Abstract
The scientific debate on the criteria guiding hospitalization of tuberculosis (TB) and COVID-19 patients is ongoing.
The aim of this review is to present the available evidence on admission for TB and TB/COVID-19 patients and discuss the criteria guiding hospitalization. Furthermore, recommendations are made as derived from recently published World Health Organization documents, based on Global Tuberculosis Network (GTN) expert opinion.
The core published documents and guidelines on the topic have been reviewed.
The proportion of new TB cases admitted to hospital ranges between 50% and 100% while for multidrug-resistant (MDR) TB patients it ranges between 85 and 100% globally. For TB patients with COVID-19 the proportion of cases admitted is 58%, probably reflecting different scenarios related to the diagnosis of COVID-19 before, after or at the same time of the active TB episode. The hospital length of stay for drug-susceptible TB ranges from 20 to 60 days in most of countries, ranging from a mean of 10 days (USA) to around 90 days in the Russian Federation. Hospitalization is longer for MDR-TB (50–180 days).
The most frequently stated reasons for recommending hospital admission include: severe TB, infection control concerns, co-morbidities and drug adverse events which cannot be managed at out-patient level. The review also provides suggestions on hospital requirements for safe admissions as well as patient discharge criteria, while underlining the relevance of patient-centred care through community/home-based care.
Keywords: TB, COVID-19, Hospital admission, Discharge, Hospitalization criteria, Infection control and prevention, Length of stay, Costs
Introduction
Tuberculosis (TB) and COVID-19 show similarities and differences, as recently discussed in the scientific literature.1, 2, 3, 4, 5, 6, 7
Both diseases have similar signs and symptoms,6, 7, 8, 9, 10 rendering their differential diagnosis difficult as they may also present concomitantly. They might require hospital admission, although justified by different reasons.
Indications of hospital admission for COVID-19 patients have been changing during the evolution of the ongoing pandemic. The occurrence of clinical deterioration11, 12 with progressive dyspnoea and desaturation requiring medical therapy (e.g., dexamethasone) and non-invasive or invasive ventilatory support represents the most important recommendation for an immediate hospitalization as in other respiratory conditions.12, 13, 14 Nevertheless, no previously used criteria or scores have been validated for COVID-19 patients to decide in favour of hospitalization or not15 and new specific criteria are being explored.16
Indications of hospital admission for TB patients are more complex, and in different countries can go far beyond the occurrence of a life-threatening condition. Moreover, they evolved over time. At the time of sanatoria and during the pre-antibiotic era17, 18 admission was used as an ‘isolation’ intervention to reduce Mycobacterium tuberculosis transmission within the community and as support measure to ensure rest, optimal nutrition and eventually to perform pneumothorax after Carlo Forlanini’s discovery in 1907.19 In addition, in children severe extra-pulmonary TB and social circumstances likely contributed to hospitalization.20
Over time, hospital admission was considered ideal to better monitor the initial phase of anti-TB treatment and eventually drug adverse events, and, in some countries, to ensure adequate adherence to the prescribed regimen.21, 22 Furthermore, in several countries hospital admission is still considered an administrative measure of infection control, as patients cannot be discharged until they achieve sputum smear and/or culture conversion21, 22 Although the World Health Organization (WHO) recommends limiting unnecessary hospitalization, this is often complicated by a’ per occupied bed’ refund mechanism prevailing in some countries22, 23, 24 and a sub-optimal Directly Observed Treatment (DOT)/patient’s support practices at outpatient settings.
The present review evaluates the available evidence on hospital admission for TB and TB/COVID-19 and discusses the criteria guiding hospitalization.
Finally, recommendations are made following a recently published WHO (Regional Office for Europe) document on how to reduce Mycobacterium tuberculosis transmission in Europe.21, 22
Methods
A non-systematic search of the scientific literature in English was carried out on PubMed without time restrictions using the following key-words: ‘hospital admission’ ‘COVID-19’, ‘tuberculosis’, ‘length of stay’, ‘ambulatory care’, ‘prevention’, ‘infection control’, and ‘workplace’.
As hospital admission implies programmatic costs, we report available information on this obtained from the literature review.
Data on TB admissions were retrieved from countries of the WHO European Region (WHO workshop: Lessons learned from finance reforms on TB control, Copenhagen, Denmark, April 26th 2016; unpublished data reporting 2014 data) and from relevant existing cohorts allowing analysis of hospital admission-related information.
They include a secondary analysis of a large GTN cohort (global bedaquiline study)25 and, for the duration of the hospital stay for TB in patients with COVID-19, from the ongoing global study on TB/COVID-1926 (interim analysis, November 27th, 2020, see references for details on the two studies).
WHO definitions of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB were adopted.27 Although not approved by WHO (but largely used by clinicians), pre-XDR TB was defined as a form of TB caused by an MDR-TB strain with additional resistance to either a fluoroquinolone or a second line-injectable agent (amikacin, capreomycin or kanamycin).25, 28
A writing committee of international experts including members of the Global Tuberculosis Network (GTN) developed the document after multiple rounds of revision. The document was then proposed for endorsement to the GTN.11, 29 The number of invited GTN members and those endorsing the document is reported under acknowledgements.
