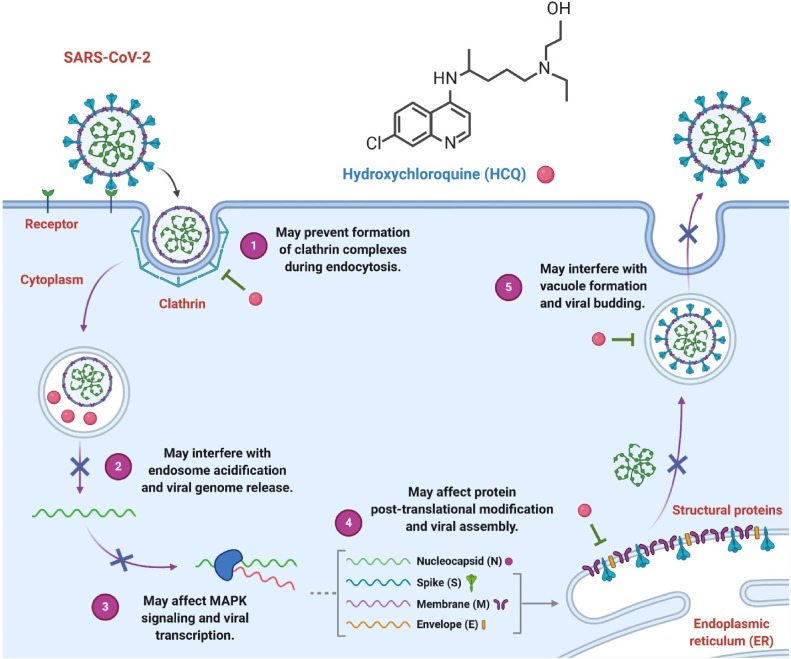
Hydroxychloroquine seems to be in the forefront of therapeutic applicants being re-used. While current prospective, randomized, regulated trials are anticipated to offer further data on Hydroxychloroquine in the immediate future (Fig. 3 ), Joseph Magagnoli et al. found in their spatial analysis have extensive details on the usage of Hydroxychloroquine with or without Azithromycin from the most organized healthcare program in the United States. In particular, they observed the usage of Hydroxychloroquine with or without Azithromycin co-administration did not increase survival or that the necessity for oxygen therapy [19]. After the announcement on April 21, 2020, of preliminary evidence from their research, two different New York groups have verified their results in broader datasets [55,56]. Their research results are comparable to the New York hospital retrospective study, which recorded no beneficial impact of Hydroxychloroquine therapy on respiratory distress or death in patients diagnosed with SARS-CoV-2 [55]. Nonetheless, although co-administered with Azithromycin, the research did not comment on the efficacy of Hydroxychloroquine. They also discuss the mortality risks and ventilation independently, unlike the report, which treated all of these findings as a combined indicator [19].
Fig. 3.
Potential mechanisms of action against replication of coronavirus, viral assembly, and viral budding by Hydroxychloroquine. By interfering with endosome-mediated viral entry or the late replication stages of enveloped viruses, Hydroxychloroquine can inhibit many viruses' replication [76,77]. For Azithromycin, this mechanism of action may also be used.
Despite the number of clinical studies currently investigating the mixture of Hydroxychloroquine and Azithromycin for SARS-CoV-2 and the possible synergistic toxicity of such medications, it is important to gain information into the results of both Hydroxychloroquine and Azithromycin-treated patients [23,27]. Their research findings are somewhat close to another latest analysis from 25 New York hospitals, which confirmed that Hydroxychloroquine, with and without Azithromycin, was not correlated with decreased fatalities but did not mention the possibility of oxygen therapy [56]. The analysis reported a higher incidence of heart failure in patients obtaining Hydroxychloroquine and Azithromycin. Previously, in patients hospitalized with Hydroxychloroquine or Chloroquine with or without macrolides, a major international registry research report found an elevated risk of death and ventricular arrhythmias [57]. While this research recorded a lack of differences in confounding factors among the groups, it regarded only two measurements of the illness's seriousness. It did not correct other significant factors for the laboratory. The outcomes of the public research by Joseph Magagnoli et al. confirmed observations from both New York regional trials. They revealed similar mortality and oxygen therapy levels to the multinational study following the tendency score improvement dependent on several variables. In contrast to these longitudinal clinical trials, no positive benefit of Hydroxychloroquine therapy was found in a previous randomized control study evaluating Hydroxychloroquine's effectiveness for mild to medium SARS-CoV-2 [58].
Hydroxychloroquine was documented to prevent in vitro replication of SARS-CoV-2, with a maximum efficient concentration of 50 % (EC50) varying from 4.5 μM to 17 μM [20]. Higher doses of Hydroxychloroquine to reach assumed concentrations of antivirals may raise the likelihood of adverse events. A randomized, regulated study of high-dose Chloroquine, the parent compound of Hydroxychloroquine, which was also confirmed to have in vitro antiviral efficacy against SARS-CoV-2 and comparable peak serum levels in people, was terminated prematurely attributable to cardiac toxicity and higher mortality rates in high-dose SARS-CoV-2 patients administered with Chloroquine [59]. No gain from Hydroxychloroquine therapy with or without Azithromycin in survival rates, oxygen therapy or duration of stay in hospitalized SARS-CoV-2 patients was reported in Joseph Magagnoli et al. after correction for many related confounding variables [19]. The fatality rates and mechanical ventilation rates in the analysis by Joseph Magagnoli et al. are close to those recorded in a broad longitudinal case series involving 5700 New York City patients [60]. Undoubtedly, it is anticipated that potential outcomes of prospective randomized clinical studies of Hydroxychloroquine performed in many countries in both hospital and outpatient settings may offer further concrete advice over the coming months.
