Abstract
The health of the individual and the population in general is the result of interaction between genetics and various environmental factors, of which diet/nutrition is the most important. The focus of this paper is on the association of high n-6 PUFA or low n-3 PUFA due to genetic variation and/or dietary intake, with changes in specialized pro-resolving mediators (SPMs), cytokine storm, inflammation-resolution and Covid-19. Human beings evolved on a diet that was balanced in the n-6 and n-3 essential fatty acids with a ratio of n-6/n-3 of 1–2/1 whereas today this ratio is 16/1. Such a high ratio due to high amounts of n-6 fatty acids leads to a prothrombotic and proinflammatory state and is associated with obesity, diabetes, cardiovascular disease, and some forms of cancer. In addition to the high intake of n-6 fatty acids that increases inflammation there is genetic variation in the biosynthesis of n-6 linoleic acid (LA) to arachidonic acid (ARA) and of linolenic (ALA) to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Present day humans have two common FADS haplotypes that differ dramatically in their ability to generate long-chain fatty acids. The more efficient, evolutionary derived haplotype increases the efficiency of synthesizing essential long-chain fatty acids from precursors and could have provided an advantage in environments with limited access to dietary long-chain fatty acids ARA, EPA and DHA. In the modern world this haplotype has been associated with lifestyle-related diseases, such as cardiovascular disease, obesity, diabetes, all of which are characterized by increased levels of inflammation. African Americans and Latino populations have increased susceptibility and higher death rates from SARS-CoV-2 than whites. These populations are characterized by increased numbers of persons (about 80%) that are fast metabolizers, leading to increased production of ARA, as well as poor intake of fruits and vegetables. The combinations of fast metabolism and high n-6 intake increases their inflammatory status and possibly susceptibility of SARS-CoV-2. In vitro and human studies indicate that the specialized pro-resolving mediators (SPM) produced from the n-3, EPA and DHA influence the resolution of inflammation, allowing the tissues to return to function and homeostasis. The SPMs each counter-regulate cytokine storms, as well as proinflammatory lipid mediators via NFκB and inflammasome down regulation and reduce the proinflammatory eicosanoids produced from ARA. The nutritional availability of dietary n-3 fatty acids from marine oils enriched with SPM intermediate precursors, along with increasing local biosynthesis of SPMs to functional concentrations may be an approach of value during SARS-CoV2 infections, as well as in prevention, and shortening their recovery from infections. It is evident that populations differ in their genetic variants and their frequencies and their interactions with the food they eat. Gene-nutrient interactions is a very important area of study that provides specific dietary advice for individuals and subgroups within a population in the form of Precision Nutrition. Nutritional science needs to focus on Precision Nutrition, genetic variants in the population and a food supply composed of Nutrients that have been part of our diet throughout evolution, which is the diet that our genes are programmed to respond.
Keywords: Inflammation, Nutrition, Essential fatty acids, Resolvins, Eicosanoids, Pro-resolving mediators
Poor Diet is one of the leading contributing factors for death in the US and worldwide (GBD 2017 Diet Collaborators, 2019). Unhealthy diets, characterized by over consumption of ultra-processed foods with an n-6 polyunsaturated (PUFA)/n-3 PUFA ratio of 11:1 are associated with increased weight gain and processed foods, and sugary drinks increase the risk of obesity, type 2 Diabetes, high blood pressure and heart disease (Hall et al., 2019).
Beyond individual deaths, the pattern of mortality among population groups can reveal both those at higher risk and the groups who bear a disproportionate burden of the pandemic. The expression of disease in patients with Covid-19 varies from asymptomatic to fatal with more severe outcomes for individuals with advancing age. In the US, Covid-19 has affected disproportionately African Americans, Hispanics, American Indians and Alaska Native persons (Centers for Disease Control and Prevention, 2020). Fineberg states, “When a pandemic reaches the health, social, and economic scale of COVID-19, regardless of the precise number of deaths that have occurred by a certain date, an intense, persistent, multipronged, and coherent response must be the order of the day and an urgent priority for the nation” (Fineberg, 2020). There have been many papers on the cause/relationship of Covid-19 and the populations involved. A significant number of these papers have been published. The fact that certain groups --- African Americans, Hispanics, Latins, American Indians, and Alaskan Natives have been infected and have succumbed to the coronavirus has led to the consideration of various reasons for health disparities such as social, economic, healthcare delivery issues, etc. What is missing specifically are papers that focus on the genetic variation of these populations and the interaction of their genes with dietary components, that have not been part of our diet during evolution, but today occur in high amounts such as the n-6 PUFA, or low amounts such as the n-3 PUFA, as well as lacking adequate amounts of fruits and vegetables, both of which are needed for proper absorption of n-3 PUFA (O’Sullivan et al., 2014). These dietary patterns lead to a high n-6/n-3 ratio and to a proinflammatory and prothrombotic state, as well as increases in fat cell size and number increasing the risk of obesity, type 2 diabetes, hypertension and heart disease (Simopoulos, 2010), which increase vulnerability to Covid-19.