Evidence on TB hospital admission
European data (2016, unpublished and WHO/ECDC tuberculosis surveillance and monitoring 2020)30
The available data (average length of stay [ALOS] and proportion of hospital admissions for drug-susceptible and MDR-TB) are presented in Figs. 1 –3, while implications for hospital admission and discharge are discussed in the following sections.
Figure 1.
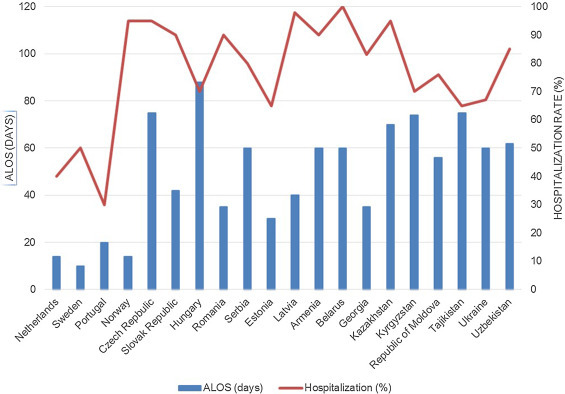
Hospitalization patterns (Average Length of Stay in days and proportion of admitted out of those treated) for newly diagnosed tuberculosis patients in Europe.
ALOS: average length of stay.
Figure 3.
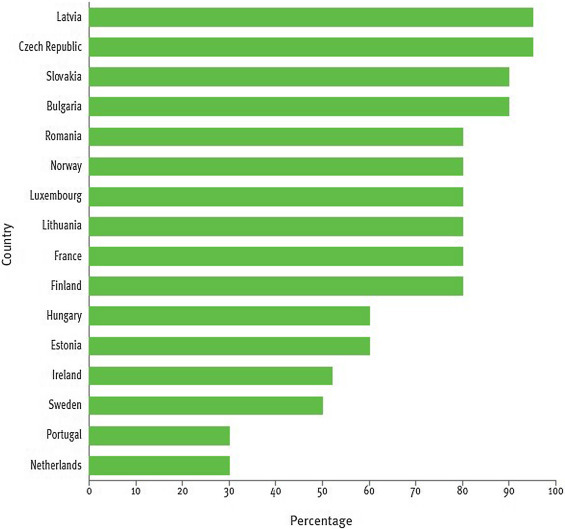
Tuberculosis surveillance and monitoring 2020, and ECDC.30
WHO EURO: World Health Organization Regional Office for Europe; ECDC: European Centre for Disease Prevention and Control.
The Netherlands, Norway, Portugal and Sweden reported the shortest length of stay (LOS) (<20 days), whereas Hungary the highest (>80 days) (Fig. 1 shows the newly diagnosed 2014 cases reported in 2016).
Several Eastern Europe and Central Asia (EECA) countries reported LOS ≥ 60 days, with only Estonia and Georgia showing a LOS < 40 days. The Netherlands, Portugal and Sweden admitted <40% of the diagnosed patients, whereas Norway the vast majority. Other countries admitted from 80% to 100%.
MDR- and XDR-TB patients had a LOS < 50 days in Norway (Fig. 2); Estonia, Georgia, Portugal, Romania, Sweden and Uzbekistan reported <100 days. Kyrgyzstan had the lowest proportion of hospitalized patients (<40%).
Figure 2.
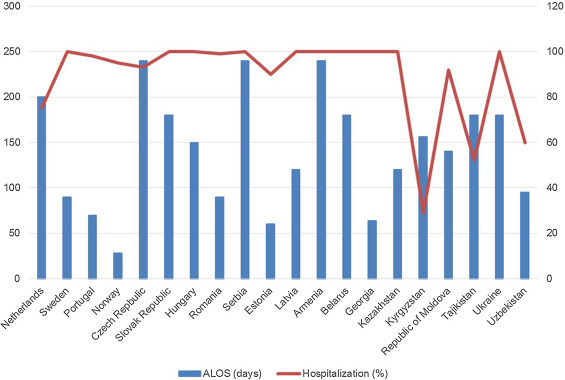
Hospitalization patterns (Average Length of Stay in days and proportion of admitted out of those treated) for patients undergoing treatment of multidrug-resistant tuberculosis in Europe.
ALOS: average length of stay.
In 2018 the Netherlands and Portugal reported the lowest proportion of admitted drug-susceptible TB cases (30%), Ireland and Sweden ∼50%, Estonia and Hungary 60%, and the remaining countries a proportion higher than 80% (Fig. 3).27, 30
The indicator ‘percentage of hospitalization for new TB patients’(1.C.1, Fig. 3) has been added to allow a comprehensive evaluation of the different aspects of TB management in the Region.