Jayalakshmi Vallamkondu et al. examined the neuroinvasive nature of coronaviruses, the threats of comorbidities, including diabetes and possible therapeutic targets and drugs [61]. Future research needs to establish the specific molecular and cellular connections to the pancreas, liver, kidney, heart, and brain between coronavirus infections and their diffusion. Such research may help physicians treat patients with weakened immune systems due to hepatic cirrhosis or acquired immunodeficiency syndrome (AIDS) [[62], [63], [64], [65]]. Medications, including Hydroxychloroquine and Remdesivir, are widely used to reduce the risk of SARS-CoV-2. Many nations also use convalescent plasma treatment around the world [61]. For therapeutic targets, the mechanism of viral entry of SARS-CoV-2, regulated by spike proteins, could also play a critical role.
Rajkumar Singh Kalra et al. provided observations into the main role of an angiotensin-converting enzyme 2 (ACE-2) in SARS-CoV-2 infection and its importance in cardiovascular systemic function. Further, they examined the effect of Hydroxychloroquine on the replication and immunomodulatory activities of SARS-CoV-2 [66]. Rajkumar Singh Kalra et al. analyzed cardiovascular threat and the advantages of Hydroxychloroquine in existing clinical settings, including readouts from clinical COVID-19 reports so far. They include more briefing on major factors in the repurposing of Hydroxychloroquine and its future viewpoints [66].
Azithromycin (Azithromycin Dihydrate), a macrolide, N-Methyl-11-aza-10-deoxo-10-dihydroerythromycin A, it demonstrated antiviral efficacy against Zika [[67], [68], [69]]. Azithromycin is a safe medication that is commonly used in the World, including, for example, 12 million therapy courses in children under the age of 19 [70]. Previous research listed these two substances as alternative therapies for SARS-CoV-2 illness, Azithromycin and Hydroxychloroquine among 97 total theoretically active agents [71]. Hydroxychloroquine and, with much higher efficacy, the mixture of Hydroxychloroquine and Azithromycin were found to be successful in decreasing the infection rate of SARS-CoV-2 in COVID-19 patients in an initial clinical study [21]. After the start of the outbreak in the Marseille area, Julien Andreani et al. have identified various strains and evaluated one of them, the SARS-CoV-2 IHUMI-3 in combination with Vero E6 cells, utilizing multiple doses of Hydroxychloroquine and Azithromycin [72]. The use of Azithromycin and Hydroxychloroquine resulted in substantial inhibition of viral replication for wells comprising 5 μM of Hydroxychloroquine in conjunction with 10 or 5 μM of Azithromycin with 97.5 % and 99.1 % of relative viral reduction, respectively [72]. Several other macrolides possibly report observations of Azithromycin’s effectiveness on RNA viruses. Clarithromycin or the non-antibiotic macrolide EM900 are selective on in vitro rhinovirus [73,74]. In vivo, Hydroxychloroquine sulfate may be indicated by reducing pro-inflammatory cytokines and by adjusting the lysosome acidification process in the regulation of the immune response [74]. In severe cases of SARS-CoVs, these factors can play a keystone function. However, cytokines storm was involved in mouse models with SARS-CoV pneumonia and lung affections [75]. Parallel to this, Azithromycin became recognized as inhibiting in vitro viral replication of the Zika virus [68]. Yet Azithromycin has been correlated with up-regulating interferons I and III in the sense of spreading viral infection [69]. As for the respiratory syncytial virus, it has also been demonstrated macrolides decrease the lysosome acidity and down-regulate the Intercellular Adhesion Molecule 1 (ICAM-1) protein. So, Azithromycin may potentialize the role of Hydroxychloroquine by the same pathway in the case of SARS-CoV-2 [72]. Julien Andreani et al. results are compatible with the treatment response of the mixture of Hydroxychloroquine and Azithromycin by Gautret et al. [21]. They endorse the therapeutic usage of this combination of medications, particularly at the preliminary phase of the COVID-19 infection before patients develop breathing difficulties with related cytokine storm and become less curable with any antiviral therapy.
5. Lopinavir/Ritonavir
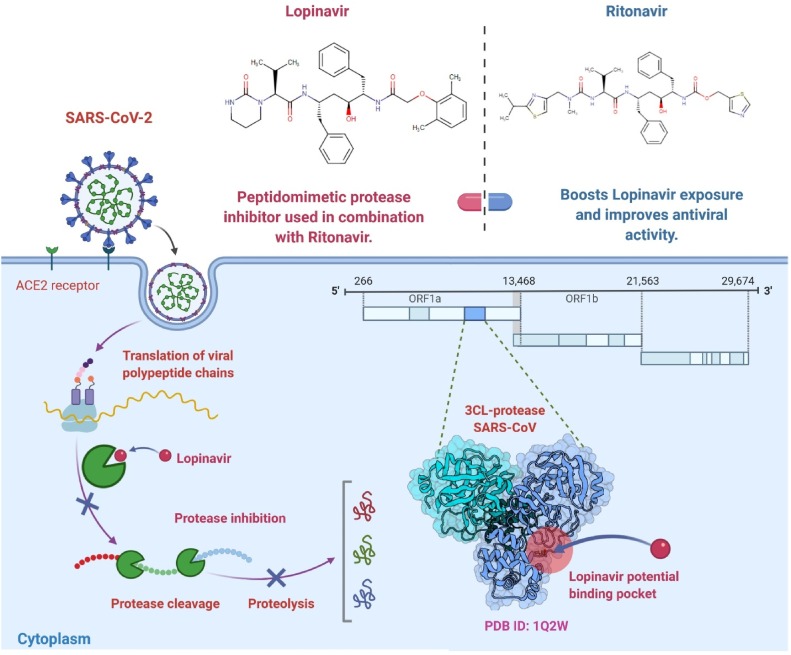
Lopinavir/Ritonavir, an oral combined drug for the treatment of human immunodeficiency virus (HIV) licensed by the US Food and Drug Administration (FDA), showed in vitro efficacy against certain novel coronaviruses by inhibiting 3-chymotrypsin-like protease (Fig. 4 ) [78,79]. There are no reported in vitro results for Lopinavir/Ritonavir on SARS-CoV-2. A systematic analysis of Lopinavir/Ritonavir for the therapy of SARS and MERS, with most of these studying SARS, identified minimal accessible research [16]. High mortality and intubation rates were consistent with clinical trials of SARS, but their historical, observational existence avoids conclusions [80]. The scheduling of implementation during the early peak period of viral replication (early 7–10 days) seems to be significant, as prolonged initiation of therapy with Lopinavir/Ritonavir has a little clinical impact [81,82]. Recent reviews of Lopinavir/Ritonavir with the SARS-CoV-2 therapy mainly case reports and few retrospective, non-randomized prospective trials, rendering it impossible to assess the causal impact of Lopinavir/Ritonavir on the treatment [81,82].