The focus of this paper is on the association of high n-6 PUFA or low n-3 PUFA due to genetic variation and/or dietary intake, with changes in specialized pro-resolving mediators (SPMs), cytokine storm, inflammation-resolution and Covid-19.
1. Evolutionary aspects of diet and n-6 PUFA and n-3 PUFA biosynthesis
The rapid nutritional changes that have taken place in developed countries after World War 2 has led to maladaptations and related human diseases never before seen in such frequencies over a short period of time (Chilton et al., 2014; Cordain et al., 2005). In fact, up to 72% of dietary calories consumed in the present Western Diet did not exist in hunter-gatherer diets (Cordain et al., 2005). These changes were driven by technological changes in food production and processing that provide high calories and refined grains. Chilton et al., 2014, Chilton et al., 2017 investigated how our current n-6 and n-3 PUFA intake interacts with our ancestral based genetic variation in the PUFA biosynthetic pathway giving rise to different PUFA levels and distinct molecular profiles that enhance disease risk for certain individuals and populations. Ameur and colleagues described the FADS haplotype patterns (ancestral and derived) with the derived haplotype found in Africa that were associated with more efficient conversion of 18 carbon PUFAs into long chain (LC)-PUFAs (Ameur et al., 2012).
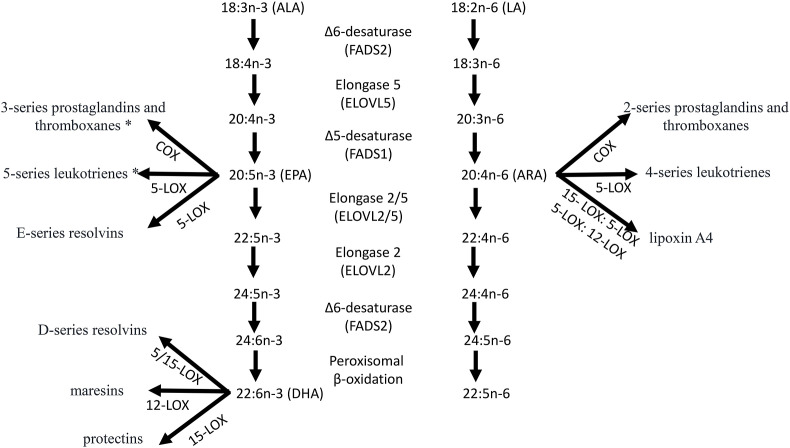
Human beings evolved on a diet that was relatively balanced in linoleic acid (18:2n-6; LA) n-6 PUFA and alpha-linolenic acid (18:3n-3; ALA) n-3 PUFA (Simopoulos, 1991). Both LA and ALA are found in similar amounts in nature (Simopoulos, 1991). Humans obtained LA from seeds and nuts with the exception of flax seeds, rapeseed, chia and perilla that are rich in ALA. ALA is also plentiful in green leafy vegetables (Simopoulos et al., 1992). Both LA and ALA are essential for health, cannot be made by humans, and must be obtained from diet. LA and ALA are the parent PUFA that in the body are metabolized to arachidonic acid (20:4n-6; ARA) or eicosapentaenoic acid (20:5n-3; EPA) and docosahexaenoic acid (22:6n-3; DHA) respectively (Fig. 1 ). ARA, EPA and DHA are precursors found in tissues of all organs in the body, that are mobilized, and enzymatically converted to potent local chemical signals of 20 carbon atoms (eicosanoids), involved in many physiologic and pathologic processes in humans. The eicosanoids biosynthesized from ARA include prostaglandins, thromboxanes, leukotrienes, as well as lipoxins. Prostaglandins, thromboxanes and leukotrienes are also produced from EPA that in general display diminished activities. EPA and DHA are each biosynthetic precursors that are enzymatically converted in inflammatory milieu to novel lipid mediator autacoids, termed resolvins and protectins/neuroprotectins (Fig. 1) (Serhan and Levy, 2018). ARA is found in meat, eggs and dairy products, whereas EPA and DHA are enriched in fish and marine oils (Simopoulos, 1991).