An important note is that the information included in Figure 1, Figure 2, Figure 3 refers to countries with different epidemiological characteristics and different rules/practices governing admission and discharge of TB patients.
Global bedaquiline study (2017)25
Although the bedaquiline study was not specifically designed to describe hospital admission (its main focus was the programmatic evaluation of safety and effectiveness of bedaquiline-containing regimens), it included detailed information on this, which is useful to report and discuss.
A total of 364/428 (85%) TB cases from 16 countries (Argentina, Australia, Belarus, Belgium, Greece, India, Italy, the Netherlands, Peru, Portugal, South Africa, Russian Federation, Spain, Sweden, and the United Kingdom) were admitted to hospital: the final anti-TB treatment outcome was reported for 224/368, with 144/368 still on treatment, while no information on hospitalization was available for 60 (14%). The proportion of patients admitted to hospital with a final outcome was: 5/224 (11%) for MDR-TB, 68/224 (65%) for pre XDR-TB, and 111/224 (24%) for XDR-TB. The proportions of admission for those still on treatment were: 31/144 (21.5%) for MDR-TB, 44/144 (30.5%) for pre XDR-TB and 69/144 (47.9%) for XDR-TB.
The median (IQR) LOS was 178.5 (93.5–302.5) days for patients who completed their treatment and 180 (91–217) days for those still on therapy.
In this study, countries from different settings had different rules/practices on TB patients’ admission and discharge. Furthermore, as the study described the early phase of bedaquilne use, a possible bias related to increased hospital admissions due to uncomplete understanding on adverse events severity cannot be excluded.
Global TB and COVID-19 project (2020, interim analysis)26
At an interim analysis (preliminary data, unpublished) on the first 381 patients, 222 (58%) were hospitalized for TB (46/222 with final anti-TB treatment outcome and 176/222 still on treatment), 118 (31%) were not hospitalized, and 41 (11%) with unavailable data.
Importantly, 141 (37%) patients were admitted for concomitant TB and COVID-19. More information on hospital admissions will be possible when the global dataset, in its final format, will be analyzed.
The median (IQR) LOS for TB patients with a final treatment outcome was 29 (10.8–36.5) days, lower than that for patients still on treatment (47, 13.5–106.5 days).
Relevant experiences from the literature in Africa, America and Europe
Africa
A cost analysis performed in South Africa31 clearly demonstrated that significant savings can be obtained by reducing the LOS of MDR-TB cases. A decentralised model of TB care improves case detection and treatment initiation rates, ensuring treatment outcomes comparable to those seen in centralised specialist centre.32 Adoption of decentralised treatment in South Africa may reduce the overall costs for the national TB programme by 15–18%.31, 33
Hospital admission could be limited to patients facing treatment-related issues (such as life-threatening conditions, drug adverse events, failure, need to use injectable medicines or other treatment measures not manageable outside the hospital), which might more frequently occur in patients with XDR- than with MDR-TB (although XDR-TB does not per se contraindicate the possibility of home management).31 Adequate referral system from primary healthcare to specialised services is needed to maintain a continuum of care.
Children are often admitted to hospital for diagnostic reasons, but long-term admission is mainly because of disease severity, including TB meningitis, TB/HIV co-infection and drug-resistant TB, social circumstances or a combination of disease severity and social circumstances.34
TB meningitis, the most devastating form of TB in children, is often treated in hospital. A study in Cape Town, South Africa showed huge cost savings and improved family interaction with effective home-based treatment with good follow-up in children deemed clinically and socially suitable for home-based TB meningitis treatment.35
America
In Montreal, Canada, a study evaluated LOS temporal trends for patients with pulmonary TB between 1993 and 2007.36 The median LOS was 17.0 days, with positive sputum-smear cases showing a longer hospital stay (≥14 days) in comparison with those without (OR = 1.90; 95% CI: 1.34–2.70). Furthermore, older age (≥50 VS. 18–49 years) was associated with longer LOS (OR = 1.66; 95% CI: 1.15–2.40). A LOS ≥ 14 days was consistent with the minimum length recommended by national guidelines but further evidence was needed to evaluate the impact of hospital admission in reducing the community risk of Mycobacterium tuberculosis transmission.
Another experience from Montreal (January 1997- May 2007) reported on 0.65 hospitalizations per TB case, with 17.8 hospital days per TB patient, and a mean LOS of 27.2 days.37
In the USA38 the overall mean annual number of hospitalization events per individual TB patient increased over time from 0.49 (1997–2000) to 0.57 (2013–2016). Moreover, the mean LOS increased from 7.3 to 11.3 days, equal to a change from 3.6 to 6.5 hospital-days per patient.
