Abstract
Fecal incontinence (FI) is defined as involuntary or uncontrollable loss of feces. Gas incontinence is defined as involuntary or uncontrollable loss of flatus, while anal incontinence is defined as the involuntary loss of feces or flatus. The prevalence of FI in people over 65 in Japan is 8.7% in the male population and 6.6% among females. The etiology of FI is usually not limited to one specific cause, with risk factors for FI including physiological factors, such as age and gender; comorbidities, such as diabetes and irritable bowel syndrome; and obstetric factors, such as multiple deliveries, home delivery, first vaginal delivery, and forceps delivery. In the initial clinical evaluation of FI, the factors responsible for individual symptoms are gathered from the history and examination of the anorectal region. The evaluation is the basis of all medical treatments for FI, including initial treatment, and also serves as a baseline for deciding the need for a specialized defecation function test and selecting treatment in stages. Following the general physical examination, together with history taking, inspection (including anoscope), and palpation (including digital anorectal and vaginal examination) of the anorectal area, clinicians can focus on the causes of FI. For the clinical evaluation of FI, it is useful to use Patient-Reported Outcome Measures (PROMs), such as scores and questionnaires, to evaluate the symptomatic severity of FI and its influence over quality of life (QoL).
Keywords: fecal incontinence, guideline, defecation disorders, anal incontinence, Japanese practice guideline
Introduction
Fecal incontinence (FI) is a defecation disorder which disturbs daily quality of life. With increased clinical practice and demand for standardization, the Japan Society of Coloproctology decided to prepare practice guidelines for FI as there is no established research base of general practices in Japan. The Guideline Preparation Committee was composed of Society members in Japan who were chosen from the experts in this field.
These guidelines were prepared not only for specialists who treat patients with FI, but also for general physicians, surgeons, and nurses. The aims of these practice guidelines are to accomplish the following: 1) to understand concepts, pathophysiology and causes, diagnosis, and comprehensive treatments for FI; 2) to promote the safety and efficacy of treatments; 3) to reduce human and economic burdens of FI in practice; and 4) to create mutual understanding between medical providers and patients.
These guidelines contain many items and much volume; therefore, we decided to report them in three parts: Part 1: Definition, Epidemiology, Etiology, Pathophysiology and Causes, Risk factors, Clinical Evaluations, and symptomatic scores and QoL questionnaire for Clinical Evaluations; Part 2: Examination and Conservative Treatment; and Part 3: Surgical Treatment and Fecal Incontinence under Special Conditions. In this Part 1 issue, Definition, Epidemiology, Etiology, Pathophysiology and Causes, Risk factors, Clinical Evaluations, and symptomatic scores and QoL questionnaire for Clinical Evaluations are described.
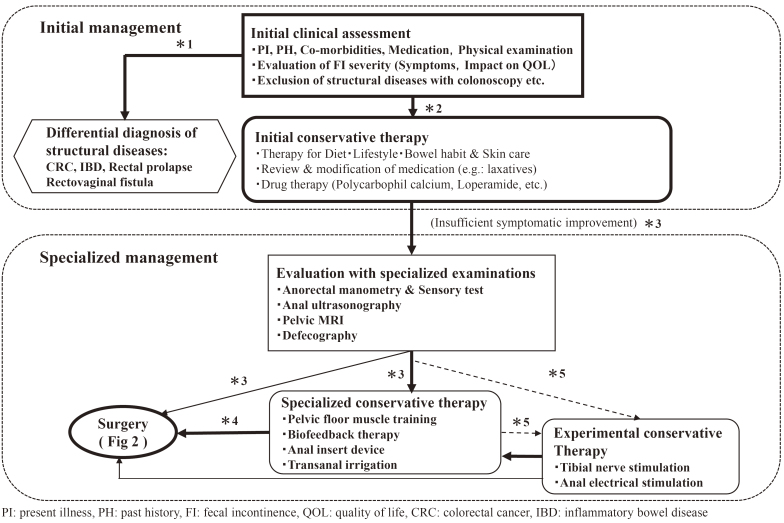
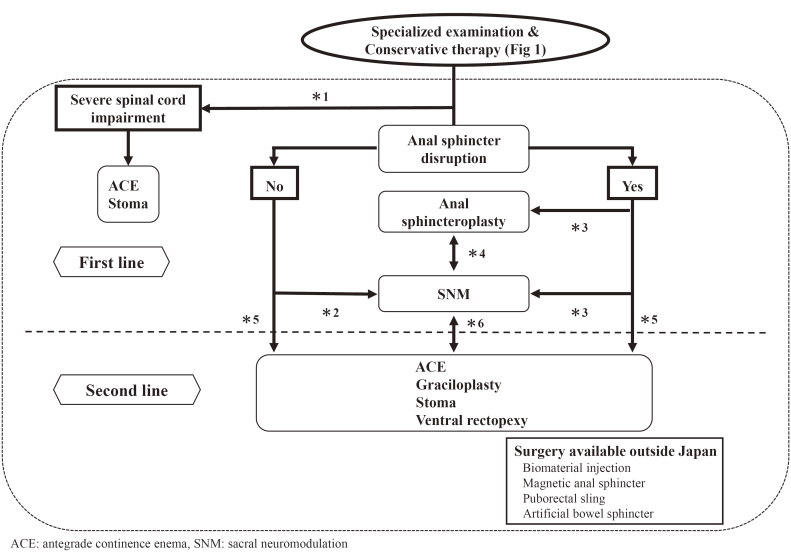
The Fecal Incontinence Guideline Preparation Committee proposed two algorithms to simplify the understanding of practice flow: an algorithm for primary and specialist practices (Figure 1), and an algorithm for surgical practice (Figure 2).
Figure 1.
Algorithm for the Management of Fecal Incontinence. Algorithm of the Initial Management and Specialized Examination & Conservative Therapy for Fecal Incontinence.
*1 If patients with fecal incontinence (FI) have some alarm signs on initial clinical assessment, including blood stool, recent changes of bowel habits, unexpected body weight loss, and palpable abdominal and/or rectal tumor, structural diseases should be differentiated with colonoscopy etc. Colonoscopy is also recommended if patients aged 50 years or over have never undergone it withing the last 3 years.
*2 If the examinations such as colonoscopy reveal some structural diseases including colorectal cancer, inflammatory bowel disease, rectal prolapse and rectovaginal fistula, they should be treated at first. Otherwise, patients with FI are to be treated with initial conservative therapies.
*3 If sufficient symptomatic improvement is not achieved with the initial conservative therapies, specialized examinations are to be performed, followed by specialized conservative therapies and/or surgery.
The bold line, thin line and broken line mean that it has higher recommendation in this order.
*4 If sufficient symptomatic improvement is not achieved with the specialized conservative therapies, surgery is to be considered.
*5 Tibial nerve stimulation and anal electrical stimulation may be performed as experimental therapies only in clinical trials.
Figure 2.
Algorithm for the Management of Fecal Incontinence. Algorithm of Surgery for Fecal Incontinence.
*1 Antegrade continence enema or stoma is to be considered if fecal incontinence (FI) is caused by sever spinal cord impairment.
*2 Sacral neuromodulation is the first line surgical therapy for FI if it is not caused by anal sphincter disruption.
*3 If FI is mainly caused by anal sphincter disruption, either anal sphincteroplasty or sacral neuromodulation is to be performed.
Its decision is to be made after full discussion with patients with FI, referring to the Clinical Question 3.
*4 If sufficient symptomatic improvement is not achieved with one of the anal sphincteroplasty and sacral neuromodulation, the other one might be performed.
*5 The surgery in the second line can be performed without the surgery in the first line being performed, depending on the preference and conditions of each patient with FI.
*6 If the first line surgical therapies fail to achieve sufficient symptomatic improvement, the surgery in the second line is to be considered. On the other hand, the second line can be tired first depending on the preference and conditions of each patient with FI. If the second line fails, the first line can follow it.