Fig. 1.
Alpha-linolenic acid (18:3n-3; ALA) is desaturated and elongated to eicosapentaenoic acid (20:5n-3; EPA) and docosahexaenoic acid (22:6n-3; DHA) while the n-6 polyunsaturated fatty acid linoleic acid (18:2n-6) produces arachidonic acid (20:4n-6; ARA). Importantly not only do ALA and LA compete for the same enzymes, but recent work demonstrates that genetic differences in the FADS genes regulates activity and tissue PUFA levels. ARA is the precursor to the prostaglandins, thromboxanes and leukotrienes, and lipoxins, while EPA * produces prostaglandins, thromboxanes and leukotrienes with diminished activity relative to those from ARA. EPA and DHA are also precursors to the specialized proresolving mediators (SPMs); see text for more details.
LA and ALA and their desaturation and elongation products use the same enzyme systems for their metabolism, have opposing properties, and are metabolically and physiologically distinct. The ALA pathway is the preferred pathway and up until the 1960's there was a balance in the amounts of LA and ALA that humans obtained from their diet. But things began to change in the 1960's after Keys did not consider that the diet of Crete was balanced in n-6 and n-3 PUFA in the Seven Countries Study for the prevention of heart disease (Keys, 1970; Simopoulos, 2001). Soon after the American Heart Association (AHA) declared cholesterol levels must be lowered to prevent heart disease, and the whole country was exposed to an enormous campaign to replace saturated fat with vegetable oils rich in LA such as corn, sunflower, safflower, soybean, and cottonseed to lower cholesterol. Almost overnight the amounts of vegetable oils and trans fats high in n-6 PUFA replaced butter despite the fact that there was no evidence from randomized controlled trials demonstrating that LA prevented heart disease (Bazinet and Chu, 2014; Ramsden et al., 2016). In fact, Ramsden et al. showed that LA will lower cholesterol by 30 mg/dl but will increase cardiovascular disease death by +22% (Ramsden et al., 2016). This dietary advice continues despite the increase in chronic diseases – obesity, type 2 diabetes, hypertension, and cardiovascular disease, all of which lead to a pro-inflammatory state due to high amounts of LA and ARA and their metabolites. Activated cells release ARA from cellular stores that is converted to high amounts of prostaglandins, leukotrienes notable pro-inflammatory mediators that amplify inflammation, with the exception of Lipoxins that are pro-resolving and anti-inflammatory, providing an environment enabling SARS-CoV-2 to thrive. Ultra-processed diets poor in fruits and vegetables and fish are the main foods available to poor neighborhoods of people of color (food deserts). EPA and DHA derived products are less thrombotic and less inflammatory than those produced from ARA. EPA and DHA are precursors to potent new autacoids that are anti-inflammatory as well as pro-resolving and participate in the resolution of inflammation and infections preventing the development of full-blown uncontrolled inflammation (Fig. 1).