Fig. 4.
Lopinavir/Ritonavir potential repurposed drug candidate for SARS-CoV-2. The 3-chymotrypsin-like protease (3CLpro) enzyme has a vital function in viral RNA processing. Since Lopinavir/Ritonavir is a protease inhibitor, the action of 3CLpro could be inhibited, thus preventing the process of viral replication and host cell release [91].
Cao et al. recently published the outcomes of an open-label randomized controlled trial (RCT) evaluating the effectiveness of Lopinavir/Ritonavir vs standard care in 199 SARS-CoV-2 patients [83]. Pertinently, the mean period from the start of the symptom to randomization was 13 days (interquartile range [IQR], 11–16), with little disparity across groups. The first result of health progress described by a 2-point increase on an ordinal or hospital discharge scale of 7 categories was comparable in all groups (16 days [IQR, 13–17] vs 16 days [IQR, 15–17]; hazard ratio [HR], 1.31 [95 % confidence interval (Cls), 0.95–1.85]; P = .09) [83]. In comparison, there were no significant variations in viral clearance or 28-day fatality rates (19.2 % vs 25.0 %; total change, −5.8 % [95 % CIs, −17.3 % to 5.7 %]) were noted [83]. While delayed initiation of care may partly describe the inefficiency of Lopinavir/Ritonavir in treating SARS-CoV-2, there was no shorter period for clinical development for patients undergoing therapy within 12 days (HR, 1.25 [95 % CIs, 0.77–2.05]) in a subgroup study [83]. Whereas further Lopinavir/Ritonavir RCTs are pending, the existing evidence indicates a restricted function for Lopinavir/Ritonavir in treating SARS-CoV-2 [80].
The most widely used and documented dosing protocol for SARS-CoV-2 therapy with Lopinavir/Ritonavir is 400 mg/100 mg twice every day for 14 days [83,84]. Considering the significant drug-drug interactions and possibly harmful drug interactions, the application of this drug needs careful study and control of concomitant medications. Lopinavir/Ritonavir negative impacts involve gastrointestinal discomforts such as vomiting and diarrhea (up to 28 %) and hepatotoxicity (up to 2 %–10 %) [85]. Such side effects can be compounded in patients with SARS-CoV-2 by combined treatment or viral infection, and at present, with SARS-CoV-2 about 20 %–30 % of patients have increased transaminases [86]. A current RCT found that about 50 % of patients with Lopinavir/Ritonavir reported an adverse reaction, and 14 % of patients abandoned treatment due to negative gastrointestinal symptoms [83]. Transaminitis triggered by the medication is of particular importance, because it may worsen liver damage incurred by SARS-CoV-2. Notably, in some SARS-CoV-2 investigational trials, the elevations of alanine transaminase are exclusion criteria, suggesting that hepatotoxicity caused by Lopinavir/Ritonavir may restrict the capacity of patients to obtain both these therapies [87].
SARS-CoV-2 cell entry has appeared to be an enticing drug repurposing strategy for COVID-19. Zhang Qi et al., integrated genetics and chemical disruption to show that ACE-2-mediated SARS-CoV and CoV-2 entry includes heparan sulfate (HS) on the cell surface as an assistant cofactor: ablation of genes involved in HS biosynthesis or incubating HS mimetic cells which both suppress Spike-mediated viral entry [88]. Zhang Qi et al., have shown that heparin/HS binds directly to Spike and promotes the binding of viral particles carrying Spike to the cell surface to encourage viral entry. They tested authorized drugs and established two groups of inhibitors to target this entry pathway through separate mechanisms. Mitoxantrone is a potent HS inhibitor among the drugs characterized, whereas Sunitinib and BNTX interrupt the actin network to abrogate HS supported viral entry indirectly [88]. Furthermore, Zhang Qi et al. demonstrate that both groups' drugs can be combined to produce a synergized activity towards the cytopathic effect caused by SARS-CoV-2. Overall, the study recognizes HS as an attachment factor that facilitates SARS coronavirus cells' entry and demonstrates drugs capable of targeting this critical process in the viral life cycle.
Murat Seyran et al. identified the structural bases underlying the pandemic potential of SARS-CoV-2, and its rapid movement over respiratory epithelia that enables rapid cellular entry is clarified [89]. They suggested that the flat sialic acid-binding domain at the N-terminal domain (NTD) of the S1 subunit contributes to more successful initial contact and association with the sialic acid layer over the epithelium based on prominent viral spike (S) protein characteristics, so this, in fact, enables faster viral 'surfing' of the epithelium and SARS-CoV-2 receptor scanning [89]. The primary entry receptor for SARS-CoV-2 is an ACE-2 protein on the epithelial surface, and protein-protein interaction assays showed high-affinity binding of the S protein to ACE-2 [89]. No high-frequency mutations have been observed to date in the S protein at the C-terminal domain (CTD) of the S1 subunit, where the receptor-binding domain (RBD) is located. Strong binding via a conserved viral RBD to ACE-2 means that the interaction between ACE2-RBD is possibly efficient. In addition, a cleavage site for furin and other proteases is contained in the viral S subunit, accelerating SARS-CoV-2 cell entry [89].