Methodology
Following a request by the Guideline Preparation Committee to the Japan Medical Library Association Medical Guidelines Working Group, a non-profit organization, PubMed and Ichushi Web were searched for relevant items published in Japanese and English between January 1983 and December 2014. In the objective and comprehensive literature search, all documents from the Cochrane Library were also searched. We also referred to domestic and foreign clinical guidelines and important past documents. Using the above procedures, we found about 320 documents, which were selected from nearly 3,360 documents discovered through document retrieval, and critically examined the entire text.
Grade of Recommendation Assessment
There are many different categorizations for recommendations. We used the most recent ones, which were adopted in the “JSSCR Guidelines 2010 for the Treatment of Colorectal Cancer”; we also used those in the “Japanese Practice Guidelines for Anal Disorders”. Therefore, in the chapters on treatment and in the CQs, we have attached the evidence for classification and recommendation assessments that have been established through a consensus of the Guideline Preparation Committee members.
Consensus of committee members was obtained through the following steps:
1. Voted “agree,” “oppose,” or “abstain” to each statement
When “oppose” and “abstain” were selected, Step 2 was omitted.
2. When “agree” was selected, members explained whether the evidence level to support the statement was high (recommendation level A) or low (recommendation level B).
The following method was used to decide the category of recommendation:
1. If all committee members agreed with the statement, the category of recommendation was determined to be A or B, according to the evidence level. The category had to be supported by a majority of members. In case of a tie, the committee chairman decided.
2. If at least one member opposed the statement, the level of recommendation was determined to be C or D. If more than 70% of members agreed, the recommendation was categorized as C, and as D if less than 70% agreed.
Grade of Recommendation A: Based on high levels of evidence, the Guideline Preparation Committee members concur in their opinions (i.e., there are a multitude of documents and some indicate a high level of evidence).
Grade of Recommendation B: Based on a low level of evidence, the Guideline Preparation Committee members concur in their opinions (i.e., only a few documents exist and some are considered to have low-level evidence).
Grade of Recommendation C: Regardless of the level of evidence, the Guideline Preparation Committee members do not agree.
Grade of Recommendation D: The Guideline Preparation Committee members have widely varying opinions.
I. Definition of FI
Statement
FI is defined as involuntary or uncontrollable loss of feces.
Gas incontinence is defined as involuntary or uncontrollable loss of flatus.
Anal incontinence is defined as involuntary loss of feces or flatus.
Discussion
FI is the term for the symptoms of loss of feces; and while consensus meetings and professional societies in Western countries have proposed several definitions, there has been no appropriate academic definition suitable for epidemiological studies or treatment indications in Japan. Despite the lack of a universal consensus, the Japan Society of Coloproctology has adopted the above distinction between FI and gas incontinence.
The International Consultation on Incontinence further distinguishes between anal incontinence and FI. In a definition which could be useful for therapeutic purposes, anal incontinence is defined as “the involuntary loss of flatus, liquid, or solid stool that is a social or hygienic problem.” When referring to the symptoms without flatus, “fecal incontinence” is used[1,2].
The American Society of Colon and Rectal Surgeons defines FI as “the uncontrolled passage of feces or gas over at least one month's duration, in an individual of at least four years of age, who had previously achieved control.” This definition does not address flatus separately; rather, it focuses on age and duration of symptoms[3-5].
The American College of Gastroenterology defines FI as “either the involuntary passage or the inability to control the discharge of fecal matter through the anus”[6]. This is the definition that most closely matches the one used in Japan, which does not address social background, age, duration of time, or involvement of flatus.
II. Prevalence of FI
Statement
The prevalence of FI in people over 65 in Japan is 8.7% in the male population and 6.6% among females.
Discussion
It is difficult to determine the actual prevalence of FI, as percentages in published studies vary dramatically between 2.2% and 25%, and patients are studied for different lengths of time, so it is difficult to say what the parameters are when discussing FI. One of the problems is that the definition of “fecal incontinence” is not the same from study to study. Secondly, questionnaires are not consistent. In an epidemiological study of FI in Japan that conducted formal interviews with 1,405 people over the age of 65, the above-stated prevalence figures of 8.7% in men and 6.6% in women were found[7].
An epidemiological study in the U.S. surveyed 6,959 people in the state of Wisconsin by telephone and reported the instances of FI, including gas incontinence, to be 2.2%. The authors reported higher instances in the female, disabled, and poor general health populations[8].
Another U.S. study queried people over 50 (778 men, 762 women) using mail-in surveys. The prevalence reported in this study was 17% in men and 24.6% in women[9]. In another large-scale postal survey in the U.S., researchers asked 15,904 people over 40 if they had experienced two or more FI episodes per month; 3% of respondents claimed they had[10]. A U.S. study using telephone interviews in a relatively young sample group of patients over the age of 29 (2,079 men, 2,229 women), showed that the prevalence of FI was 7.7% in men (6.0-9.4%) and 8.8% in women (7.1%-10.4%)[11].
A postal survey in the Netherlands of 5,748 local residents indicated the prevalence of FI in those over age 60 to be 9%[12]. A survey of 1,253 women in Taiwan revealed the prevalence of FI to be 2.8%, and the prevalence of gas incontinence was 8.6%[13].
III. Pathogenesis and Etiology of FI
Statement
The etiology of FI is usually not limited to one specific cause (Table 1).
Table 1.
Pathogenesis and Etiology of Fecal Incontinence.
| Pathogenesis | Etiology |
|---|---|
| Idiopathic anal sphincter dysfunction | Impaired internal anal sphincter dysfunction due to aging |
| Traumatic anal sphincter dysfunction | Childbirth injury |
| Anal surgery | |
| Rectal cancer surgery | |
| Anorectal trauma (from an accident) | |
| Neurogenic anal sphincter dysfunction | Pudendal neuropathy after childbirth |
| Postoperative (postop) autonomic neuropathy, rectal cancer | |
| Autonomic neuropathy from diabetes | |
| Spinal neuropathy (injury, tumor, spina bifida, meningocele, etc.) | |
| Congenital anorectal disorders | Imperforate anus (postop) |
| Hirschsprung disease (postop) | |
| Acquired anorectal disorders | Rectal prolapse |
| Rectocele | |
| Rectal intussusception | |
| Impaired recognition | Multiple sclerosis |
| Dementia | |
| Cerebral infarction | |
| Diabetes | |
| Rectal reservoir dysfunction | Rectal cancer surgery (low anterior resection) |
| Ulcerative colitis surgery (restorative total proctocolectomy) | |
| Radiation | |
| Inflammatory bowel disease (e.g. Rectal lesion from Crohn’s disease) | |
| Bowel habits issues (chronic diarrhea) | Irritable bowel syndrome |
| Inflammatory bowel diseases | |
| Postop cholecystectomy | |
| Collagenous colitis | |
| Functional diarrhea | |
| Laxative abuse | |
| Overflow fecal incontinence | Fecal impaction |
| Encopresis in children |
Discussion
The pathogenesis and etiology of FI are not uniform; factors vary from person to person and a single individual may experience multiple factors simultaneously[14]. Symptoms may fail to appear as expected with a specific risk factor when other compensating factors exist. Among the causes of FI are consistency of feces, dysfunction of the anorectal nerves, or dysfunction of the central nervous system, resulting in impaired recognition of feces, while, in general, several factors may affect FI, such as anal sphincter or rectal reservoir dysfunction, resulting in impaired rectal sensation, capacity, or compliance.
Anal sphincter dysfunction has two components: internal anal sphincter dysfunction presents as decreased resting pressure, while external anal sphincter dysfunction presents as decreased squeeze pressure. Both traumatic physical dysfunction and non-traumatic neural dysfunction cause anal sphincter dysfunction[15-17]. The most frequent causes of physical sphincter dysfunction are childbirth and anal surgery. Conversely, the most common neural dysfunctions causing FI are congenital spinal disorders, spinal injuries, multiple sclerosis, peripheral nerve impairment, and/or autonomic nerve dysfunction resulting from diabetes. Pudendal neuropathy caused by pressure and traction during delivery also cause FI[16].