1.1. Controlling infectious inflammation and its resolution
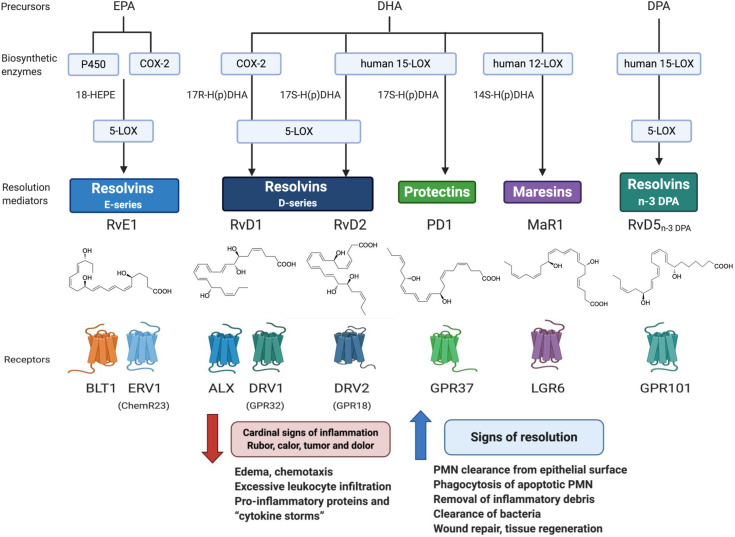
The acute inflammatory response is protective aimed toward neutralizing invading microbes on barrier breaks. The host also mounts this primordial response on injury from within and surgery-induced organ injury. When uncontrolled, excessive inflammation contributes to many widely occurring diseases in all organs of the body e.g., neurodegenerative diseases, cardiovascular diseases, diabetes to name just a few as well as to aging and cancer. This acute response is ideally self-limited, meaning that it resolves on its own, a design that prevents us from melting down on each minor injury or microbial invasion. The acute inflammatory response is classically divided into two phases, initiation and resolution (Delano and Ward, 2016). In this initiation phase of the acute response, n-6 ARA is mobilized to enzymatically produce prostaglandins and leukotrienes by leukocytes, platelets and surrounding damaged tissues (Samuelsson, 2012). These local chemical signals evoke the well-known cardinal signs of inflammation heat, redness, swelling and pain and when uncontrolled these mediators amplify inflammation that can lead to chronic inflammation or abscess formation. The n-3 PUFA, namely EPA, DPA and DHA, are enzymatically transformed during the resolution phase in self-limited sites of inflammation (i.e., pus) into potent local chemical signals. These novel signals are mediators that locally control the inflammatory response by limiting its duration, and magnitude. This is achieved by the ability of these novel mediators to a) limit the further tissue infiltration of neutrophils, e.g. while host protective, when in excess within tissues, neutrophils can contribute to collateral tissue damage and b) by enhancing clearance of apoptotic cells, debris and microbes from the site(s) of inflammation enabling the tissue to return to function and homeostasis (Serhan, 2014). These are the cellular signs of resolution governed by the n-3 PUFA chemical mediator superfamily comprised of the resolvin, protectin and maresin families that together are collectively coined specialized proresolving mediators or SPM. Their precise individual stereochemistry is critical in stimulating cellular functions via specific cell surface receptors (Serhan, 2014; Chiang and Serhan, 2020) that stimulate resolution and protect organs (Fig. 2 ). This precise stereochemistry of the SPMs arises from the chemistry inherited from their n-3 PUFA precursors present in our diets. The SPMs each counter-regulate cytokine storms as well as proinflammatory lipid mediators via NFkB and inflammasome down-regulation (Serhan, 2014; Chiang and Serhan, 2020) and unlike anti-inflammatory agents that eventually become immunosuppressive, SPMs control both the killing and clearance of microbes.
Fig. 2.
Pro-Resolving Mediators Network: Biosynthesis, Receptors and Functions. The SPMs depicted are biosynthesized from EPA, n-3 DPA and DHA by human leukocytes (see text for further details and reviewed in (Serhan, 2014; Serhan and Levy, 2018)\). The E series resolvins are produced from EPA and D-series from DHA. Both the protectin family and maresin family are also biosynthesized from DHA. The complete stereochemistry and pro-resolving actions of each SPM are established and confirmed by total organic synthesis and commercially available for research. These include SPMs biosynthesized from n-3 DPA. The known enzymes depicted here, and receptors are reviewed in (Chiang and Serhan, 2020) and the original citations within not cited here for brevity. Each of the SPMs reduce and counter-regulate the Cardinal signs of inflammation and stimulate as agonists the major signs of resolution by definition, thus serving as immunoresolvents (Serhan, 2014).