The mean (standard deviation [SD]) LOS in three Brazilian hospitals in 2013 was 28.2 (32.6) days, without statistically significant differences between HIV-positive and -negative TB patients.24
Of a cohort of 3991 TB/HIV co-infected patients in Rio de Janeiro, Brazil,39 evaluated between 2007 and 2013, 46.6% were hospital admitted (10.44 per 100 person-years). An annual decrease was recorded for both the hospitalization rate (incidence rate ratio 0.92) and LOS (median of 15 days in 2007 VS. 11 days in 2013; p-value for trend <0.001).
Europe
A survey conducted in 1999 in an Italian sample recruiting 203 pulmonology centres showed a median LOS of 34 days for sputum smear-positive, 20 days for sputum smear-negative, and 21.5 days for extra-pulmonary TB cases. Sputum smear conversion to negative was considered the key criterion for discharge in 61% of the centres.40 A switch from hospital- to home-based treatment (limiting hospital admission to severe cases) was recommended based on cost-effectiveness.41 In the study no information of the patients’ drug resistance profile was available.
In a Portuguese sample of 15,296 TB cases (4,415, 28.9% admitted) enrolled between 2008 and 2013, hospital admission did not influence treatment outcomes, with 13.8% unfavourable outcomes among hospitalized patients compared with 7.6% among non-hospitalized ones.42, 43
In the Russian Federation, as published in 2007, the LOS was rather longer both before (86 days) and after (90 days) the implementation of the WHO Directly observed treatment, short-course (DOTS) Strategy.44 This LOS refers to patients with drug-susceptible TB.
In Spain 41% of cases were managed in 2014–2015 as outpatients,45 with a mean LOS of 11.3 (±7) days. Out of 319 patients included in the study, only 14 (4.5%) were TB drug-resistant. The authors concluded that an important proportion of the overall management cost of TB patients was related to hospital admission, and recommended to reduce the hospital stay. This prospective study involved 19 hospitals belonging to SEPAR (Sociedad Española de Aparato Respiratorio) network.
A recent study from Switzerland46 estimated a TB hospitalization rate of 81% between 2002 and 2015, with a median (IQR) LOS of 14 days (6–22). LOS increased in case of miliary TB, older age, and for some hospital locations. The most prevalent comorbidities were HIV infection, liver disease, anaemia, malnutrition, and genito-urinary tract disease. LOS was specifically evaluated in the following sub-groups: 1) malnutrition, cachexia and anaemia (median, IQR: 20, 13–31, days); 2) alcoholic liver disease and hepatitis (median, IQR: 23, 14–37.5, days) and 3) adverse drug events (median, IQR: 20, 13–30, days).
Summary of the findings
-
1)
The proportion of newly diagnosed admitted TB cases ranges between 50% and 100% (47% in Brazil, 57% in USA, 65% in Canada, >80% in the WHO European Region where the proportion of patients hospitalized is higher than in the other settings evaluated;
-
2)
The proportion of MDR-TB admitted patients is ∼100% in Europe and 85% in the bedaquiline study;
-
3)
The proportion of TB and COVID-19 admitted patients is around 58% according to the limited evidence available, which included about 80% of drug-susceptible TB patients.26 Different reasons are likely behind this figure, including the need to admit patients after simultaneous diagnosis of both diseases, or to re-hospitalize TB cases already discharged after initial anti-TB treatment and worsened because of COVID-19 (or COVID-19 appearing in already hospitalized TB patients).
-
4)
LOS of newly diagnosed cases ranges from 20 to 60 days in Europe, similarly to Canada (mean 17 and median 27 days), Brazil (median 15 days), and Italy (not included in the European dataset: 31 days for sputum smear-positive patients and 20 days for other cases). Studies from USA report the lowest LOS (mean 10 days) and the Russian Federation the highest (∼90 days).
-
5)
LOS of MDR-TB patients was 50–100 days in most of European countries, ∼180 days in the bedaquiline study (which reported severe cases with high proportion of XDR-TB) and 29–47 days for TB and COVID patients.
The duration of hospital stay depends on bacteriological conversion in several countries.
TB/COVID-19 admissions
Global TB/COVID-19 project (2020, interim analysis)
An interim analysis was conducted on 381 patients from 40 countries: 263/381 (69%) were hospitalized for COVID-19, 31/263 (12%) were still hospitalized, 201/263 (76%) were discharged and 31/263 (12%) died, whereas 113/381 (30%) were not hospitalized. Information on hospital admission was missing in 5 (1%) patients. As reported under ‘TB admissions’, a total of 141/381 (37%) patients underwent hospital admission for concomitant TB and COVID-19.
The median (IQR) LOS attributed to COVID-19 was 16 (10–22) days.
Hospital admission data for COVID-19 is not yet comprehensively and systematically reported by Member States to WHO. Following different sources of already existing data inputs, however, hospital stay duration can differ widely depending on numerous factors, including age and co-morbidities among others.
