Abstract
Objectives
Hypoalbuminemia is a negative acute phase reactant which has been associated with inflammatory response and poor outcome in infectious diseases. The aim of this study was to analyze the value of hypoalbuminemia on admission as a predictor of mortality and adverse events in COVID-19 patients.
Methods
We analyzed retrospective data from a cohort of 609 consecutive patients, with confirmed diagnosis of COVID-19, discharged from hospital (deceased or alive). Demographic characteristics, previous comorbidities, symptoms and laboratory findings on admission were collected. Comorbidities were assessed by Charlson-Age Comorbidity Index.
Results
Hypoalbuminemia on admission (<34 g/L) was more frequent in nonsurvivors than survivors (65.6% vs. 38%, p < 0.001) and was significantly associated with the development of sepsis, macrophage activation syndrome, acute heart failure, acute respiratory distress syndrome and acute kidney injury, regardless of Charlson-Age Comorbidity Index. Hypoalbuminemia was a predictor of mortality in multivariable Cox regression analysis (HR 1.537, 95% CI 1.050–2.250, p = 0.027), independently of Charlson-Age Index, gender, lymphocyte count <800/μL, creatinine, high-sensitivity C- reactive protein >8 mg/L, lactate dehydrogenase >250 U/L, bilateral infiltration on chest X-ray and q-SOFA ≥2.
Conclusions
Hypoalbuminemia was an early predictor of in-hospital mortality in COVID-19, regardless of age, comorbidity and inflammatory markers. It also had significant association with severe adverse events, independently of Charlson-Age Comorbidity Index. Our results suggest that serum albumin determination on admission may help to identify patients with SARS-CoV-2 infection at high risk of developing potential life-threatening conditions and death.
Keywords: Hypoalbuminemia, COVID-19, SARS-CoV-2, Predictors of mortality, Predictors of adverse events
Abstract
Objetivos
La hipoalbuminemia es un reactante de fase aguda negativo que ha sido asociado a la respuesta inflamatoria y mal resultado en enfermedades infecciosas. El objetivo de este estudio fue analizar el valor de la hipoalbuminemia en el momento del ingreso, como factor predictivo de mortalidad y episodios adversos en los pacientes de COVID-19.
Métodos
Analizamos los datos retrospectivos de una cohorte de 609 pacientes consecutivos, con diagnóstico confirmado de COVID-19, que abandonaron el hospital (fallecidos o vivos). Se recopilaron las características demográficas, comorbilidades previas, síntomas y hallazgos de laboratorio en el momento del ingreso. Las comorbilidades se asociaron al índice de comorbilidad de Charlson-Age.
Resultados
La hipoalbuminemia en el momento del ingreso (< 34 g/l) fue más frecuente en los no supervivientes que en los supervivientes (65,6 vs. 38%; p < 0,001) y estuvo significativamente asociada a desarrollo de sepsis, síndrome de activación macrofágica, insuficiencia cardiaca aguda, síndrome de distrés respiratorio agudo e insuficiencia renal aguda, independientemente del índice de comorbilidad de Charlson-Age. La hipoalbuminemia fue un factor predictivo de la mortalidad en el análisis multivariable de regresión de Cox (HR: 1,537; IC 95%: 1,050-2,250; p = 0,027), independientemente del índice de Charlson-Age, sexo, recuento linfocítico < 800/μl, creatinina, proteína C reactiva de alta sensibilidad > 8 mg/l, lactato deshidrogenasa > 250 U/l, infiltración bilateral en la placa de tórax y q-SOFA ≥ 2.
Conclusiones
La hipoalbuminemia fue un factor predictivo temprano de la mortalidad intrahospitalaria en la COVID-19, independientemente de la edad, de la comorbilidad y de los marcadores inflamatorios. También tuvo una asociación significativa con episodios adversos graves, independientemente del índice de comorbilidad de Charlson-Age. Nuestros resultados sugieren que determinar la albúmina sérica en el momento del ingreso podría ayudar a identificar a los pacientes con infección por SARS-CoV-2 con alto riesgo de desarrollar situaciones potencialmente mortales y muerte.
Palabras clave: Hipoalbuminemia, COVID-19, SARS-CoV-2, Factores predictivos de la mortalidad, Factores predictivos de episodios adversos
Introduction
Coronaviruses are enveloped, RNA viruses which belong to the family Coronaviridae. They can infect many different host species, including humans. First described in the 1960s, human coronaviruses (HCoVs) can cause respiratory and intestinal tract infections with a wide range of clinical manifestations which vary from common cold (more frequent in low pathogenic HCoVs like: HCoV-229E, HCoVOC43, HCoV-NL63 and HCoV-HKU) to acute respiratory distress syndrome (in high pathogenic HCoVs like SARSCoV and MERSCoV, which caused severe epidemics in 2002 and 2017).1 In December 2019, a new Betacoronavirus was identified in a cluster of patients presenting with pneumonia in Wuhan, China.2 It was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease which causes, coronavirus disease 2019 (COVID-19).