Diabetes can present with these factors that cause FI, such as internal anal sphincter dysfunction, and autonomic neuropathy, as well as impaired rectal sensation, with FI maybe resulting from a variety of other chronic conditions, including multiple sclerosis, dementia, meningocele, spinal injury, etc. In addition to rectal surgery, inflammatory bowel diseases and irradiation to the rectum may cause impaired rectal capacity and compliance.
Fecal impaction is usually seen in the elderly population, but it may also be seen among children in which case it is called encopresis, and sometimes it causes overflow incontinence. The mechanism of overflow FI is internal anal sphincter relaxation due to the recto-anal inhibitory reflex, followed by proximal soft or liquid feces.
Idiopathic FI, occurring mostly among older adults, may result from unknown factors, such as internal anal sphincter dysfunction or impaired anal/rectal sensation[18], with the condition being called idiopathic FI if definitive causes are not identifiable. This type of FI is primarily attributed to aging.
IV. FI Risk Factors
Statement
Risk factors for FI include: physiological factors, such as age and gender; comorbidities, such as diabetes and irritable bowel syndrome; and obstetric factors, such as multiple deliveries, home delivery, first vaginal delivery, and forceps delivery (Table 2).
Table 2.
Risk Factors for Fecal Incontinence.
| 1. | Physiological conditions |
| age, gender, obesity, poor general condition, physical disability | |
| 2. | Comorbidities |
| diabetes mellitus, irritable bowel syndrome, inflammatory bowel diseases (ulcerative colitis, Crohn’s disease) | |
| 3. | Obstetric conditions |
| multiple deliveries, home delivery, first vaginal delivery, forceps delivery, heavy infant | |
| (>4,000 g), prolonged second labor |
Discussion
Certain physiological factors have been reported as risk factors of FI. Among them, age is a definitive factor. Epidemiological studies including adults of all ages showed that there is a concrete relationship between increased age and FI[7-10,19,20]. This is due to decreased physical abilities such as muscle weakness and compromised recognition ability, as well as increased comorbidities which could be risk factors for FI. With obesity (BMI>30)[21,22], poor general health[21,23], and physical disability[8,24] having been reported as possible physiological factors related to FI, gender is considered a relatively weak risk factor, with few studies having reported that FI is more prevalent in women than in men[8,9,19,20,25] and other studies having showed that no significant difference has been found between the sexes[7,10,12,23].
Specific chronic diseases may also be risk factors for FI. A higher prevalence has been noted among diabetes patients, for example, and a relationship between blood sugar control and severity of FI has been reported[26]. Patients with irritable bowel syndrome[27] and inflammatory bowel diseases are also more likely to suffer from FI[22]. Constipation is the most frequent cause of FI in children[28]. Urinary incontinence[9,21], overactive bladder[27], and pelvic organ prolapse[13,21] are also relevant to FI. Neurological conditions such as dementia[29] and spinal cord injury[24] have also been reported as risk factors for FI.
Certain obstetric conditions, such as multiple deliveries[13,19], home delivery[30], first vaginal delivery[31,32], and forceps delivery[33], are also risk factors for FI. In addition, high infant birth weight (> 4,000 g)[31,33]and/or prolonged second labor[34] could also be risk factors for FI.
V. Initial Clinical Evaluation for FI
The initial clinical evaluation of FI is the basis of all medical treatments for FI, including initial treatment, and also serves as a baseline for deciding the need for a specialized defecation function test and selecting treatment in stages, with the factors responsible for individual symptoms being gathered from the history and examination of the anorectal region.
A. Medical History Taking
1. Present history
Statement
Since it is often possible to predict the factors that cause FI from the medical history, and because it is useful for coping with this issue in daily life and the selection of initial treatment, it is recommended that the procedure for taking medical history regarding FI be standardized.
Discussion
FI is classified as urge, passive, or mixed based on major complaints[6]. Urge incontinence is “a symptom of feeling stool but spilling feces without being able to reach the toilet,” and passive incontinence is “a symptom of fecal spilling without awareness.” Changes in anal sphincter and pelvic floor muscle functions, rectal retention, stool type, and changes in neurological functions are recognized as risk factors for the development of FI, which is rarely caused by a single factor, with these multiple factors often being interrelated[35]. (See “3. Causes and pathologies of FI”).
The anal sphincter is not always impaired in incontinence patients. However, if anal sphincter disorders are the major cause of FI, decreased internal anal sphincter function causes decreased anal resting pressure and leaky FI, and decreased external anal sphincter function causes anal failure. Decrease in voluntary pressure in the duct leads to urinary incontinence[36]. In contrast, even if the anal sphincter is perfectly normal, when the rectal sensation is disturbed and the feces in the rectum are not felt, then fecal impaction may occur, which can lead to overflow incontinence. One theory about overflow FI is that the feces remaining in the rectum spill out after defecation[37], but the feces in the rectum cannot be completely evacuated due to rectocele or rectal intussusception. Furthermore, the intussuscepted mucosa induces the recto-anal inhibitory reflex to lower the anal canal resting pressure, resulting in FI. Alternatively, in patients with irritable bowel syndrome, which causes increased rectal sensation and contractility, urge incontinence may occur even if the anal sphincter muscle is completely normal.
Although a logbook is insufficient to identify the pathophysiology of incontinence, it is critical for working out the specialized functional tests that will be required to determine treatment, and patients should be encouraged to maintain a logbook of daily bowel habits, eating habits and details of FI[6,38,39].
1) Questions about daily bowel habits
・What were the previous bowel movements like?
・How and when did bowel movements change?
・When and for how long have you been using oral medications such as laxatives, enemas or suppositories?
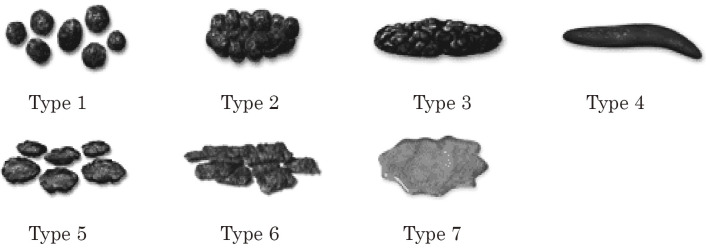
・What are your usual stool types (from Bristol stool form scale)? (Figure 3)
Figure 3.
Bristol Stool Form Scale.
・Do you strain when defecating? How long does it take to have a bowel movement?
・Is it possible to distinguish gas from stool and to distinguish between liquid and solid stool?
・Do you feel abdominal pain or bloating before defecation?
・Do you need finger or hand assistance for defecation?
・Is it possible to wipe yourself clean after defecation?
・What are your usual activities of daily life?
2) Questions that focus on fecal incontinence
・Are you aware of the incontinence, or is the incontinence so bad that you cannot tolerate it?
・Is it flatus, mucus, liquid stool, or solid stool? How often does it occur?
・Can you tolerate defecation, and if so, for how long?
・Can you tolerate the flatus, and if so, for how long?
・When did the first instance of incontinence occur, and how has it changed over time?
・How much and what type of feces spillover are you experiencing?
・Are there any triggers for incontinence?
・Does incontinence occur during sleep?
・Does incontinence occur after defecation?
・Does incontinence affect your daily life? What kind of trouble does it cause, and how often?
・Do you need to use a pad? And if so, how often?