Sk. Sarif Hassan et al., evaluated the vulnerability of other species, whether they have the potential to be a possible host of SARS-CoV-2 [90]. They selected nineteen different species (Bos taurus, Capra hircus, Danio rerio, Equus caballus, Felis catus, Gallus gallus, Homo sapiens, Macaca mulatta, Manis javanica, Mesocricetus auratus, Mustela putorius furo, Pelodiscus sinensis, Pteropus alecto, Pteropus vampyrus, Pan troglodytes, Rattus norvegicus, Rhinolophus ferrumequinum, Salmo salar, and Sus scrofa) and studied the ACE-2 protein sequence compared to the human ACE-2 sequence from eighteen non-human species and calculated the degree of variation through which the sequences varied from each other [90]. In addition to the phylogenetic analysis, Sk. Sarif Hassan et al. conducted a detailed bioinformatics analysis focused on full-length sequence homology, polarity along with individual domain sequence homology, and secondary structure estimation of such protein sequences. Six independent clusters of nineteen species may have established these results based on a collective study and thus predicted the transmission of SARS-CoV-2 inter-species [90].
6. Nafamostat mesylate
Nafamostat mesylate has been known as a strong inhibitor of MERS-CoV entry into human epithelial cells [92]. Previously, it has been demonstrated that Nafamostat mesylate inhibits SARS-CoV-2 entry into human epithelial cells at EC50 of ∼10 nM [28,93]. Nafamostat mesylate was utilized clinically in Japan; for preventing acute pancreatitis and disseminated intravascular coagulation. Its blood concentrations are preserved between 30–240 nM by intravenous injection, which is adequate to prevent the virus's entry [93]. Nafamostat mesylate, combined with Favipiravir, could block virus entry and replication and repress pathogenic host response, i.e., hyper-coagulopathy [94]. While most patients in this case report were relatively low, such a limited fatality rate indicates that Favipiravir and Nafamostat mesylate combined therapy could be successful for patients with seriously ill COVID-19 patients. A clinical trial (jRCTs031200026) will be conducted in Japan for the combination therapy of Nafamostat mesylate and Favipiravir against COVID-19 [94].
Camostat and Nafamostat are effective drug applicants for a drug treatment plan for COVID-19. In order to uncover the molecular activity hypothesis of these medicines and provide evidence that can help advance them further, Tim Hempel et al. have combined cell-based assays, detailed molecular experiments, and Markov modeling [95]. Prior studies provided evidence that both blockers work directly on Transmembrane serine protease 2 (TMPRSS2) and that Nafamostat is more active than Camostat, and this qualitative discrepancy is consistent with additional purified protein and cell-entry assays in addition to in vitro assays [28,96,97]. Tim Hempel et al. observe that between such three assay forms, the absolute IC50 s amounts differ, indicating variations in experimental parameters and which feature has been inhibited and calculated. Although no TMPRSS2 crystallographic structure is usable, they provide comprehensive all-atom molecular dynamics (MD) calculations of 280 microseconds beginning from a homology model that produces stable assemblies of the two protein-drug clusters of the optimum structure. Throughout the protein binding site, these simulations sample several association/dissociation occurrences and distinct drug poses [95]. Their analysis demonstrates that the non-covalent compounds of Camostat and Nafamostat with TMPRSS2 are comparatively short-lived, implying that the key inhibitory activity is attributed to the interaction guanidinobenzoyl ligand of the compound and the catalyzed serine of TMPRSS2 of the long-lived covalent acyl-enzyme complex. Two groups of Nafamostat can link into the S1 envelope, while there is only one group of Camostat. Nevertheless, Tim Hempel et al. found that Camostat and Nafamostat are identical in the population of S1 related states, indicating that non-covalent prevention is probably to become a slight contributor to TMPRSS2 as the whole repression [95]. Nafamostat mesylate attenuates respiratory inflammation in a murine asthma model by suppressing NF-kB activation, the essential transcription factor for forming inflammatory cytokines [98]. Nafamostat mesylate is also anticipated to have some treatment consequences. Since Nafamostat mesylate has been used in Japan for several years and has accrued sufficient safety clinical data, Mizuki Yamamoto et al. propose that it should be tested in patients with COVID-19 alone or combined with other antiviral drugs targeting the different steps required for the production of viruses.
7. Current status of vaccines and their clinical readouts
A vaccine against COVID-19 is desperately required, considering the unparalleled morbidity and fatalities associated with the COVID-19 pandemic [99,100]. The FDA will likely grant emergency use licenses for two vaccines, Pfizer and BioNTech jointly developed one, and the other by Moderna against SARS-CoV-2 [101]. Both vaccines have demonstrated about 95 % effectiveness in combating symptomatic COVID-19 infections in phase III trials, referring to company press reports and data made accessible by the FDA, without significant safety issues that could delay FDA authorization [102]. Additional phase III trials of vaccines produced by Janssen and AstraZeneca are ongoing; preliminary findings from those trials might not be far away, with rapidly increasing case numbers in the US. These trials equate the occurrence of symptomatic infection with that of a placebo control group of vaccine receivers [101]. Nevertheless, conducting placebo-controlled trials of subsequent vaccine candidates could become difficult once approved vaccines become readily accessible [103]. There is a need for alternative methods to test these vaccines and equate their safety and effectiveness with approved drugs.