In SARS-CoV-2 activated human macrophages, Resolvin D1 and Resolvin D2 each dramatically reduce the cytokine storm diminishing the levels of key pro-inflammatory cytokines including TNF-α, IL-8 and MIP-1 (Recchiuti et al., 2020). SPMs also reduce eicosanoids (Samuelsson, 2012) that amplify and are mediators of inflammation, namely prostaglandins and leukotrienes from ARA (Serhan, 2014; Chiang and Serhan, 2020) that are each present in peripheral blood of Covid-19 patients along with resolvins that dynamically change with disease severity (Schwarz et al., 2020). The lung is a major organ where cytokine and eicosanoid storms contribute to the Covid-19 host evoked response that increases the likelihood of a poor outcome for many of these patients. Bronchoalveolar lavages from severe Covid-19 patients show high amounts of ARA produced pro-inflammatory and pro-thrombotic eicosanoids such as thromboxane metabolite (TXB2), prostaglandin E2 as well as the potent leukocyte chemoattractant leukotriene B4 (Samuelsson, 2012) compared to healthy subjects indicating their origins in lung tissues of these Covid-19 patients marking the dynamic pro-coagulant and inflammatory environment within the lungs of patients with severe Covid-19 (Archambault et al., 2020). Also present in these lavages from severe Covid-19 patients were reported high amounts of pro-resolving mediators, namely, SPMs identified using tandem mass spectroscopy (LC-MS/MS) interrogated lung lavage samples. These lavages demonstrated the presence of lipoxin A4 and many of the resolvins of the D-series notably resolvin D1, resolvin D2, resolvin D4, resolvin D5 and the precursor intermediates, 18-HEPE and 17HDHA that are also known to be bioactive whereas other SPMs, e.g., resolvin E1 and maresins, were absent in these lavage samples. The presence of proinflammatory eicosanoids and specifically resolvins and other SPMs in lung lavages at biologically active concentrations from severe Covid-19 patients suggests these mediators may have functional roles in the lipid mediator storm of SARS-CoV-2 infections (Archambault et al., 2020).
In a recent randomized double-blind placebo-controlled study in healthy volunteers, oral administration of marine oil enriched with 17-HDHA,18-HEPE and 14-HDHA showed a time- and dose-dependent increase in peripheral blood SPMs likely from endogenous enzymatic conversion of these precursors (Fig. 2) (Souza et al., 2020). This increase in SPM correlates with rapid reprograming of blood cells that include rapid changes in the leukocyte transcriptome and increased bacterial phagocytosis (Souza et al., 2020). These changes were apparent within hours, increasing SPMs that in turn enhance both neutrophil and monocyte ability to phagocytose E. coli as well as regulate leukocyte transcripts of interest in infections. Hence, the nutritional availability of dietary n-3 PUFA and marine oils enriched with the SPM intermediate precursors (e.g., 17-HDHA, 18HEPE), along with increasing local biosynthesis of SPMs (Pal et al., 2020) to functional concentrations may be an approach of value during Covid-19 as well as in prevention and shortening their recovery phase from infections.
1.2. Genetic variation: inflammation and increase susceptibility to Covid-19
So far, we focused on the development of the proinflammatory state as a result of the dietary change that led to high LA and ARA intake in the absence of scientific data. There is however another very important factor that exacerbates the inflammatory state created by the high LA and ARA diet. It is the genetic variants that are found in higher frequency in African Americans and Hispanics. The fatty acid desaturase (FADS1 and FADS2 located at chromosome 12.2–13.1) and elongase genes (ELOVL2 at chromosome 6p24.2 and ELOVL5 located at chromosome 6p12.1) mediate the endogenous biosynthesis of ARA, EPA, and DHA. Ameur and colleagues investigated the importance of Genetic variability in PUFA biosynthesis (Ameur et al., 2012). They studied FADS1 and FADS2, which encode rate-limiting enzymes for PUFA conversion from 18 carbon PUFA to LC-PUFA. They performed genome-wide genotyping in various populations of the FADS region in 5 European population cohorts. In addition, they analyzed available genomic data from other human populations, archaic, hominids and more distant primates. Their results show that present-day humans have two common FADS haplotypes-defined by 28 closely linked SNPs across 38.9 kB that differ in their ability to generate LC-PUFA.
The ancestral haplotype (A) is characterized by a slow biosynthetic pathway whereas the derived (D) has a fast or rapid biosynthetic pathway and is specific to humans. This haplotype shows evidence of positive selection in African populations, in which it is presently almost fixed. This haplotype is less frequent outside of Africa. Ameur et al. proposed that “the haplotype that provides a more efficient biosynthesis of LC-PUFA might act as a thrifty genotype and represents a risk factor for lifestyle-related diseases such as coronary heart disease.” Haplotype D increases the efficiency of synthesizing essential LC-PUFA from precursors and thereby might have provided an advantage in environments with limited access to dietary LC-PUFAs. In the modern world, this haplotype has been associated with lifestyle-related diseases, such as coronary heart disease” (Ameur et al., 2012).