3) Questions about daily life related to incontinence
・Diet style, including favorite foods such as coffee, alcohol, etc.;
・Smoking history, change in body weight (BMI);
・Medication, including laxatives and psychotropic drugs;
・Environment, including toilet
The stool form is an important factor that influences the continence mechanism. Diarrhea causes FI; in contrast, constipation with hard pellet stools causes chronic rectal extension, which results in decreased rectal sensation, leading to passive FI. Since bowel movements, including FI, vary by individual, we should confirm individual daily bowel habits and their changes. The stool types should be described on the universally used Bristol stool form scale (Figure 3)[39]. Medical history taking should be focused on risk factors and the course and grade of FI. Regarding the factors related to FI in daily life, we confirmed not only physical restriction and ability of recognition[35], but also general condition, including basic activity[40], as well as living environment and bathroom habits. The details of prescribed medications should be carefully checked because they may be involved in the onset of FI. Coffee and alcohol also affect intestinal motility and stool types, and smoking leads to atrophy of the external anal sphincter, causing urge incontinence[40], with laxatives often being the cause of FI, and psychotropic drugs may act on intestinal motility and peripheral nerves, resulting in FI[41]. Similarly, obesity causes FI, so patients should be careful of changes in body weight[42].
2. Past history・underlying condition
Statement
Investigate past medical history and underlying conditions related to the risk of FI.
Discussion
Risk factors related to the onset of FI include the following diseases and physical conditions: (See “3. Causes and pathologies of fecal incontinence”)
・Diseases and physical conditions that affect changes in intestinal motility; diarrhea, constipation, enteritis, irritable bowel syndrome, diabetes, cholecystectomy, etc.
・Diseases / physical conditions that affect rectal sensory function; childbirth (due to nerve over extension), spinal nerve disease and injury, chronic constipation, anal malformation, diabetes, dementia, etc.
・Diseases and physical conditions that have a damaging effect on anal sphincter function; childbirth (sphincter injury), anal surgery, trauma, spinal nerve disease, aging, etc.
・Disorders and physical conditions that cause changes in rectal volume and extensibility; rectal and anal cancer, history of irradiation, inflammatory bowel disease, history of intra-pelvic surgery such as hysterectomy, constipation (chronic distension of the rectum), etc.
・Diseases and physical conditions that reduce the performance of evacuation; restriction due to cerebral infarction, aging, dementia, etc.
・Other diseases and physical conditions; hemorrhoids, anal fistula, rectal prolapse, intussusception, inflammation of the rectum and perineum, fecal impaction, side effects of medications, etc.
1) Labor history (history of childbirth delivery)
Confirm the delivery method (vaginal, Caesarean, forceps or suction), the number of deliveries, history of birth trauma and its grade, the weight of the baby, postpartum defecation status, etc.
Sphincter injury is observed in 20%-30% of vaginal deliveries, but most patients are asymptomatic and often only show FI with age[43]. Sphincter injury is more common in multiparous women (32.3%) than in primiparous women (21.7%), especially in forceps delivery (49.1%) and suction delivery (45.2%). As a result, the risk of developing FI increases after forceps delivery (odds ratio 4.75) and after aspiration delivery (odds ratio 3.51)[33,44,45]. In addition, factors such as the method of delivery and fetal weight may cause FI and its pathological conditions. However, these factors are not established as evidence because causality and frequency differ depending on the report[33,46-48].
2) History of surgery and irradiation
History of hysterectomy, anal surgery, and rectal surgery are associated with FI. Furthermore, the history of other surgeries, such as cholecystectomy, and the history of radiation therapy to the pelvic region are also relevant.
Hyper-extension of the anal canal during surgery can also be a cause of FI[3], as well as direct surgical manipulation of sphincter muscles during anal surgery, surgery of anal fissure, anal fistula, hemorrhoidectomy, etc. Rectal surgery (anterior rectal resection, ileo-anal anastomosis in ulcerative colitis, and familial adenomatous polyposis, etc.) can result in sphincter injury and diminished retention capacity associated with rectal resection, as well as changes in fecal characteristics and perceived sensation, which may cause incontinence[49]. Hysterectomy causes not only urinary incontinence, but also FI[32]. Further increasing the risk of FI, radiation therapy may be used in combination with surgery for malignant tumors to treat prostate cancer, rectal and anal cancer, etc.[39,50]. In addition to surgery in the anus or pelvic cavity, diarrhea after cholecystectomy has also been listed as a risk factor[39].
3) History of spinal disease and pelvic trauma
Spinal disorders (spinal cord injury, spina bifida, spinal canal stenosis, etc.) and history of spinal surgery and pelvic trauma directly affect sensory and motor nerves in the pelvis.
Furthermore, diarrhea and constipation caused by autonomic neuropathy via the spinal nerve also cause FI[51-53].
4) Diabetes
Diabetes mellitus is recognized as a risk factor for FI because it is accompanied by systemic peripheral neuropathy and impairs intestinal motility as well as sensory nerves of the rectum and motor nerves of the anal sphincter muscle[22,40,43]. In a survey report of 8,657 subjects, symptoms were correlated with blood glucose control[54].
5) Neuromuscular disease
In neuromuscular diseases such as Parkinson's disease, multiple sclerosis, lateral sclerosis, and scleroderma, autonomic nerves, and muscle contraction and relaxation are systemically impaired. When the central nervous system is affected, such as from cerebral infarction, FI is often caused not only by direct impaired rectal anal function, but also by movement restriction[39,41]), while, in addition, peripheral neuropathy occurs in the rectum and anal sphincter and pelvic floor muscles[55], and with rectal volume, compliance, and sensation being diminished and contraction of the sphincter being disturbed, FI results[39,40].
6) Urinary incontinence
Urinary incontinence and FI have similar pathogenesis, resulting from conditions such as pelvic floor muscle and nerve disorders. Postpartum urinary incontinence and FI prevalence is 6% to 8%, and the risk is higher in primiparous women over 35 years old who give birth by forceps and suction deliveries[43,56,57]. About one third of female urinary incontinence patients who have undergone childbirth have FI, which is a higher proportion than that of males[32]. The onsets of urinary incontinence and FI are correlated with depression (odds ratio 2.3), and it is believed that psychological factors are also involved in the onset of incontinence[35].
7) Other diseases with abnormal bowel movement, such as constipation and diarrhea
Chronic enteritis, irritable bowel syndrome[27,58], and other conditions that cause motility disorders, such as constipation and diarrhea, are also risk factors for FI[59]. Irritable bowel syndrome in particular has a high odds ratio of 2.4 for FI and is considered to be a risk factor[22].
B. Anorectal Examination and Evaluation
Inspection (including anoscope) and palpation (including digital anorectal and vaginal examination) of the anorectal area focusing on the factors of FI should be conducted following the general physical examination and taking of medical history.
1. Inspection
Statement
Visual inspection is conducted to observe the shape of the anus at rest, the condition of the surrounding skin, the appearance of scars, and the movements of the anus and perineum during contraction of the anal sphincter.
Discussion
During inspection, check the symmetry of the anus, observe whether there is stool on the area, measure the distance between the vagina and anus, and inspect the perineal surface lesions (dermatitis, flare reaction, avulsion, maceration), including the details of previous incisional scars[60-62]. While the evidence for its significance has not yet been established, it has been reported that lateral perineal incision reduces the risk of anal sphincter injury compared with median incision[46]. The distance between the vaginal orifice and anus is normally 3 cm or more. If the distance is 2 cm or less, the sphincter muscle may have been damaged during childbirth[63]. Observe the condition of anus closure and the presence of visible anal lesions, such as mucosal prolapse, hemorrhoids, etc. During anal sphincter contraction, inspect whether the anus is closed circumferentially and uniformly, or if dimples appear. After checking the anal lesions using an anoscope, inspect the perineal bulge through digital rectal examination (described below in section 2b). Perineal descent suggests that the pelvic floor muscles are flaccid, which is a cause of FI[64].
2. Palpation - a. palpation outside the anus
Statement
Push the skin around the anus with a finger to determine whether there is a defect in the muscle tissue, and confirm that the patient maintains sensation. In women, confirm the health of the tissue between the anus and vagina.