8. Prospective strategies for investigating upcoming vaccines against SARS-CoV-2
Randomly selected, blinded clinical trials that equate the vaccines with placebo controls are the best clinically reliable way to investigate vaccines that have not yet reached clinical trials. Influential organizations have advocated for utilizing these trials to test potential candidates and collect initial vaccine data, even after being accepted or licensed [104,105]. This strategy could be sustainable for a short period of time. However, accessibility to emergency-authorized vaccinations is limited, and only selected subpopulations could be vaccinated, including front-line healthcare staff and nursing home occupants [106]. Nevertheless, it might no longer be practical or acceptable to involve certain individuals in placebo-controlled trials once approved vaccines become accessible in adequate quantities to start vaccinating larger populations at high risk of serious illnesses, like community-dwelling older people and people with comorbidities. Considering the significance of testing vaccines' effectiveness and safety in high-risk subgroups, removing them from placebo-controlled trials would dramatically reduce such trials' validity.
The second strategy that is considered by the FDA in its guidelines once immunological effectiveness correlates including such levels of vaccine antigen antibodies are identified, is to administer test vaccines to groups of individuals and then decide based on proxy measures to approve or authorize such vaccines [107]. Nevertheless, to establish what comprises a validated substitute indicator of vaccine effectiveness, sufficient data is still not accessible. Furthermore, assessing effectiveness alone would not be sufficient; it is similarly important to assess safety, including comparable trials containing large numbers of participants. Therefore, trials focused on substitute efficiency tests, at least in the near term, are not a feasible strategy [108].
The third strategy is to perform random head-to-head trials contrasting a potential candidate vaccine with such a vaccine which has already earned an urgent or full license. In such experiments, non-inferiority models may be used to successfully label the new vaccine if symptomatic COVID-19 disease or other primary endpoints are not higher than the analogue group's occurrence by a certain defined margin. Such trials may also promote direct safety correlations between the new vaccine and its established analog; these correlations are of special significance, due to the widespread public safety issues. Understanding such trials will need to trust that the analog vaccine's historical effectiveness predictions from previous trials also held in the present trial. The high recorded effectiveness of Pfizer/BioNTech and Moderna vaccines reassures that if the level of infection in the new vaccine group is seen to be no lower than the level in the analog group, it could be inferred that the new vaccine was successful even without the placebo control group enabling clear conclusions (In other phrases, the trial would have the sensitivity of the test) [109]. The conduct of such a trial will rely on collaboration between both the producer of the new vaccine and the producer of the vaccine in dispute, a necessity which presents both logistical and financial difficulties.
The fourth strategy will be most beneficial from a scientific and global health viewpoint. It will launch a multi-group pilot trial that might test accepted or licensed vaccines against new candidates for unauthorized vaccines [110]. Such a trial performed throughout Operation Warp Speed's auspices could directly enable both proven and investigational vaccines to compare safety and effectiveness directly. Investigational vaccine groups will be introduced to the network as soon as the candidate vaccines in smaller, earlier-phase trials reached safety and immunogenicity thresholds. Candidate vaccines will be removed from the platform based on interim safety, and efficacy evaluations and their development might be terminated utilizing an adaptive design if they appeared to have inferior efficiency or major safety issues as assessed against benchmark comparators. On the other hand, once comforting initial safety and effectiveness data were accessible; it was possible to extend enrollment in novel vaccine groups to children and other communities that were largely exempted from the current phase III trials. In other COVID-19-related settings, adaptive designs have been utilized, including the assessment of therapeutic drugs for hospitalized patients with serious illness [111].
9. Conclusion
Several antiviral therapies, individually or in combination, previously utilized against SARS and MERS, FDA-approved and laboratory tests, have been examined against COVID-19. Nevertheless, even with significant efforts, no effective COVID-19 antiviral drug or vaccine is yet accessible [112,113]. Thus, there is an immediate need to battle this extremely infectious disease, which needs a thorough understanding of the disease's pathology and essential techniques for new treatment detection. Alternative treatments, like the antiviral activity of natural killer cells, mesenchymal stem cells, immunotherapy, and conventional herbal medicine, could also be studied, in addition to currently authorized antiviral drugs [[114], [115], [116]]. While many new therapeutic techniques are developing in these desperate times, in order to ensure the safety and effectiveness of new drugs, more clinical trials are required [117]. The drug repurposing strategy rapidly brings the drug discovery process on track and has drawn scientists' interest in a wide variety of research disciplines [118,119]. Drug development periods are reduced because all these crucial processes can be circumvented due to the existence of in vitro and in vivo profiling reports, complete chemical preparation, toxicity tests, bulk production, suitable and sufficient assessment, and pharmacokinetic studies of FDA-approved drugs [120,121]. Besides, greater expenditures are not needed, and repurposed drugs have been shown to be effective in clinical trials, thus also reducing attrition rates [118,122]. Therefore, drug repurposing's key benefits are related to the proven safety of the approved candidate drugs, dramatically shortened periods for production, and the expenses involved with progressing a candidate into clinical studies.
Drug repurposing has become a successful drug development approach because of the costly and time-consuming conventional drug development method with higher failure rates. Drug repurposing allows the discovery in shorter periods of new old medications applications, while being cost-effective with lower turnover rates, thereby benefiting patients and the overall healthcare system. Day by day, with a growing multitude of new viral infections, it is important to discover therapeutics in parallel. While many therapeutics have been demonstrated to be successful against different respiratory viral infections, such as influenza and some coronavirus strains, the critical challenge is developing resistance to established antiviral drugs. In addition, new types of viruses like SARS-CoV-2 are generating a disease outbreak scenario. A very effective way of exploiting drugs with proven safety profiles to tackle the coronavirus epidemic is to repurpose FDA-approved therapies. Nevertheless, it is important to choose the correct delivery method and delivery route for drug administration and deliver repurposed drugs directly to the target site. Repurposing may be a great possibility with clinical delivery intervention. Furthermore, the integration of pharmaceutics and toxicology is necessary in order to resolve dosing and safety concerns.