A study conducted in 25 European countries by the members of the Paediatric Tuberculosis Network European Trials Group on COVID-19, hospital admissions in children and adolescents identified 582 individuals during April 2020 with polymerase chain reaction (PCR) test confirmed of SARS-CoV-2 infection with a median (IQR) age of 5·0 years (0·5–12·0): TB was not documented as comorbidity in any of these individuals.47
Recommendations on hospital admission
Several documents consistently reported recommendations for TB-related hospital admission in Europe21, 22, 48 summarised as follows:
-
a)
TB-related conditions requiring hospital treatment (i.e., respiratory failure and surgical emergencies e.g -haemorrhage, pneumothorax, and pleural effusion);
-
b)
Severe forms of TB and/or pre-existing co-morbidities exacerbated by TB which cannot be managed in outpatient settings (e.g., coma, liver or renal disease, uncontrolled diabetes, etc.)49;
-
c)
Life-threatening or severe adverse effects associated to anti-TB drugs (e.g., arrhythmias, psychosis, renal failure, hearing loss), particularly in fragile/non-self-sufficient patients.
Additional considerations on hospitalization include:
-
1)
Patients where effective and safe treatment cannot be ensured in outpatient, community, and home settings (e.g., homelessness, overcrowding, exposure of children aged <5 years and pregnant women in households, children with severe TB and unreliable caregivers) or difficult geographical accessibility (e.g., long distance to outpatient facilities or problematic travel conditions);
-
2)
Involuntary isolation of non-adherent patients once all other healthcare options have been unsuccessfully tried.
It was recommended to care for admitted patients in single rooms. In hospital settings, people with presumed infectious TB or confirmed pulmonary TB should be assessed rapidly for MDR-TB, using existing rapid diagnostic methods to allow adequate management and reducing the risk of transmission, especially to those patients with immune-compromised conditions/status.
Importantly, several pre-conditions have been proposed to allow safe in-hospital management:
-
a)
Appropriate infection control and prevention measures should be in place and continuously assessed50;
-
b)
Respiratory isolation rooms should be available for TB patients until they are smear/culture-negative;
-
c)
Staff should be trained and adequately supervised, and adhere to administrative measures included in the facility’s infection control and prevention plan;
-
d)
Trained staff should be available to ensure quality patient-centred care;
-
e)
Open and safe space should be available for patients to socialize according to infectiousness status and resistance patterns (ideally not mixing infectious with non-infectious patients or drug-susceptible and drug-resistant patients);
-
f)
“Friendly” but effective administrative procedures should be in place to facilitate regular access of visitors;
-
g)
Protocols should be in place for effective communication and coordination, detailing terms of reference for involved staff including accountability and responsibility, for laboratories providing services during treatment and for peripheral units receiving patients after hospital discharge;
-
h)
Reference clinical centres located in low TB incidence countries should ensure quality services based on a minimum number of patients necessary to maintain proficiency.
Specific recommendations on hospitalization of TB/COVID-19 patients are not yet available. As of today, we need to consider the existing criteria for the most severe prevalent condition. If TB is the most relevant condition, the criteria defined above can be used to guide decisions. COVID-19 hospitalization will be needed when the disease is clinically severe, i.e. in presence of respiratory failure (desaturations, and need for oxygen supplementation or mechanical ventilation) or, in the future, when the patient will meet specific clinical-radiological scores that are under study.12
Criteria guiding hospital discharge
Criteria for hospital discharge of co-infected (SARS-COV-2 and TB) patients should be based mainly on the TB clinical course given that COVID-19 is a disease with a fast clinical evolution and it can stop requiring hospitalization more rapidly than TB.
The criteria for hospital discharge are:
-
a)
Clinical improvement of TB (e.g. improvement of signs and symptoms, radiological improvement, weight gain);
-
b)
Clinical improvement of the co-morbidities and/or of the adverse events precipitating the hospital admission;
-
c)
Effective treatment and continuity of care ensured with a patient-centred model of care as per WHO and national guidelines.
Bacteriological conversion per se is not a mandatory criterion for hospital discharge, given that precautions are recommended to educate family members on basic infection control and prevention requirements and to monitor the continuation of treatment at home. Adequate treatment can render patients rapidly non-infectious, although sputum smear positivity can be present for a longer period (dead bacilli).21, 22 Nevertheless, transmission can occur if an ineffective regimen is prescribed – for example, when a first-line or sub-optimal regimen is used in MDR-TB patients. Thus, MDR- and XDR-TB regimens need to be guided by rapid drug susceptibility testing.21, 22, 29, 51 , 52
Typically, patients have already infected their household members prior to diagnosis.21, 22 Therefore, contact-tracing of household members and other close contacts is essential to rapidly diagnose and treat them and reduce transmission of Mycobacterium tuberculosis within the community.
In summary, ambulatory treatment of TB (regardless of smear and drug-susceptibility patterns) is possible from the beginning to reduce the risk of hospital transmission and improve treatment adherence. Importantly, outpatient care should be organised following strict infection control and prevention measures.
Conclusions
Existing evidence from WHO guidance and the literature review indicate that a high proportion of TB cases continue to be admitted in hospitals, and for relatively long length of stay. This is particularly evident in Europe.