Ameur et al. studied the frequencies of haplotypes A and D in native populations distributed all over the world. The most common haplotype D was associated with high lipid levels, whereas the less common haplotype A was associated with low levels. Persons homozygotes for haplotype D had 24% higher levels of DHA and 43% higher levels of ARA, than those homozygous for haplotype A. The two FADs haplotypes differ both in transcription levels and in their ability to synthesize ARA and DHA from their precursor (Ameur et al., 2012).
In the African Human Genome Diversity Project (HGDP) population, haplotype A is essentially absent (less than 1% of the chromosomes, whereas in Europe, West, South, and East Asia, and Oceania, it occurs at a frequency of 25%–50%. Among the 126 Native Americans included in HGDP, haplotype A accounts for 97% of the chromosomes. A complementary analysis of haplotype frequencies for population samples from HapMap (Altshuler et al., 2010) and the 1000 Genomes Project (Durbin et al., 2010) confirmed that haplotype A occurs at a very low frequency among individuals of African descent, whereas it is present at moderate high frequencies in populations of European and Asian ancestry. Among individuals of African ancestry, 49% carry mixed FADS haplotypes with a higher resemblance to haplotype D than to haplotype A, consistent with a decay of haplotype D by recombination in African populations. The FADS region is among the top five candidate-gene clusters that have been under positive selection in African populations (Pickrell et al., 2009).
Individuals with haplotype D have a rapid biosynthesis from LA to ARA and therefore have higher amounts of ARA circulating in the blood, far and above the amounts expected from the high intake of LA in the diet. During evolution, as the populations in Africa moved from marine environments to inland areas, the gene with the rapid biosynthesis was essential to obtain DHA from ALA for brain development. This same gene is found in 80% of African American ancestry and in about 43% of Europeans. It's the higher frequency of genes and the current US diet high in LA that makes African Americans and Hispanics to become susceptible to obesity, type 2 diabetes, high blood pressure, and heart disease – all of which have inflammation as a basis for their development. It's possible that the combination of an ultra-processed Western type of diet plus the higher frequency of haplotype D that magnifies vulnerability of African Americans and Hispanics to Covid-19. In Africa there are fewer deaths due to Covid-19 despite having the same genetic variants possibly because their diet is not as high in n-6 PUFA and ultra-processed foods (Nordling, 2020).
There are also other populations that carry genes that have lower biosynthesis of ARA from LA or EPA and DHA from ALA, such as American Natives. These populations have high LA and ARA because of the US diet and are relatively low in ALA and DHA. The Indigenous people of Arctic regions are another group, but they eat a lot of fish and thus do not become deficient in n-3 PUFA (Fumagalli et al., 2015).
1.3. The need for precision nutrition
It is evident that populations differ in their genetic variants and their frequencies and in their interactions with the food they eat. Gene/Nutrient interactions is a very important area of study that provides specific dietary advice for individuals and subgroups within a population in the form of Precision Nutrition. The current recommendation of the American Heart Association to increase LA intake to 10% of energy could be detrimental for those in the US population that are fast metabolizers, specifically African Americans and Hispanics. Under natural conditions of dietary intake of no processed foods and a balanced n-6/n-3 ratio, the fast metabolizers will do well because they start their metabolism with balanced n-6 and n-3 PUFA. In a “melting pot” type of population like the US, what the AHA recommends might actually be harmful. Importantly, new research is also identifying enzymatic metabolites from LA that may regulate nociception (Domenichiello et al., 2020), and initial clinical trials testing if lowering dietary LA can alter nociceptive lipid mediators in a manner that decreases headache pain are underway (Mann et al., 2018). There is no scientific basis or evidence for the AHA statement to increase LA to 10% of energy for all Americans.
Nutritional Science needs to focus on Precision Nutrition, genetic variants in the population and a food supply composed of Nutrients that have been part of our diet throughout evolution, which is the diet that our genes are programmed to respond. In today's diet 72% of calories come from foods that were not present during evolution (Cordain et al., 2005). In the meantime, physicians should consider measuring PUFAs and their potent cellular effectors, both the eicosanoids and SPMs, in patients with Covid-19 and in patients with chronic diseases as in refs (Archambault et al., 2020; Souza et al., 2020; Pal et al., 2020; Durbin et al., 2010), specifically obesity, type 2 diabetes, hypertension and CHD by measuring LA, ALA, and the LA/ALA ratio, ARA + EPA + DHA and ARA/EPA + DHA ratio in red blood cell membrane phospholipids (RBCs) and aim for balance in the dietary intake of n-6 and n-3 PUFA. FDA should not continue to list PUFA on the food label and should replace the term “PUFA” with actual amounts of the individual molecules, namely LA, ALA, ARA, EPA and DHA, and eventually industry should adopt an n-6/n-3 “ratio” per serving in all processed foods (Hall and Grummon, 2020).