Discussion
The level of damage to spinal segments can be diagnosed by palpation and by consideration of the distribution of the spinal nerves to the skin. For example, L1 = base of penis and upper scrotum; L1-2 = labia minor and central scrotum; L3 = anterior knee; S1 = sole; and S1-3 = perineum and perianal area[62,65,66]. The anal wink is an anal skin reflex in which the anal sphincter contracts when the skin on both sides of the anus is touched. This reaction does not appear in S2-4 neuropathy.
2. Palpation – b. digital anorectal examination (including digital vaginal and bidigital examinations)
Statement
Digital anorectal examination assesses tonus, homogeneity, symmetry, and length of the anal canal, the anorectal angle, and also confirms whether there is an unusual intra-rectal condition such as fecal impaction, residual feces, etc. The anal canal should be closed voluntarily, and the contraction of the anal sphincter and puborectalis should be evaluated. The relaxation of these muscles should then be confirmed by applying abdominal pressure. The Digital Rectal Examination Scoring System (DRESS) score (Table 3) is useful for the evaluation and recording of data.
Table 3.
The Digital Rectal Exam Scoring System (DRESS) [68].
| Resting Score | |
|---|---|
| 0 | No discernable tone at rest, an open or patulous anal canal |
| 1 | Very low tone |
| 2 | Mildly decreased tone |
| 3 | Normal |
| 4 | Elevated tone, snug |
| 5 | Very high tone, a tight anal canal, difficult to insert a finger |
| Squeeze Score | |
| 0 | No discernable increase in tone with squeezing effort |
| 1 | Slight increase |
| 2 | Fair increase but below normal |
| 3 | Normal |
| 4 | Strong squeeze |
| 5 | Very strong squeeze, to the point of being painful to the |
| examiner | |
Quated from Orkin BA., Sinykin SB, Lloyd PC. The Digital Rectal Examination Scoring System (DRESS). Dis Colon Rectum. 2010 Dec; 53 (12): 1656-60 (68). The permission for using this table is not required.
Discussion
Digital rectal anal examination should be used to check for presence, volume, and type of stool in the rectum. In the elderly, as well as in patients with chronic constipation, as the rectal sensation becomes less sensitive, a large amount of stool is retained and fecal impaction may occur, causing reflexive relaxation of the internal sphincter, resulting in overflow FI[67]. In women, rectocele is suspected if pushing the anterior wall of the rectum with the finger results in protrusion into the vagina[66].
The evaluation of anal sphincter tonus is essential for the pathophysiological diagnosis of FI. Attempts are being made to standardize objective evaluation and status recording, as digital rectal examination can give an overview of resting pressure and squeeze pressure, but the accuracy of the assessment depends on the examiner. Therefore, The DRESS score (Table 3) has been confirmed to be useful in multi-center comparative evaluations[68]. The anal sphincter is bilaterally symmetric and palpable for whole circumferential muscle tone, but if it is asymmetric, or if there is a part where no contraction is observed, sphincter injury is suspected. However, since the evaluation of sphincter injury by digital examination is unreliable, if anal sphincter injury is suspected based on symptoms and history, more specialized anorectal function tests, such as anorectal ultrasound, should be performed[61,67]. It is possible to use palpation not only to evaluate the level of muscle relaxation, but also to diagnose perineal descent, mucosal prolapse, rectal prolapse, and pelvic organ prolapse.
Vaginal and bidigital examinations can be done along with rectal anal examination. The fragility of the rectovaginal septum and the ptosis of the uterus and bladder due to abdominal pressure through digital examination of the vagina should be checked. Enterocele and sigmoidocele can be diagnosed by performing the bidigital examination[69].
VI. Patient-reported Outcome Measures To Evaluate FI Symptoms and FI-specific Quality of Life
Statements
・In clinical practice, Cleveland Clinic Florida Fecal Incontinence Score (CCFIS) is to be used to evaluate the symptomatic severity of FI and FI-specific quality of life (QoL);
・In clinical practice, St. Mark's score is to be used specifically to evaluate fecal urgency in addition to the symptomatic severity of FI and FI-specific QoL;
・In clinical research, the Fecal Incontinence Severity Index (FISI) and the Japanese version of the Fecal Incontinence Quality of Life Scale (JFIQL) should be used to evaluate FI symptoms and FI-specific QoL, respectively;
・For low anterior resection syndrome (LARS), LARS score and JFIQL should be used to evaluate symptoms of disordered bowel function and FI-specific QoL, respectively. Additionally, the Japanese version of the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire (EORTC QLQ-C30) and the Japanese version of the EORTC Colorectal Cancer-Specific Quality of Life Questionnaire 38 (EORTC QLQ-CR38) should be used to evaluate general health-related QoL, including the QoL associated with disordered bowel function in LARS.
Discussion
For the clinical evaluation of FI, it is useful to use Patient-Reported Outcome Measures (PROMs), such as scores and questionnaires, to evaluate the symptomatic severity of FI and its influence over QoL[61]. Many scales have been developed, including Kirwan classification[70], Miller score[71], Pescatori score[72], CCFIS score[73], St. Mark's score[74], and FISI[75]. Currently, the most frequently used scales are the CCFIS (Table 4), St. Mark's score (Table 5), and FISI (Table 6)[76].
Table 4.
Cleveland Clinic Florida Fecal Incontinence Score [73].
| Type of
Incontinence |
Frequency | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Usually | Always | |
| Solid | 0 | 1 | 2 | 3 | 4 |
| Liquid | 0 | 1 | 2 | 3 | 4 |
| Gas | 0 | 1 | 2 | 3 | 4 |
| Wear pad | 0 | 1 | 2 | 3 | 4 |
| Lifestyle
alteration |
0 | 1 | 2 | 3 | 4 |
(CCFIS = Wexner score)
0 = perfect.
20 = complete incontinence.
Never = 0 (never).
Rarely = < 1 / month.
Sometimes = < 1 / week, > 1 / month.
Usually = < 1 / day, > 1 / week.
Always = > 1 / day
The continence score is determined by adding points from the above table, which takes into account the type and frequency of incontinence and the extent to which it alters the patient’s life.
Quated from Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993 Jan; 36 (1): 77-97 (73). The permission for using this table is not required.
Table 5.
St. Mark’s Score [74] (=Vaizey score).
| Never | Rarely | Sometimes | Weekly | Daily | |
|---|---|---|---|---|---|
| Incontinence for solid stool | 0 | 1 | 2 | 3 | 4 |
| Incontinence for liquid stool | 0 | 1 | 2 | 3 | 4 |
| Incontinence for gas | 0 | 1 | 2 | 3 | 4 |
| Alteration in lifestyle | 0 | 1 | 2 | 3 | 4 |
| No | Yes | ||||
| Need to wear a pad or plug | 0 | 2 | |||
| Taking constipating medicines | 0 | 2 | |||
| Lack of ability to defer defecation for 15 minutes | 0 | 4 |
Never, no episodes in the past four weeks; rarely, 1 episode in the past four weeks; sometimes, >1 episode in the past four weeks but <1 a week; weekly, 1 or more episodes a week but <1 a day; daily, 1 or more episodes a day.
Add one score from each row: minimum score = 0 = perfect continence; maximum score = 24 = totally incontinent
Quated from Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999 Jan; 44 (1): 77-80 (74). The permission for using this table is not required.
Table 6.
Fecal Incontinence Severity Index (FISI) [75].
| Patient Ratings
of Fecal Incontinence |
Never | 1 to 3
Times a Month |
Once
a Week |
2 or More
Times a Week |
Once a
Day |
2 or More
Times a Day |
|---|---|---|---|---|---|---|
| Solid | 0 | 8 | 10 | 13 | 16 | 18 |
| Liquid | 0 | 8 | 10 | 13 | 17 | 19 |
| Mucus | 0 | 3 | 5 | 7 | 10 | 12 |
| Gas | 0 | 4 | 6 | 8 | 11 | 12 |
Add one score from each row: minimum score = 0 (no fecal incontinence); maximum score = 61 (worst fecal incontinence)
Quated from Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999 Dec; 42 (12): 1525-32 (75). The permission for using this table is not required.