Cytokines and chemokines play an important role in inflammatory response during virus infections. However, some viruses like HCoVs can induce an excessive cytokine/chemokine response, known as “cytokine storm”, which can lead to a diminished T-cell response.3 This circumstance has also been described in SARS-CoV-2 infection and leads to a dysregulated inflammatory response associated with high morbidity and mortality.4 Hypoalbuminemia is associated with inflammatory response in critical illness. The release of cytokines and chemokines induces an increase in capillary leakage which alters the distribution of albumin between intravascular and extravascular compartments.5 The aim of this study was to analyze the predictive value of hypoalbuminemia in the occurrence of adverse events and mortality in hospitalized COVID-19 patients.
Methods
Study design and measurements
We performed a retrospective analysis of data from a cohort of 609 consecutive patients discharged (deceased or alive) from a general hospital between March and April 2020, with diagnosis of COVID-19 confirmed by a positive result on real-time reverse transcription polymerase chain reaction (RT-PCR) for the presence of SARS-CoV-2 in nasal and pharyngeal swab specimens. Exclusion criteria were patient's explicit refusal to participate or lack of measurement of serum albumin within first days from admission. The study was approved by our local Ethics Research Committee. Written informed consent was waived by the Ethics Committee due to the design of the study.
The following information was collected for each patient: demographic characteristics; previous treatments; symptoms, vital signs, chest X-ray findings and laboratory findings on admission; in-hospital adverse events and support treatment. Comorbidities were assessed by Charlson-Age Comorbidity Index6 which includes 19 medical conditions weighted 1 to 6 points (acute coronary syndrome, heart failure, peripheral arterial disease, cerebrovascular disease, dementia, chronic lung disease, connective disease, gastroduodenal ulcer, mild or moderate-severe chronic liver disease, diabetes with or without target organ damage, hemiplegia, moderate-severe chronic renal failure, solid cancer with or without metastasis, leukemia, lymphoma, acquired immunodeficiency syndrome); adding one point for each decade of life over the age of 50 (score from 0 to 43).
For each patient quick-Sequential Organ Failure Assessment score7 (q-SOFA) was calculated. Acute Respiratory Distress Syndrome (ARDS) was diagnosed according to the Berlin definition.8 Sepsis was defined according to “The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)”.9 Macrophage activation syndrome (MAS) was diagnosed when three or more of the following conditions were present: sustained fever (>38 °C), hepatosplenomegaly, hyperferritinemia (>1000 μg/L), pancytopenia (at least two of hemoglobin <12 g/L, white blood cell count <5.0 × 109/L or platelet count <150 × 109/L), hypofibrinogenemia (<250 mg/dL), hypertriglyceridemia (>300 mg/dL), high sensitivity C-reactive protein (hs-CRP >150 mg/L), aspartate aminotransferase (AST >50 U/L) and exclusion of severe systemic bacterial infection (procalcitonin <0.5 ng/mL). These criteria were based on HLH (hemophagocytic lymphohistiocytosis)-2004 guidelines10 after multidisciplinary consensus in our institution. Primary outcome was all-cause in-hospital mortality. Serum albumin concentration was analyzed using the BCG (bromocresol green) colorimetric dye-binding method on an ArchitectTM c16000 (Abbott Laboratories). Laboratory reference range is 34 to 50 g/L, with a lower detection limit of 3.1 g/L and an inter-assay coefficient of variation (CV) of 0.7–1.3%. We used serum albumin assessed on admission and when it was not available, first albumin assessed within first days of hospitalization.