The FDA has now approved Hydroxychloroquine and Remdesivir, but recent studies on comorbidities patients remain controversial [123]. To eliminate SARS-CoV-2 infection, several physicians have repurposed Ebola, SARS-CoV, MERS-CoV, and HIV medications. The clinical treatment of the disorder also uses IL-6 and convalescent therapy [123]. Ramesh Kandimalla et al. describes both the scope of possibilities and the sharp lack of drug pathways, effectiveness, and consequences knowledge [123]. Therefore, a fast emphasis on drug repurposing and effective therapeutic delivery methods and inhaler devices is important to treat diseases caused by multiple viruses, including SARS-CoV-2, and the development of vaccines needs to be accelerated in order to save lives.
Author contributions
AA designed research, wrote the manuscript, and revised the manuscript. YLW conceived of the study. YT revised the manuscript. WZ designed the study, revised the manuscript, and provided funding support. All authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
We gratefully acknowledge the support by the National Natural Science Foundation of China (No. 21877101), the Zhejiang Leading Innovation and Entrepreneurship Team (2018R01015), and the Emergency Project of Key Research and Development Plan of Zhejiang Province (2020C03124).
References
- 1.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-41020-42008-41583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awadasseid A., Wu Y., Tanaka Y., Zhang W. SARS-CoV-2 variants evolved during the early stage of the pandemic and effects of mutations on adaptation in Wuhan populations. Int. J. Biol. Sci. 2020;17 doi: 10.7150/ijbs.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awadasseid A., Wu Y., Tanaka Y., Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int. J. Biol. Sci. 2020;16:1846–1860. doi: 10.7150/ijbs.45018. http://www.ijbs.com/v1816p1846.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Kutateladze T.G. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat. Commun. 2020;11:1–4. doi: 10.1038/s41467-41020-16779-41464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundstrom K., Seyran M., Pizzol D., Adadi P., Mohamed Abd El-Aziz T., Hassan S., Soares A., Kandimalla R., Tambuwala M.M., Aljabali A.A. Origin of SARS-CoV-2. Viruses. 2020;12(11):1203. doi: 10.3390/v12111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://covid19.who.int/.
- 7.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., Van Doremalen N., Leighton I., Yinda C.K., Pérez-Pérez L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.1104.1115.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.41038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remdesivir. 2020. (Accessed 15 May 2020, at https://clinicaltrials.gov/ct2/results?cond=&term=remdesivir&cntry=&state=&city=&dist=).
- 11.2020. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. (Accessed 15 May 2020, at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment) [Google Scholar]
- 12.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-41019-13940-41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.-F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020:104786. doi: 10.1016/j.antiviral.102020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-41020-40282-41420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 17.Nagata T., Lefor A.K., Hasegawa M., Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med. Public Health Prep. 2015;9:79–81. doi: 10.1017/dmp.2014.1151. [DOI] [PubMed] [Google Scholar]
- 18.Costanzo M., De Giglio M.A.R., Roviello G.N. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 19.Magagnoli J., Narendran S., Pereira F., Cummings T.H., Hardin J.W., Sutton S.S., Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med. 2020 doi: 10.1016/j.medj.2020.1006.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-41020-40156-41420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.101016/j.ijantimicag.102020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Food and Drug Administration . 2020. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Hyroxychlroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of COVID-19 in Certain Hospitalized Patients.https://www.fda.gov/media/136537/download [Google Scholar]
- 23.Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020:1–2. doi: 10.1038/s41591-41020-40888-41592. [DOI] [PubMed] [Google Scholar]
- 24.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., Song S., Zhang D. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19, Zhejiang da xue xue bao. Yi xue ban= Journal of Zhejiang University. Med. Sci. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.-A., Lescure F.-X. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L., Cour M. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.00754-00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020;5:1–10. doi: 10.1038/s41392-41020-40191-41391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169 doi: 10.101016/j.antiviral.102019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruijssers A.J., George A.S., Schäfer A., Leist S.R., Gralinksi L.E., Dinnon K.H., III, Yount B.L., Agostini M.L., Stevens L.J., Chappell J.D. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020 doi: 10.101016/j.celrep.102020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksson S. Is the expression of deoxynucleoside kinases and 5’-nucleotidases in animal tissues related to the biological effects of nucleoside analogs? Curr. Med. Chem. 2013;20:4241–4248. doi: 10.2174/0929867311320340004. [DOI] [PubMed] [Google Scholar]
- 35.Koczor C.A., Torres R.A., Lewis W. The role of transporters in the toxicity of nucleoside and nucleotide analogs. Expert Opin. Drug Metab. Toxicol. 2012;8:665–676. doi: 10.1517/17425255.17422012.17680885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 doi: 10.101016/j.phrs.102020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., Stevens L.J. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 doi: 10.1016/S0140-6736(1020)31022-31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.2020. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Remdesivir (GS-5734™). (Accessed 15 May 2020, at https://www.gilead.com/-/media/files/pdfs/remdesivir/eua-fact-sheet-for-hcps_01may2020.pdf) [Google Scholar]
- 40.Menachery V.D., Yount B.L., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.-Y., Donaldson E.F. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menachery V.D., Yount B.L., Sims A.C., Debbink K., Agnihothram S.S., Gralinski L.E., Graham R.L., Scobey T., Plante J.A., Royal S.R. SARS-like WIV1-CoV poised for human emergence. Proc. Natl. Acad. Sci. U. S. A. 2016;113:3048–3053. doi: 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Medicines Agency. EMA committee for human medicinal products compassionate use opinion on remdesivir; [cited 2020 May 22]. Available from: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf.
- 43.Saint-Raymond A., Sato J., Kishioka Y., Teixeira T., Hasslboeck C., Kweder S. Remdesivir emergency approvals: a comparison of the US, Japanese, and EU systems. Expert Rev. Clin. Pharmacol. 2020;13:1095–1101. doi: 10.1080/17512433.17512020.11821650. [DOI] [PubMed] [Google Scholar]
- 44.Lamb Y.N. Remdesivir: first approval. Drugs. 2020:1–9. doi: 10.1007/s40265-40020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Medicines Agency. EMA committee for human medicinal products opinion summary on Veklury; [cited 2020 May 22]. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/veklury.