Among the different reasons behind this finding, existing legislation on infection control (e.g. patients cannot be discharged until bacteriological conversion has been achieved) and funding mechanisms (focus on per bed occupancy) play a role, slowing down the ‘reduction of unnecessary hospital admission’ recommended by WHO.
A collaboration of all stakeholders involved in TB prevention, diagnosis and treatment is necessary to support the WHO recommendations in order to reduce patient suffering, TB transmission and lower costs within a patient-centred vision.
Declarations of interest
None.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Out of 39 GTN members responding, a single member, although agreeing with the paper, was unable to endorse it as needing the institutional clearance. Of the remaining 38, 37 endorsed the document (97.4%).
This article belongs to the scientific activities of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2017-2020- GBM/RC/LDA and of the European Research Initiative (ERI).
The Authors wish to thank Szabolcs Szigeti and Alexandre Lourenco for providing data on hospital admission in Europe as well as Alberto L. Garcia-Basteiro (Barcelona Institute for Global Health, Barcelona, Spain) and Askar Yedilbayev (WHO Regional Office for Europe, Copenhagen, Denmark) for their useful comments on the manuscript.
References
- 1.Alagna R., Besozzi G., Codecasa L.R., Gori A., Migliori G.B., Raviglione M. Celebrating World Tuberculosis Day at the time of COVID-19. Eur Respir J. 2020;55(4):2000650. doi: 10.1183/13993003.00650-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dara M., Sotgiu G., Reichler M.R., Chiang C.-Y., Chee C.B.E., Migliori G.B. New diseases and old threats: lessons from tuberculosis for the COVID-19 response. Int J Tuberc Lung Dis. 2020;24(5):544–545. doi: 10.5588/ijtld.20.0151. [DOI] [PubMed] [Google Scholar]
- 3.Zumla A., Marais B.J., McHugh T.D., Maeurer M., Zumla A., Kapata N. COVID-19 and tuberculosis-threats and opportunities. Int J Tuberc Lung Dis. 2020;24(8):757–760. doi: 10.5588/ijtld.20.0387. [DOI] [PubMed] [Google Scholar]
- 4.Leung C.C., Lam T.H., Cheng K.K. Let us not forget the mask in our attempts to stall the spread of COVID-19. Int J Tuberc Lung Dis. 2020;24(4):364–366. doi: 10.5588/ijtld.20.0124. [DOI] [PubMed] [Google Scholar]
- 5.Visca D., Tiberi S., Pontali E., Spanevello A., Migliori G.B. Tuberculosis in the time of COVID-19: quality of life and digital innovation. Eur Respir J. 2020;56 doi: 10.1183/13993003.01998-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motta I., Centis R., D’Ambrosio L., García-García J.M., Goletti D., Gualano G. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–240. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . 2020. WHO Information Note on Tuberculosis and COVID-19. 12 May. https://www.who.int/docs/default-source/documents/tuberculosis/infonote-tb-covid-19.pdf. Last access 15 December 2020. [Google Scholar]
- 8.Visca D., Ong C.W.M., Tiberi S., Centis R., D’Ambrosio L., Chenc B. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2020.12.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadolini M., Codecasa L.R., García-García J.M., Blanc F.X., Borisov S., Alffenaar J.W. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stochino C., Villa S., Zucchi P., Parravicini P., Gori A., Raviglione M.C. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong C.W.M., Migliori G.B., Raviglione M., MacGregor-Skinner G., Sotgiu G., Alffenaar J.W. Epidemic and pandemic viral infections: impact on tuberculosis and the lung. A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN) and members of ESCMID Study Group for Mycobacterial Infections (ESGMYC) Eur Respir J. 2020;56(4):2001727. doi: 10.1183/13993003.01727-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Raya B., Migliori G.B., O’Ryan M., Edwards K., Torres A., Alffenaar J.-W. Coronavirus Disease-19: an interim evidence synthesis of the World Association for Infectious Diseases and Immunological Disorders (Waidid) Front. Med. 2020;7 doi: 10.3389/fmed.2020.572485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winck J.C., Ambrosino N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology. 2020;26(4):213–220. doi: 10.1016/j.pulmoe.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliberti S., Messinesi G., Gamberini S., Maggiolini S., Visca D., Galavotti V. Non-invasive mechanical ventilation in patients with diffuse interstitial lung diseases. BMC Pulm Med. 2014;14:194. doi: 10.1186/1471-2466-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley P., Frost F., Tharmaratnam K., Wootton D.G., NW Collaborative Organisation for Respiratory Research Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir Res. 2020;7(1):e000729. doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozafari A., Miladinia M., Sabri A., Movaseghi F., Baeis M.G. The challenge of deciding between home-discharge versus hospitalization in COVID-19 patients: the role of initial imaging and clinicolaboratory data. Clin Epidemiol Glob Health. 2020 doi: 10.1016/j.cegh.2020.11.006. Dec 3. doi: 10.1016/j.cegh.2020.11.006.Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwick E.D., Pepperell C.S. Tuberculosis sanatorium treatment at the advent of the chemotherapy era. BMC Infect Dis. 2020;20(1):831. doi: 10.1186/s12879-020-05539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migliori G.B., Sotgiu G., Lange C., Centis R. Extensively drug-resistant tuberculosis: back to the future. Eur Respir J. 2010;36(3):475–477. doi: 10.1183/09031936.00025910. [DOI] [PubMed] [Google Scholar]
- 19.Mazzarello P. A Physical Cure for Tuberculosis: Carlo Forlanini and the Invention of Therapeutic Pneumothorax. Appl. Sci. 2020;10(9):3138. doi: 10.1001/jamadermatol.2014.559. [DOI] [Google Scholar]
- 20.Roberts C.A., Bernard M.C. Tuberculosis: a biosocial study of admissions to a children’s sanatorium (1936-1954) in Stannington, Northumberland, England. Tuberculosis (Edinb) 2015;95(Suppl 1):S105–8. doi: 10.1016/j.tube.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Regional Office For Europe Copenhagen, World Health Organization Regional Office For Europe; 2018. Guiding principles to reduce tuberculosis transmission in the WHO European region. http://www.euro.who.int/__data/assets/pdf_file/0008/377954/ic-principles-eng.pdf Last access 15 December 2020.