The CCFIS is also called the Wexner score, after Dr. S.D. Wexner who developed the questionnaire[73]. The CCFIS consists of five items; three items address the frequency of incontinence from gas, liquid stool, and solid stool, and the other two items address the frequency of wearing a pad for protection and lifestyle alterations due to FI. Scores range from 0 (no FI) to 20 points (worst FI). The CCFIS should be called “anal incontinence score” rather than “fecal incontinence score”, because it includes the evaluation of gas incontinence. St. Mark's score and FISI are also traditionally called “fecal incontinence scores” even though they include the evaluation of gas incontinence. One of the advantages of the CCFIS is that it can evaluate components of QoL, such as the influence of FI over lifestyle, as well as FI symptoms, using a relatively small number of questions. Another advantage of the CCFIS is that it has been used both historically and internationally. On the other hand, it has some disadvantages. Firstly, it does not weigh FI symptoms differently. For example, it gives the same four points to daily leakage, whether it is gas or formed stool. Another disadvantage of the CCFIS is that it does not necessarily reflect the FI symptoms alone because it also evaluates the influence of FI over QoL. Even if FI symptoms are completely cured, anxiety about FI continues to affect the daily life of some patients; for example, they may continue to wear pads. For these patients, the CCFIS will reflect a score of eight points at most. This is why FI sometimes falls under “symptoms of anxiety”. A third disadvantage of the CCFIS is that it is not able to evaluate fecal urgency, which is one of the main concerns of some patients with FI, even if it does not lead to actual FI.
St. Mark's score is also called the Vaizey score, named after Dr. C.J. Vaizey, who developed it. This questionnaire consists of seven items, including the five items on the CCFIS, as well as two items for “taking constipating medicines” and “lack of ability to defer defecation for 15 minutes”. The score ranges from 0 (no FI) to 24 points (worst FI)[74]. The advantage of St. Mark's score is that it can evaluate fecal urgency in addition to the five items evaluated by CCFIS. St. Mark's score has the same disadvantages of CCFIS; it does not weigh each FI symptom, and its score does not necessarily reflect the FI symptoms alone because it evaluates both FI symptoms and their influence over QoL.
The FISI consists of four items addressing the frequency of incontinence from gas, mucus, liquid stool, and solid stool, ranging from 0 (no FI) to 61 points (worst FI)[75]. This questionnaire has two advantages: firstly, it evaluates only FI symptoms, unlike the CCFIS and St. Mark's scores, and secondly, each FI symptom is weighted. However, the symptoms-only nature of the questionnaire can also be a disadvantage, since it does not permit evaluation of any other FI-related factors. Therefore, when the FISI is used for the clinical evaluation of FI, it should be used in conjunction with other PROMs to evaluate FI-specific QoL, because the ultimate purpose of the management of FI is to improve the QoL by alleviating symptoms.
Examples of FI-specific QoL questionnaires include the Fecal Incontinence Quality of Life Scale (FIQL)[77] and the Modified Manchester Health Questionnaire[78]. The FIQL is the most widely used internationally.
The FIQL consists of 29 questions, which are categorized into four scales. Scores are expressed as the average response to the answered questions, and the higher the score, the better the QoL[77]. The number of questions and the range of points in each Scale are as follows: Lifestyle (10 questions, 1-4 points); Coping/Behavior (9 questions, 1-4 points); Depression/Self Perception (7 questions, 1-4.4 points); and Embarrassment (3 questions, 1-4 points). The original FIQL was developed in English in the U.S, but its translated versions have been validated in various languages, including French, Portuguese, Italian, Spanish, Turkish, Norwegian, German, Chinese, and Dutch. Two Japanese versions of the FIQL were also validated, and both allow the subscale as well as the total score (1-4.1 points) to be evaluated[79,80]. In high quality studies, such as randomized controlled trials, it is recommended that researchers use both the FISI and the FIQL so that the separate evaluation of FI symptoms and FI-specific QoL can be performed.
LARS is defined as disordered bowel function after rectal resection leading to a detriment in QoL. For LARS, it is important to evaluate not only FI but also other LARS-specific symptoms, such as frequent bowel motions within a short period of time, or “clustering.” The LARS score (Table 7) was developed as a simple tool for the clinical evaluation of LARS, and it can evaluate more LARS-specific symptoms than the CCFIS and the FISI[81]. While the FI-specific QoL can be evaluated with the Japanese version of the FIQL, the Japanese version of the EORTC QLQ-C30[82] and the Japanese version of the EORTC QLQ-CR38[83]are recommended for the evaluation of general health-related QoL. Although the modified FI QoL scale (mFIQL) was developed in Japan to evaluate FI-specific QoL in patients with LARS[84], it is not recommended for evaluating patients with LARS. This is because 10 questions (out of the original 29) were excluded during its development. These excluded questions addressed Depression/Self Perception and Embarrassment. Consequently, the mFIQL cannot evaluate the QoL associated with depression and embarrassment that are generally very important for patients with FI.
Table 7.
Low Anterior Resection Syndrome Score (LARS score).
| Add the scores from each of the 5 questions to obtain one final score. | ||
| Do you ever have occasions when you cannot control your flatus (wind) ? | ||
| □ | No, never | 0 |
| □ | Yes, less than once per week | 4 |
| □ | Yes, at least once per week | 7 |
| Do you ever have any accidental leakage of liquid stool? | ||
| □ | No, never | 0 |
| □ | Yes, less than once per week | 3 |
| □ | Yes, at least once per week | 3 |
| How often do you open your bowels? | ||
| □ | More than 7 times per day (24 hours) | 4 |
| □ | 4 ‐ 7 times per day (24 hours) | 2 |
| □ | 1 ‐ 3 times per day (24 hours) | 0 |
| □ | Less than once per day (24 hours) | 5 |
| Do you ever have to open your bowels again within one hour of the last bowel opening? | ||
| □ | No, never | 0 |
| □ | Yes, less than once per week | 9 |
| □ | Yes, at least once per week | 11 |
| Do you ever have such a strong urge to open your bowels that you have to rush to the toilet? | ||
| □ | No, never | 0 |
| □ | Yes, less than once per week | 11 |
| □ | Yes, at least once per week | 16 |
| Total Score: | ||
| Interpretation: | ||
| 0 - 20: No LARS | ||
| 21 - 29: Minor LARS | ||
| 30 - 42: Major LARS | ||
Conflicts of Interest
There are no conflicts of interest.
Source of funding
Support came from the Japan Society of Coloproctology.
Author Contributions
KM, MS, HK, YT, AT, TM, TY, and KY: Substantial contribution to the conception or design of the work, and the acquisition and interpretation of data for the work.
Disclaimer
Hidetoshi Katsuno, Tetsuo Yamana and Kazuhiko Yoshioka are Associate Editors of Journal of the Anus, Rectum and Colon and on the journal's Editorial Board. They were not involved in the editorial evaluation or decision to accept this article for publication at all.
This article is based on a study first reported in “Japanese Practice guidelines for Fecal Incontinence 2017 version”[85].
The original version is available at https://www.nankodo.co.jp/g/g9784524258963/.
The Editor-in-Chief of Journal of the Anus, Rectum and Colon, the Japan Society of Coloproctology and the publisher of the original version have permitted the publication of this manuscript.
Acknowledgements
We would like to thank Japan Society of Coloproctology for funding and Editage (www.editage.com) for English language editing.