- 46.Li G., De Clercq E. Nature Publishing Group; 2020. Therapeutic Options for the 2019 Novel Coronavirus (2019-nCoV) [DOI] [PubMed] [Google Scholar]
- 47.De Clercq E. Dancing with chemical formulae of antivirals: a personal account. Biochem. Pharmacol. 2013;86:711–725. doi: 10.1016/j.bcp.2013.1007.1012. [DOI] [PubMed] [Google Scholar]
- 48.De Clercq E. Dancing with chemical formulae of antivirals: a panoramic view (Part 2) Biochem. Pharmacol. 2013;86:1397–1410. doi: 10.1016/j.bcp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.1149.1123.1981-1986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem.– Asian J. 2019;14:3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.1103.1117.20037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G., De Clercq E. Nature Publishing Group; 2020. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) [DOI] [PubMed] [Google Scholar]
- 53.Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir–a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6:45. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.1103.1117.20037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(1020)31180-31186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3:e208857. doi: 10.201001/jamanetworkopen.202020.208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vallamkondu J., John A., Wani W.Y., Ramadevi S., Jella K.K., Reddy P.H., Kandimalla R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020 doi: 10.1016/j.bbadis.2020.165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lleo A., Invernizzi P., Lohse A.W., Aghemo A., Carbone M. Highlights for management of patients with Autoimmune Liver Disease during COVID-19 pandemia. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tapper E.B., Asrani S.K. COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao Y., Pan H., She Q., Wang F., Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol. Hepatol. 2020;5:528–529. doi: 10.1016/S2468-1253(20)30080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalra R.S., Tomar D., Meena A.S., Kandimalla R. SARS-CoV-2, ACE2, and hydroxychloroquine: cardiovascular complications, therapeutics, and clinical readouts in the current settings. Pathogens. 2020;9:546. doi: 10.3390/pathogens9070546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Retallack H., Di Lullo E., Arias C., Knopp K.A., Laurie M.T., Sandoval-Espinosa C., Leon W.R.M., Krencik R., Ullian E.M., Spatazza J. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14408–14413. doi: 10.11073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosseboeuf E., Aubry M., Nhan T., De Pina J., Rolain J., Raoult D., Musso D. Azithromycin inhibits the replication of Zika virus. J. Antivirals Antiretrovirals. 2018;10:6–11. doi: 10.4172/1948-5964.1000173. [DOI] [Google Scholar]
- 69.Li C., Zu S., Deng Y.-Q., Li D., Parvatiyar K., Quanquin N., Shang J., Sun N., Su J., Liu Z. Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00394-00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleming-Dutra K.E., Demirjian A., Bartoces M., Roberts R.M., Taylor T.H., Jr., Hicks L.A. Variations in antibiotic and azithromycin prescribing for children by geography and specialty—United States, 2013. Pediatr. Infect. Dis. J. 2018;37:52. doi: 10.1097/INF.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nabirotchkin S., Peluffo A.E., Bouaziz J., Cohen D. 2020. Focusing on the Unfolded Protein Response and Autophagy Related Pathways to reposition Common Approved Drugs Against COVID-19. [DOI] [Google Scholar]
- 72.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.-M., Colson P., La Scola B. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020:104228. doi: 10.101016/j.micpath.102020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgene M.F., Maurin C., Pillet S., Berthelot P., Morfin F., Pozzetto B., Botelho-Nevers E., Verhoeven P.O. HaCaT epithelial cells as an innovative novel model of rhinovirus infection and impact of clarithromycin treatment on infection kinetics. Virology. 2018;523:27–34. doi: 10.1016/j.virol.2018.1007.1025. [DOI] [PubMed] [Google Scholar]
- 74.Lusamba Kalonji N., Nomura K., Kawase T., Ota C., Kubo H., Sato T., Yanagisawa T., Sunazuka T., Ōmura S., Yamaya M. The non‐antibiotic macrolide EM 900 inhibits rhinovirus infection and cytokine production in human airway epithelial cells. Physiol. Rep. 2015;3:e12557. doi: 10.14814/phy12552.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(1003)00806-00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., Kao R., Poon L., Wong C., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., Van Nieuwkoop S., Bestebroer T.M., Van Den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-03014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 81.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease‐19 treatment option. J. Med. Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan K., Lai S., Chu C., Tsui E., Tam C., Wong M., Tse M., Que T., Peiris J., Sung J. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. https://www.hkmj.org/abstracts/v9n6/399.htm [PubMed] [Google Scholar]
- 83.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Health Commission and State Administration of Traditional Chinese Medicine Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. https://www.chinalawtranslate.com/wp-content/uploads/2020/03/Who-translation.pdf (Accessed 18 March 2020)
- 85.2016. Lopinavir/ritonavir [database online]. Hudson (OH): Lexicomp Inc.http://online.lexi.com (Accessed 17 March 2020) [Google Scholar]
- 86.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.ClinicalTrials.gov. (Accessed 18 March 2020). https://clinicaltrials.gov/.