- 22.Migliori G.B., Nardell E., Yedilbayev A., D’Ambrosio L., Centis R., Tadolini M. Reducing tuberculosis transmission: a consensus document from the World Health Organization Regional Office for Europe. Eur Respir J. 2019;53(6):1900391. doi: 10.1183/13993003.00391-2019. [DOI] [PubMed] [Google Scholar]
- 23.Davtyan K., Hayrapetyan A., Dara M., Gillini L., Davtyan H., Centis R. Key role of tuberculosis services funding mechanisms in tuberculosis control and elimination. Eur Respir J. 2015;45(1):289–291. doi: 10.1183/09031936.00118514. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves M.J., Ferreira A.A. Factors associated with length of hospital stay among HIV positive and HIV negative patients with tuberculosis in Brazil. PLoS One. 2013;8(4):e60487. doi: 10.1371/journal.pone.0060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borisov S.E., Dheda K., Enwerem M., Romero Leyet R., D’Ambrosio L., Centis R. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):1700387. doi: 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- 26.The TB/COVID-19 Global Study Group TB and COVID-19 co-infection: rationale and aims of a global Study. Int J Tuberc Lung Dis. 2021 doi: 10.5588/ijtld.20.0786. in press. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . World Health Organization; Geneva: 2020. Global tuberculosis report 2020. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020. Last access 15 December 2020. [Google Scholar]
- 28.Borisov S., Danila E., Maryandyshev A., Dalcolmo M., Miliauskas S., Kuksa L. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54(6):1901522. doi: 10.1183/13993003.01522-2019. [DOI] [PubMed] [Google Scholar]
- 29.Migliori G.B., Tiberi S., Zumla A., Petersen E., Chakaya J.M., Wejse C., members of the Global Tuberculosis Network MDR/XDR-TB management of patients and contacts: Challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. Int J Infect Dis. 2020;92S:S15–S25. doi: 10.1016/j.ijid.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 30.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2020 – 2018 data. ECDC; Stockholm: 2020. https://www.ecdc.europa.eu/sites/default/files/documents/TB-Surveillance-report_24March2020.pdf Last access 15 December 2020. [Google Scholar]
- 31.Ramma Sinanovic L., Vassall A., Azevedo V., Wilkinson L., Ndjeka N. Impact of reduced hospitalisation on the cost of treatment for drug-resistant tuberculosis in South Africa. Int J Tuberc Lung Dis. 2015;19(2):172–178. doi: 10.5588/ijtld.14.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox H., Hughes J., Daniels J., Azevedo V., McDermid C., Poolman M. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis. 2014;18(4):441–448. doi: 10.5588/ijtld.13.0742. [DOI] [PubMed] [Google Scholar]
- 33.Pooran A., Pieterson E., Davids M., Theorn G., Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0054587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soeters M., De Vries A.-M., Kimpen J.L.L., Donald P.R., Schaaf H.S. Clinical features and outcome in children admitted to a tuberculosis hospital in the Western Cape Province: the influence of HIV infection and drug resistance. S Afr Med J. 2005;95:602–606. [PubMed] [Google Scholar]
- 35.Van Elsland S.L., van Dongen S.I., Bosmans J.E., Schaaf H.S., van Toorn R., van Furth A.M. Cost-effectiveness of home-based versus in-hospital treatment of paediatric tuberculous meningitis. Int J Tuberc Lung Dis. 2018;22(10):1188–1195. doi: 10.5588/ijtld.18.0236. [DOI] [PubMed] [Google Scholar]