To put precision nutrition into practice, targeted LC-MS/MS-based lipidomic profiling of fatty acids and SPMs combined with genetic variation and markers of inflammation would identify candidate targets for intervention. Interventions, especially dietary, should be monitored to confirm that both substrates and SPMs reach functional concentrations and could be considered in the management of patients with Covid-19, as well as in preventing and shortening their recovery phase from infections.
In terms of Research priorities, the emphasis should be in measuring validated biomarkers and understanding the mechanisms and the effects/impact of gene-nutrient interactions in growth and development and in the prevention and management of diseases. Precision Nutrition must be the driving force in making dietary recommendations.
Declaration of competing interest
The authors report no Conflict of Interest disclosures.
Acknowledgments
We thank Dr. Nan Chiang of the CETRI at BWH-Harvard Medical School for help in preparing Fig. 2. We would also like to thank Mary A. Baez of the Center for Genetics, Nutrition and Health for the completion and typing of the manuscript and researching the references. We also acknowledge the following support: CNS's research is supported by the National Institutes of Health grants program project P01GM095467 and R01GM038765. RPB acknowledges funding from the Canadian Institutes of Health Research and RPB holds the Canada Research Chair in Brain Lipid Metabolism.
References
- Altshuler D.M., Gibbs R.A., Peltonen L., Altshuler D.M., Gibbs R.A., Peltonen L., Dermitzakis E., Schaffner S.F., Yu F., Peltonen L. International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameur A., Enroth S., Johansson A., Zaboli G., Igl W., Johansson A.C., Rivas M.A., Daly M.J., Schmitz G., Hicks A.A., Meitinger T., Feuk L., van Dujin C., Oostra B., Pramstaller P.P., Rudan I., Wright A.F., Wilson J.F., Campbell H., Gyllensten U. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012;90(5):809–820. doi: 10.1016/j.ajhg.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault A.S., Zaid Y., Rakotoarivelo V., Doré É., Dubuc I., Martin C., Amar Y., Cheikh A., Fares H., El Hassani A., Tijani Y., Laviolette M., Boilard É., Flamand L., Flamand N. Lipid Storm within the Lungs of Severe COVID-19 Patients: Extensive Levels of Cyclooxygenase and Lipoxygenase-Derived Inflammatory Metabolites. medRxiv. 2020 12.04.20242115. [Google Scholar]
- Bazinet R.P., Chu M.W. Omega-6 polyunsaturated fatty acids: is a broad cholesterol-lowering health claim appropriate? CMAJ (Can. Med. Assoc. J.) 2014;186(6):434–439. doi: 10.1503/cmaj.130253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention COVID-19 hospitalization and death by race/ethnicity. 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html Accessed September 22.
- Chiang N., Serhan C.N. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443–462. doi: 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton F.H., Dutta R., Reynolds L.M., Sergeant S., Mathias R.A., Seeds M.C. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients. 2017;9(11):1165. doi: 10.3390/nu9111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton F.H., Murphy R.C., Wilson B.A., Sergeant S., Ainsworth H., Seeds M.C., Mathias R.A. Diet-gene interactions and PUFA metabolism: a potential contributor to health disparities and human diseases. Nutrients. 2014;6(5):1993–2022. doi: 10.3390/nu6051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O'Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- Delano M.J., Ward P.A. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016;274(1):330–353. doi: 10.1111/imr.12499. PMID: 27782333; PMCID: PMC5111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenichiello A.F., Sapio M.R., Loydpierson A.J., Maric D., Goto T., Horowitz M.S., Keyes G.S., Yuan Z.X., Majchrzak-Hong S.F., Mannes A.J., Iadarola M.J., Ramsden C.E. Molecular pathways linking oxylipins to nociception in rats. J. Pain. 2020;20:30077–30078. doi: 10.1016/j.jpain.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin R.M., Abecasis G.R., Altshuler D.L., Auton A., Brooks L.D., Gibbs R.A., Hurles M.E., McVean G.A. 1000 Genomes Project Consortium A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg H.V. The toll of Covid-19. J. Am. Med. Assoc. 2020;324(15):1502–1503. doi: 10.1001/jama.2020.20019. [DOI] [PubMed] [Google Scholar]