- 88.Zhang Q., Chen C.Z., Swaroop M., Xu M., Wang L., Lee J., Wang A.Q., Pradhan M., Hagen N., Chen L. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020;6:1–14. doi: 10.1038/s41421-41020-00222-41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seyran M., Takayama K., Uversky V.N., Lundstrom K., Palù G., Sherchan S.P., Attrish D., Rezaei N., Aljabali A.A., Ghosh S. The structural basis of accelerated host cell entry by SARS‐CoV‐2. FEBS J. 2020 doi: 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hassan S., Ghosh S., Attrish D., Choudhury P.P., Aljabali A.A., Uhal B.D., Lundstrom K., Rezaei N., Uversky V.N., Seyran M. Possible transmission flow of SARS-CoV-2 based on ACE2 features. Molecules. 2020;25:5906. doi: 10.3390/molecules25245906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorward J., Gbinigie K. 2020. Lopinavir/ritonavir: A Rapid Review of Effectiveness in COVID-19.https://www.cebm.net/covid-19/lopinavir-ritonavir-a-rapid-review-of-the-evidence-for-effectiveness-in-treating-covid/ [Google Scholar]
- 92.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J.-i., Matsuda Z. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K. The anticoagulant nafamostat potently inhibits SARS-CoV-2 infection in vitro: an existing drug with multiple possible therapeutic effects. bioRxiv. 2020 doi: 10.1101/2020.1104.1122.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doi K., Ikeda M., Hayase N., Moriya K., Morimura N. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series. Crit. Care. 2020;24:1–4. doi: 10.1186/s13054-13020-03078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hempel T., Raich L., Olsson S., Azouz N.P., Klingler A.M., Rothenberg M.E., Noé F. Molecular mechanism of SARS-CoV-2 cell entry inhibition via TMPRSS2 by Camostat and Nafamostat mesylate. bioRxiv. 2020 doi: 10.1039/d0sc05064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shrimp J.H., Kales S.C., Sanderson P.E., Simeonov A., Shen M., Hall M.D. An enzymatic TMPRSS2 assay for assessment of clinical candidates and discovery of inhibitors as potential treatment of COVID-19. ACS Pharmacol. Transl. Sci. 2020 doi: 10.1021/acsptsci.1020c00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C.-L., Wang S.-D., Zeng Z.-Y., Lin K.-J., Kao S.-T., Tani T., Yu C.-K., Wang J.-Y. Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma. J. Allergy Clin. Immunol. 2006;118:105–112. doi: 10.1016/j.jaci.2006.1002.1047. [DOI] [PubMed] [Google Scholar]
- 99.Awadasseid A., Wu Y., Tanaka Y., Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2020;17 doi: 10.7150/ijbs.52569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(1020)30843-30844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Joffe S. Evaluating SARS-CoV-2 vaccines after emergency use authorization or licensing of initial candidate vaccines. JAMA. 2020 doi: 10.1001/jama.2020.25127. [DOI] [PubMed] [Google Scholar]
- 102.Vaccines and Related Biological Products Advisory Committee December 10, 2020, meeting announcement. US Food and Drug Administration. (Accessed 9 December 2020). https://www.fda.gov/advisory-committees/advisory-committeecalendar/vaccines-and-related-biological-productsadvisory-committee-december-10-2020meetingannouncement#event-materials.
- 103.Zimmer C., Weiland N. New York Times; 2020. Should Volunteers Who Got Placebo be First to Get the Real Thing? p. A7. December 3. [Google Scholar]
- 104.2020. Statement on Continuation of Vaccine Trials. International Coalition of Medicines Regulatory Authorities. Published.http://www.icmra.info/drupal/covid-19/statement_on_continuation_of_vaccine_trials (Accessed 2 December 2020) [Google Scholar]
- 105.WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation Placebo-controlled trials of Covid-19 vaccines—why we still need them. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2033538. Published online December 2. [DOI] [PubMed] [Google Scholar]
- 106.Goodnough A. New York Times; 2020. Everything You Wanted to Know About the Shot; p. A7. December 3. [Google Scholar]
- 107.2020. Center for Biologics Evaluation and Research. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. US Food and Drug Administration. Published June.https://www.fda.gov/media/139638/download (Accessed 2 December 2020) [Google Scholar]
- 108.Avorn J., Kesselheim A. Regulatory decision-making on COVID-19 vaccines during a public health emergency. JAMA. 2020;324:1284–1285. doi: 10.1001/jama.2020.17101. [DOI] [PubMed] [Google Scholar]
- 109.Temple R., Ellenberg S.S. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann. Intern. Med. 2000;133:455–463. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 110.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 111.Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., van Bentum-Puijk W., Berry L., Bhimani Z., Bonten M. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.2220.23034. [DOI] [PubMed] [Google Scholar]
- 113.Song Y., Zhang M., Yin L., Wang K., Zhou Y., Zhou M., Lu Y. COVID-19 treatment: close to a cure?–a rapid review of pharmacotherapies for the novel coronavirus. Int. J. Antimicrob. Agents. 2020:106080. doi: 10.101016/j.ijantimicag.102020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspective. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.102020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.22222020.21729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H., Du Q. 2020. Potential Natural Compounds for Preventing SARS-CoV-2 (2019-nCoV) Infection. Preprints. [DOI] [Google Scholar]
- 117.Xie M., Chen Q. Insight into 2019 novel coronavirus—an updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.071.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cha Y., Erez T., Reynolds I., Kumar D., Ross J., Koytiger G., Kusko R., Zeskind B., Risso S., Kagan E. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018;175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.1168. [DOI] [PubMed] [Google Scholar]
- 120.Pillaiyar T., Meenakshisundaram S., Manickam M., Sankaranarayanan M. A medicinal chemistry perspective of drug repositioning: recent advances and challenges in drug discovery. Eur. J. Med. Chem. 2020 doi: 10.111016/j.ejmech.112020.112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov. Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. https://www.sciencedirect.com/science/article/abs/pii/S1359644618302599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talevi A., Bellera C.L. Taylor & Francis; 2020. Challenges and Opportunities With Drug Repurposing: Finding Strategies to Find Alternative Uses of Therapeutics. [DOI] [PubMed] [Google Scholar]
- 123.Kandimalla R., John A., Abburi C., Vallamkondu J., Reddy P.H. Current status of multiple drug molecules, and vaccines: an update in SARS-CoV-2 therapeutics. Mol. Neurobiol. 2020:1–11. doi: 10.1007/s12035-020-02022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]