References
- 1.Norton C, Whitehead WE, Bliss DZ, et al. Conservative and pharmacological management of faecal incontinence in adults. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth: Health Publications; 2005. 1521-63. [Google Scholar]
- 2.Whitehead WE, Wald A, Norton NJ. Treatment options for fecal incontinence. Dis Colon Rectum. 2001 Jan; 44(1): 131-44. [DOI] [PubMed] [Google Scholar]
- 3.Tjandra JJ, Dykes SL, Kumar RR, et al. Standards practice task force of the American society of Colon and Rectal surgeons. Practice parameters for the treatment of fecal incontinence. Dis Colon Rectum. 2007 Oct; 50(10): 1497-507. [DOI] [PubMed] [Google Scholar]
- 4.Wald A. Clinical practice. Fecal incontinence in adults. N Engl J Med. 2007 Apr; 356: 1648-55. [DOI] [PubMed] [Google Scholar]
- 5.Madoff RD, Parker SC, Varma MG, et al. Faecal incontinence in adults. Lancet. 2004 Aug; 364(9434): 621-32. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS. American College of Gastroenterology Practice Parameters Committee. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004 Aug; 99(8): 1585-604. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi N, Tatara K, Naramura H, et al. Urinary and fecal incontinence in a community-residing older population in Japan. J Am Geriatr Soc. 1997 Feb; 45(2): 215-9. [DOI] [PubMed] [Google Scholar]
- 8.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA. 1995 Aug; 274: 559-61. [PubMed] [Google Scholar]
- 9.Roberts RO, Jacobsen SJ, Reilly WT, et al. Prevalence of combined fecal and urinary incontinence: a community-based study. J Am Geriatr Soc. 1999 Jul; 47(7): 837-41. [DOI] [PubMed] [Google Scholar]
- 10.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002 Apr; 50(4): 480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adult: epidemiology and risk factors. Gastroenterol. 2009 Aug; 137(2): 512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teunissen TA, van den Bosch WJ, van den Hoogen HJ, et al. Prevalence of urinary, fecal and double incontinence in the elderly living at home. Int Urogynecol J Pelvic Floor Dysfunct. 2004 Jan-Feb; 15(1): 10-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen GD, Hu SW, Chen YC, et al. Prevalence and correlations of anal incontinence and constipation in Taiwanese women. Neurourol Urodyn. 2003 Oct; 22: 664-9. [DOI] [PubMed] [Google Scholar]
- 14. Mimura T, Yamana T, Takao Y, et al. Current Situation of the Management of Fecal Incontinence in Japanese Institutions -Diagnosis and Treatment-. J Jpn Soc Coloproctol. 2012; 65(3): 101-8. [Google Scholar]
- 15.Walker WA, Rothenberger DA, Goldberg SM. Morbidity of internal sphincterotomy for anal fissure and stenosis. Dis Colon Rectum. 1985 Nov; 28(11): 832-5. [DOI] [PubMed] [Google Scholar]
- 16.Kamm MA, Faecal incontinence. BMJ. 1998 Feb; 316: 528-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003 Nov; 90: 1333-7. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005 Apr; 54(4): 546-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLennan AH, Taylor AW, Wilson DH, et al. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000 Aug; 107(12): 1460-70. [DOI] [PubMed] [Google Scholar]
- 20.Walter S, Hallbook O, Gotthard R, et al. A population-based study on bowel habits in a Swedish community: prevalence of faecal incontinence and constipation. Scand J Gastroenterol. 2002 Aug; 37(8): 911-6. [DOI] [PubMed] [Google Scholar]
- 21.Fornell EU, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004 Apr; 83(4): 383-9. [DOI] [PubMed] [Google Scholar]
- 22.Varma MG, Brown JS, Creasman JM, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006 Jun; 49(6): 841-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005 Apr; 53(4): 629-35. [DOI] [PubMed] [Google Scholar]
- 24.Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996 Jun; 347(9016): 1651-3. [DOI] [PubMed] [Google Scholar]
- 25.Damon H, Guye O, Seigneurin A, et al. Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol. 2006 Jan; 30(1): 37-43. [DOI] [PubMed] [Google Scholar]
- 26. Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001 Sep; 161(16): 1989-96. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, Sandler RS, Broom CM, et al. Urgency and fecal soiling in people with bowel dysfunction. Dig Dis Sci. 1986 Nov; 31(11): 1221-5. [DOI] [PubMed] [Google Scholar]
- 28.Lowery SP, Srour JW, Whitehead WE, et al. Habit training as treatment of encopresis secondary to chronic constipation. J Pediatr Gastroenterol Nutr. 1985 Jun; 4(3): 397-401. [DOI] [PubMed] [Google Scholar]
- 29.Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998 Oct; 41(10): 1226-9. [DOI] [PubMed] [Google Scholar]
- 30.Roman H, Robillard PY, Payet E, et al. Factors associated with fecal incontinence after childbirth. Prospective study in 525 women. J Gynecol Obstet Biol Reprod. 2004 Oct; 33(6 Pt 1): 497-505. [DOI] [PubMed] [Google Scholar]
- 31.Zetterstrom J, Lopez A, Anzen B, et al. Anal sphincter tears at vaginal delivery: risk factors and clinical outcome of primary repair. Obstet Gynecol. 1999 Jul; 94(1): 21-8. [PubMed] [Google Scholar]
- 32.Borello-France D, Burgio KL, Richter HE, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006 Oct; 108(4): 863-72. [DOI] [PubMed] [Google Scholar]
- 33.Fenner DE, Genberg B, Brahma P, et al. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2004 Jan; 189(6): 1543-9. [DOI] [PubMed] [Google Scholar]
- 34.Hatem M, Pasquier JC, Fraser W, et al. Factors associated with postpartum urinary/anal incontinence in primiparous women in Quebec. J Obstet Gynaecol Can. 2007 Mar; 29(3): 232-9. [DOI] [PubMed] [Google Scholar]
- 35.Wu JM, Matthews CA, Vaughan CP, et al. Urinary, fecal, and dual incontinence in older U.S. Adults. J Am Geriatr Soc. 2015 May; 63(5): 947-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis. 1995; 10(3): 152-5. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins AT, Olariu AG, Savitt LR, et al. Impact of rising grades of internal rectal intussusception on fecal continence and symptoms of constipation. Dis Colon Rectum. 2016 Jan; 59(1): 54-61. [DOI] [PubMed] [Google Scholar]
- 38.Meyer I, Richter HE. An Evidence-Based Approach to the Evaluation, Diagnostic Assessment and Treatment of Fecal Incontinence in Women. Curr Obstet Gynecol Rep. 2014 Sep; 3(3): 155-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014 Jul; 109(8): 1141-57. [DOI] [PubMed] [Google Scholar]
- 40.Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013 Jan; 108(1): 113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quander CR, Morris MC, Melson J, et al. Prevalence of and factors associated with fecal incontinence in a large community study of older individuals. Am J Gastroenterol. 2005 Apr; 100(4): 905-9. [DOI] [PubMed] [Google Scholar]
- 42.Parés D, Vallverdú H, Monroy G, et al. Bowel habits and fecal incontinence in patients with obesity undergoing evaluation for weight loss: the importance of stool consistency. Dis Colon Rectum. 2012 May; 55(5): 599-604. [DOI] [PubMed] [Google Scholar]
- 43.Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014 Apr; 12(4): 636-43. [DOI] [PubMed] [Google Scholar]
- 44.Johnson JK, Lindow SW, Duthie GS. The prevalence of occult obstetric anal sphincter injury following childbirth-literature review. J Matern Fetal Neonatal Med. 2007 Jul; 20(7): 547-54. [DOI] [PubMed] [Google Scholar]