- Fumagalli M., Moltke I., Grarup N., Racimo F., Bjerregaard P., Jørgensen M.E., Korneliussen T.S., Gerbault P., Skotte L., Linneberg A., Christensen C., Brandslund I., Jorgensen T., Huerta-Sánchez E., Schmidt E.B., Pedersen O., Hansen T., Albrechtsen A., Nielsen R. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–1347. doi: 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., Chung S.T., Costa E., Courville A., Darcey V., Fletcher L.A., Forde C.G., Gharib A.M., Guo J., Howard R., Joseph P.V., McGehee S., Ouwerkerk R., Raisinger K., Rozga I., Stagliano M., Walter M., Walter P.J., Yang S., Zhou M. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metabol. 2019;30(1):226. doi: 10.1016/j.cmet.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.G., Grummon A.H., et al. Nutrient warnings on unhealthy foods. J. Am. Med. Assoc. 2020 Oct 1 doi: 10.1001/jama.2020.18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A. Coronary heart disease in seven countries. Circulation. 1970;41(Suppl. l):1–211. [PubMed] [Google Scholar]
- Mann J.D., Faurot K.R., MacIntosh B., Palsson O.S., Suchindran C.M., Gaylord S.A., Lynch C., Johnston A., Maiden K., Barrow D.A., Hibblen J.R., Ramsden C.E. A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA+DHA in adults with episodic migraine: study protocol. Prostaglandins Leukot. Essent. Fatty Acids. 2018;128:41–52. doi: 10.1016/j.plefa.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordling L. Africa’s pandemic puzzle: why so few cases and deaths? Science. 2020;369(6505):756–757. doi: 10.1126/science.369.6505.756. [DOI] [PubMed] [Google Scholar]
- O’Sullivan A., Armstrong P., Schuster G.U., Pedersen T.L., Allayee H., Stephensen C.B., Newman J.W. Habitual diets rich in dark-green vegetables are associated with an increased response to omega-3 fatty acid supplementation in Americans of African Ancestry. J. Nutr. 2014;144(2):123–131. doi: 10.3945/jn.113.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Gowdy K.M., Oestreich K.J., Beck M., Shaikh S.R. Obesity-driven deficiencies of specialized pro-resolving mediators may drive adverse outcomes during SARS-CoV-2 infection. Front. Immunol. 2020;11:1997. doi: 10.3389/fimmu.2020.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell J.K., Coop G., Novembre J., Kudaravalli S., Li J.Z., Absher D., Srinivasan B.S., Barsh G.S., Myers R.M., Feldman M.W., Pritchard J.K. Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 2009;19:826–837. doi: 10.1101/gr.087577.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden C.E., Zamora D., Majchrzak-Hong S., Faurot K.R., Broste S.K., Frantz R.P., Davis J.M., Ringel A., Suchindran C.M., Hibbeln J.R. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73) BMJ. 2016;353:i1246. doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A., Patruno S., Mattoscio D., Isopi E., Pomilio A., Lamolinara A., Iezzi M., Pecce R., Romano M. Resolvin D1 and D2 reduce SARS-Cov-2-induced inflammation in cystic fibrosis macrophages. bioRxiv. 2020 doi: 10.1101/2020.08.28.255463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem. 2012;287(13):10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B., Sharma L., Roberts L., Peng X., Bermejo S., Leighton I., Casanovas-Massana A., Minasyan M., Farhadian S., Ko A.I., Yale IMPACT Team, Dela Cruz C.S., Bosio C.M. Cutting edge: severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome resulting in dysregulation of eicosanoid immune mediators. Preprint. Res Sq. 2020;3 doi: 10.21203/rs.3.rs-42999/v1. rs-42999. Published 2020 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001;131:3065S–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. Genetic variants in the metabolism of omega-6 and omega-3fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P., Norman H.A., Gillaspy J.E., Duke J.A. Common purslane: a source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr. 1992;11(4):374–382. doi: 10.1080/07315724.1992.10718240. [DOI] [PubMed] [Google Scholar]
- Souza P.R., Marques R.M., Gomez E.A., Colas R.A., De Matteis R., Zak A., Patel M., Collier D.J., Dalli J. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ. Res. 2020;126(1):75–79. doi: 10.1161/CIRCRESAHA.119.315506. [DOI] [PubMed] [Google Scholar]