- 36.Dehghani K., Allard R., Gratton J., Marcotte L., Rivest P. Trends in Duration of Hospitalization for Patients with Tuberculosis in Montreal, Canada from 1993 to 2007. Can J Public Health. 2011;102(2):108–111. doi: 10.1007/BF03404157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronald L.A., FitzGerald J.M., Benedetti A., Boivin J.F., Schwartzman K., Bartlett-Esquilant G. Predictors of hospitalization of tuberculosis patients in Montreal, Canada: a retrospective cohort study. BMC Infect Dis. 2016;16(1):679. doi: 10.1186/s12879-016-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada P.Y., Lee-Rodriguez C., Hung Y.Y., Skarbinski J. Burden of Active Tuberculosis in an Integrated Health Care System, 1997-2016: Incidence, Mortality, and Excess Health Care Utilization. Open Forum Infect Dis. 2020;7(1) doi: 10.1093/ofid/ofaa015. ofaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coelho L.E., Ribeiro S.R., Veloso V.G., Grinsztejn B., Luz P.M. Hospitalization rates, length of stay and in-hospital mortality in a cohort of HIV infected patients from Rio de Janeiro. Brazil. Braz J Infect Dis. 2017;21(2):190–195. doi: 10.1016/j.bjid.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutini S., Fiorenti F., Codecasa L.R., Casali L., Besozzi G., Di Pisa G. Hospital admission policy for tuberculosis in pulmonary centres in Italy: a national survey. AIPO Tuberculosis Study Group. Italian Association of Hospital Pulmonologists. Int J Tuberc Lung Dis. 1999;3(11):985–991. PMID: 10587320. [PubMed] [Google Scholar]
- 41.Migliori G.B., Ambrosetti M., Besozzi G., Farris B., Nutini S., Saini L. Cost-comparison of different management policies for tuberculosis patients in Italy. AIPO TB Study Group. Bull World Health Organ. 1999;77(6):467–476. [PMC free article] [PubMed] [Google Scholar]
- 42.Boaventura R., Santos J.V., Viana J., Freitas A., Duarte R. Does hospitalization influence tuberculosis’ treatment outcome? A Portuguese nationwide study. Eur Respir J. 2018;52(Suppl. 62) doi: 10.1183/13993003.congress-2018.PA530. PA530. [DOI] [Google Scholar]
- 43.Galego M.A., Santos J.V., Viana J., Freitas A., Duarte R. To be or not to be hospitalised with tuberculosis in Portugal. Int J Tuberc Lung Dis. 2019;23(9):1029–1034. doi: 10.5588/ijtld.18.0617. [DOI] [PubMed] [Google Scholar]
- 44.Marx F.M., Atun R.A., Jakubowiak W., McKee M., Coker R.J. Reform of tuberculosis control and DOTS within Russian public health systems: an ecological study. Eur J Public Health. 2007;17(1):98–103. doi: 10.1093/eurpub/ckl098. [DOI] [PubMed] [Google Scholar]
- 45.Gullón J.A., García-García J.M., Villanueva M.Á, Álvarez-Navascues F., Rodrigo T., Casals M. Grupo de Trabajo del Programa Integrado de Investigación en Tuberculosis (PII TB) Tuberculosis Costs in Spain and Related Factors. Arch Bronconeumol. 2016;52(12):583–589. doi: 10.1016/j.arbres.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Tonko S., Baty F., Brutsche M.H., Schoch O.D. Length of hospital stay for TB varies with comorbidity and hospital location. Int J Tuberc Lung Dis. 2020;24(9):948–955. doi: 10.5588/ijtld.19.0759. [DOI] [PubMed] [Google Scholar]
- 47.Götzinger F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I. ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization Regional Office for Europe . first edition. World Health Organization Regional Office for Europe; Copenhagen: 2017. A people-centred model of TB care. Blueprint for EECA countries.https://www.euro.who.int/__data/assets/pdf_file/0004/342373/TB_Content_WHO_PRO_eng_final.pdf Last access 15 December 2020. [Google Scholar]
- 49.Singla R., Raghu B., Gupta A., Caminero J.A., Sethi P., Tayal D. Risk factors for early mortality in patients with pulmonary tuberculosis admitted to the emergency room. Pulmonology. 2021;27(1) doi: 10.1016/j.pulmoe.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Ippolito M., Vitale F., Accurso G., Iozzo P., Gregoretti C., Giarratano A. Medical masks and Respirators for the Protection of Healthcare Workers from SARS-CoV-2 and other viruses. Pulmonology. 2020;26(4):204–212. doi: 10.1016/j.pulmoe.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization . World Health Organization; Geneva: 2020. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/332397. Last access 9 December 2020. [PubMed] [Google Scholar]
- 52.Nahid P., Mase S.R., Migliori G.B., Sotgiu G., Bothamley G.H., Brozek J.L. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. Erratum in: Am J Respir Crit Care Med. 2020 Feb 15;201(4):500-501. [DOI] [PMC free article] [PubMed] [Google Scholar]