- 45.Gregory WT, Nygaard I. Childbirth and pelvic floor disorders.Clin Obstet Gynecol. 2004 Jun; 47(2): 394-403. [DOI] [PubMed] [Google Scholar]
- 46.de Leeuw JW, de Wit C, Kuijken JP, et al. Mediolateral episiotomy reduces the risk for anal sphincter injury during operative vaginal delivery. BJOG. 2008 Jan; 115(1): 104-8. [DOI] [PubMed] [Google Scholar]
- 47.MacArthur C, Glazener C, Lancashire R, et al. Exclusive caesarean section delivery and subsequent urinary and faecal incontinence: a 12-year longitudinal study. BJOG. 2011 Jul; 118(8): 1001-7. [DOI] [PubMed] [Google Scholar]
- 48.Bols EM, Hendriks EJ, Berghmans BC, et al. A systematic review of etiological factors for postpartum fecal incontinence. Acta Obstet Gynecol Scand. 2010 Mar; 89(10): 302-14. [DOI] [PubMed] [Google Scholar]
- 49.Juul T, Ahlberg M, Biondo S, et al. International validation of the low anterior resection syndrome score. Ann Surg. 2014 Apr; 259(4): 728-34. [DOI] [PubMed] [Google Scholar]
- 50.Bentzen AG, Guren MG, Vonen B, et al. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013 Jul; 108(1): 55-60. [DOI] [PubMed] [Google Scholar]
- 51.Krogh K, Nielsen J, Djurhuus JC, et al. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997 Oct; 40(10): 1233-9. [DOI] [PubMed] [Google Scholar]
- 52.Burgell RE, Bhan C, Lunniss PJ, et al. Fecal incontinence in men: coexistent constipation and impact of rectal hyposensitivity. Dis Colon Rectum. 2012 Jan; 55(1): 18-25. [DOI] [PubMed] [Google Scholar]
- 53.Hocevar B, Gray M. Intestinal diversion (colostomy or ileostomy) in patients with severe bowel dysfunction following spinal cord injury. J Wound Ostomy Continence Nurs. 2008 Mar-Apr; 35(2): 159-66. [DOI] [PubMed] [Google Scholar]
- 54.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001 Sep; 161(16): 1989-96. [DOI] [PubMed] [Google Scholar]
- 55.Nelson RL. Epidemiology of fecal incontinence. Gastroenterology. 2004 Jan; 126(1): S3-7. [DOI] [PubMed] [Google Scholar]
- 56.Espuña-Pons M, Solans-Domènech M, Sánchez E. Double incontinence in a cohort of nulliparous pregnant women. Neurourol Urodyn. 2012 Nov; 31(8): 1236-41. [DOI] [PubMed] [Google Scholar]
- 57.Matthews CA. Risk factors for urinary, fecal, or double incontinence in women. Curr Opin Obstet Gynecol. 2014 Oct; 26(5): 393-7. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhary BN, Chadwick M, Roe AM. Selecting patients with faecal incontinence for anal sphincter surgery: the influence of irritable bowel syndrome. Colorectal Dis. 2010 Jul; 12(8): 750-3. [DOI] [PubMed] [Google Scholar]
- 59.Scarlett Y. Medical management of fecal incontinence. Gastroenterology. 2004 Jan; 126(1): S55-63. [DOI] [PubMed] [Google Scholar]
- 60.Dobben AC, Terra MP, Deutekom M, et al. Anal inspection and digital rectal examination compared to anorectal physiology tests and endoanal ultrasonography in evaluating fecal incontinence. Int J Colorectal Dis. 2007 Nov; 22(7): 783-90. [DOI] [PubMed] [Google Scholar]
- 61.Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' Clinical Practice Guideline for the Treatment of Fecal Incontinence. Dis Colon Rectum. 2015 Jul; 58(7): 623-36. [DOI] [PubMed] [Google Scholar]
- 62.Rao SS, Sun WM. Current techniques of assessing defecation dynamics. Dig Dis. 1997; 15(1): 64-77. [DOI] [PubMed] [Google Scholar]
- 63. Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1994; 83: 12-8. [PubMed] [Google Scholar]
- 64.Harewood GC, Coulie B, Camilleri M, et al. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999 Jan; 94(1): 126-30. [DOI] [PubMed] [Google Scholar]
- 65.Tuteja AK, Rao SS. Review article: Recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther. 2004 Mar; 19: 829-40. [DOI] [PubMed] [Google Scholar]
- 66.Cundiff GW, Fenner D. Evaluation and treatment of women with rectocele: focus on associated defecatory and sexual dysfunction. Obstet Gynecol. 2004 Dec; 104(6): 1403-21. [DOI] [PubMed] [Google Scholar]
- 67.Keating JP, Stewart PJ, Eyers AA, et al. Are special investigations of value in the management of patients with fecal incontinence? Dis Colon Rectum. 1997 Aug; 40(8): 896-901. [DOI] [PubMed] [Google Scholar]
- 68.Orkin BA., Sinykin SB, Lloyd PC. The Digital Rectal Examination Scoring System (DRESS). Dis Colon Rectum. 2010 Dec; 53(12): 1656-60. [DOI] [PubMed] [Google Scholar]
- 69.de Mello Portella P, Feldner PC Jr, da Conceição JC, et al. Prevalence of and quality of life related to anal incontinence in women with urinary incontinence and pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2012 Feb; 160(2): 228-31. [DOI] [PubMed] [Google Scholar]
- 70.Kirwan WO, Turnbull RB Jr., Fazio VW, et al. Pull-through operation with delayed anastomosis for rectal cancer. Br J Surg. 1978 Oct; 65(10): 695-8. [DOI] [PubMed] [Google Scholar]
- 71.Miller R, Bartolo DC, Locke-Edmunds JC, et al. Prospective study of conservative and operative treatment for faecal incontinence. Br J Surg. 1988 Feb; 75(2): 101-5. [DOI] [PubMed] [Google Scholar]
- 72.Pescatori M, Anastasio G, Bottini C, et al. New grading and scoring for anal incontinence. Evaluation of 335 patients. Dis Colon Rectum. 1992 May; 35(5): 482-7. [DOI] [PubMed] [Google Scholar]
- 73.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993 Jan; 36(1): 77-97. [DOI] [PubMed] [Google Scholar]
- 74.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999 Jan; 44(1): 77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999 Dec; 42(12): 1525-32. [DOI] [PubMed] [Google Scholar]
- 76. Mimura T. What are the severity and classifications of fecal incontinence? In: Maeda K, editor. Bowel management nursing Q& A. Sogo Igaku Sha, Tokyu; 2006. 78-9. [Google Scholar]
- 77.Rockwood TH, Church JM, Fleshman JW, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000 Jan; 43(1): 9-16. [DOI] [PubMed] [Google Scholar]
- 78.Kwon S, Visco AG, Fitzgerald MP, et al. Pelvic Floor Disorders Network P et al. Validity and reliability of the Modified Manchester Health Questionnaire in assessing patients with fecal incontinence. Dis Colon Rectum. 2005 Feb; 48(2): 323-31. [DOI] [PubMed] [Google Scholar]
- 79.Ogata H, Mimura T, Hanazaki K. Validation study of the Japanese version of the Faecal Incontinence Quality of Life Scale. Colorectal Dis. 2012 Feb; 14(2): 194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsunoda A, Yamada K, Kano N, et al. Translation and validation of the Japanese version of the fecal incontinence quality of life scale. Surg Today. 2013 Nov; 43(10): 1103-8. [DOI] [PubMed] [Google Scholar]
- 81.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012 May; 255(5): 922-8. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer. 1998 May; 34(5): 810-5. [DOI] [PubMed] [Google Scholar]
- 83.Tsunoda A, Yasuda N, Nakao K, et al. Validation of the Japanese version of EORTC QLQ-CR38. Qual Life Res. 2008 Mar; 17(2): 317-22. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto H, Shiokawa H, Funahashi K, et al. Development and validation of a modified fecal incontinence quality of life scale for Japanese patients after intersphincteric resection for very low rectal cancer. J Gastroenterol. 2010 Sep; 45(9): 928-35. [DOI] [PubMed] [Google Scholar]
- 85.The Japan Society of Coloproctology. Practice guidelines for fecal incontinence 2017 version. Tokyo: Nankodo; 2017. [Google Scholar]