Statistical analysis
Continuous variables were presented as median and interquartile range (IQR) or mean ± standard deviation (SD) as appropriate, according to the Kolmogorov–Smirnov test, while categorical variables were presented as % (n/total). Continuous variables were compared using t test or Mann–Whitney U test as appropriate and categorical variables using χ 2 test. We used binary logistic regression to identify variables associated with in-hospital mortality and to analyze the association of hypoalbuminemia with the development of adverse events. Results were reported as crude odds ratio (OR) with 95% confidence interval (CI) and adjusted to Charlson-Age Index OR. For the purpose of the analysis and clinical interpretation, albumin, lymphocyte count, hs-CRP and lactate dehydrogenase (LDH) were dichotomized and entered into the multivariable model as binary variables, using as cut-off point the upper limit of reference interval defined for each variable by our local biochemistry laboratory. Hypoalbuminemia was defined as serum albumin concentration below 34 g/L. For lymphocyte count, we used Youden's J statistic to identify the optimal cut-off point. Kaplan–Meier survival analysis was used to assess survival differences between patients with and without serum hypoalbuminemia on hospital admission. A multivariable Cox proportional hazards regression model was performed to find independent predictors for in-hospital mortality. Prespecified independent variables included in Cox model were those with significant adjusted OR for all-cause in-hospital mortality. Patients with missing values were not included in multivariable analysis. The relationship between serum albumin levels and the predicted in-hospital mortality was graphically represented after modeling this association using fractional polynomials. The IBM SPSS Statistics 21.0 software package (IBM Corp; IBM SPSS Statistics) was used for all calculations and STATA 14.2 software package (StataCorp) for graphic design. Differences were considered statistically significant if the null hypothesis could be rejected with >95%Cl. STROBE11 guidelines for observational studies were followed.
Results
Baseline characteristics
Baseline characteristics of the 609 patients included in the study are depicted in Table 1 . Median age was 71 years (IQR: 58–82), 60.3% (367) were male and median Charlson-Age Index of the whole population was 3 (IQR: 2–5). During hospitalization 128 patients (21%) died. Compared to survivors, deceased patients were older and had a higher prevalence of hypertension, dyslipidemia, and diabetes mellitus, with no significant difference for smoking status. Chronic heart disease, including atrial fibrillation/flutter, history of cancer, immunosuppressive conditions, chronic kidney disease and dementia were more frequent in deceased patients, without any significant difference in chronic lung disease prevalence between groups. Median Charlson-Age Index for nonsurvivors was 5 (IQR: 4–6).
Table 1.
Baseline characteristics for alive and deceased patients.
| Total (n = 609) | Alive (n = 481) | Deceased (n = 128) | p | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Gender: male | 60.3% (367/609) | 58.6% (282/481) | 66.4% (85/128) | 0.110 |
| Age | 71 (IQR: 58–82) | 67 (IQR: 55.0–77.0) | 81 (IQR: 71.2–88.0) | 0.000 |
| BMI (Kg/m2) | 28.90 (IQR: 25.94–32.04) | 28.98 (IQR: 26.33–32.04) | 28.64 (IQR: 23.61–31.56) | 0.461 |
| Institucionalization | 10.3% (63/609) | 7.7% (37/481) | 20.3% (26/128) | 0.000 |
| Duration of hospitalization | 8 (IQR: 5–12) | 8 (IQR: 6–12) | 7 (IQR: 5–12) | 0.371 |
| ICU admission | 4.3% (26/609) | 1.0% (5/481) | 16.4% (21/128) | 0.000 |
| Comorbidities | ||||
| Charlson-Age Index | 3 (IQR: 2–5) | 3 (IQR: 1–4) | 5 (IQR: 4–6) | 0.000 |
| Smoking | ||||
| Nonsmokers | 72.5% (441/608) | 74.2% (357/481) | 66.1% (84/127) | 0.070 |
| Active smokers | 5.8% (35/608) | 6.0% (29/481) | 4.7% (6/127) | 0.575 |
| Previous smokers | 27.7% (132/608) | 19.8% (95/481) | 29.1% (37/127) | 0.022 |
| Hypertension | 55.0% (335/609) | 49.9% (240/481) | 74.2% (95/128) | 0.000 |
| Dyslipidemia | 43.5% (265/609) | 41.0% (197/481) | 53.1% (68/128) | 0.014 |
| Diabetes | 25.3% (154/609) | 22.5% (108/481) | 35.9% (46/128) | 0.002 |
| Chronic heart disease | 30.2% (184/609) | 24.9% (120/481) | 50.0% (64/128) | 0.000 |
| Atrial fibrillation/flutter | 14.4% (88/609) | 10.2% (49/481) | 30.5% (39/128) | 0.000 |
| Chronic lung disease | 23.6% (144/609) | 22.2% (107/481) | 28.9% (37/128) | 0.115 |
| History of any cancer | 15.8% (96/609) | 14.1% (68/481) | 21.9% (28/128) | 0.033 |
| Cerebrovascular disease | 8.7% (53/609) | 7.9% (38/481) | 11.7% (15/128) | 0.173 |
| Any immunosuppressive condition | 8.5% (52/609) | 6.9% (33/481) | 14.8% (19/128) | 0.004 |
| Chronic kidney disease | 7.4% (45/609) | 4.8% (23/481) | 17.2% (22/128) | 0.000 |
| Dementia | 6.2% (38/609) | 5.0% (24/481) | 10.9% (14/128) | 0.013 |
| Chronic liver disease | 4.8% (29/609) | 5.0% (24/481) | 3.9% (5/128) | 0.609 |
| Connective disease | 2.6% (16/609) | 2.5% (12/481) | 3.1% (4/128) | 0.692 |
| Peripheral arterial disease | 2.5%/15/609) | 2.1% (10/481) | 3.9% (5/128) | 0.236 |
| Previous treatments | ||||
| Previous ACEI/ARB | 38% (228/600) | 36% (170/472) | 45.3% (25/128) | 0.055 |
| Previous statins | 27.4% (167/609) | 27.0% (130/481) | 28.9% (37/128) | 0.672 |
| Previous anticoagulation | 15.6% (95/609) | 11.2% (54/481) | 32.0% (41/128) | 0.000 |
| Symptoms on admission | ||||
| High Temperature | 77.8% (474/609) | 78% (375/481) | 77.3% (99/128) | 0.881 |
| Cough | 70.6% (430/609) | 73.2% (352/481) | 60.9% (78/128) | 0.007 |
| Dyspnea | 66.8% (407/609) | 62.8% (302/481) | 82.0% (105/128) | 0.000 |
| Asthenia | 60.1% (366/609) | 64.7% (311/481) | 43% (55/128) | 0.000 |
| Arthralgia/myalgia | 31.4% (191/609) | 37.4% (180/481) | 8.6% (11/128) | 0.000 |
| Diarrhea | 21.5% (131/609) | 22.9% (110/481) | 16.4% (21/128) | 0.114 |
| Vomiting | 9.9% (60/609) | 10.2% (49/481) | 8.6% (11/128) | 0.591 |
| Dysgeusia/anosmia | 7.9% (48/609) | 9.6% (46/481) | 1.6% (2/128) | 0.003 |
| Vital signs on admission | ||||
| Tachypnea (<22 bpm) | 51.2% (310/605) | 45.7% (218/477) | 71.9% (92/128) | 0.000 |
| Blood oxygen saturation <92% | 37.4% (228/609) | 28.9% (139/481) | 69.5% (89/128) | 0.000 |
| Disorder of consciousness | 9.4% (57/609) | 5.6% (27/481) | 23.4% (30/128) | 0.000 |
| SBP<90 or/and DBP<60 mmHg | 7.1% (43/609) | 5.0% (24/481) | 14.8% (19/128) | 0.000 |
| q-SOFA≥2 | 7.1% (43/609) | 4.0% (19/481) | 18.8% (24/128) | 0.000 |
| Chest X-ray on admission | ||||
| Bilateral infiltration | 73.9% (447/605) | 72.2% (346/479) | 80.2% (101/126) | 0.071 |
| Unilateral infiltration | 14.7% (89/605) | 16.1% (77/479) | 9.5% (12/126) | 0.064 |
| No significant findings | 11.4% (69/605) | 11.7% (56/479) | 10.3% (13/126) | 0.667 |
| Laboratory findings on admission | ||||
| Serum albumin (g/L) | 34.4 (±4.03) | 35.0 (±3.82) | 32.3 (±4.11) | 0.000 |
| Albumin <34 g/dL | 43.8% (267/609) | 38.0% (183/481) | 65.6% (84/128) | 0.000 |
| Days from admission to albumin assesment | 1 (IQR: 1–1) | 2 (IQR: 1–3) | 1 (IQR: 1–2.75) | 0.258 |
| Days from symptoms onset to albumin assesment | 8 (IQR: 5–11) | 8 (IQR: 6–11) | 6 (IQT: 2–9) | 0.000 |
| Leucocyte count (/μL) | 6800 (IQR: 5100–8900) | 6700 (IQR: 5000–8600) | 7100 (IQR: 5500–10100) | 0.038 |
| Lymphocyte count(/μL) | 900 (IQR: 600–1200) | 900 (IQR: 700–1200) | 700 (IQR: 400–1000) | 0.000 |
| Lymphocytes ≤800/μL | 49.3% (300/608) | 44.2% (212/480) | 68.8% (88/128) | 0.000 |
| Hemoglobin (g/dL) | 13.8 (IQR: 12.4–15.0) | 13.9 (IQR: 12.9–15.0) | 13.0 (IQR: 12.0–15.8) | 0.000 |
| Platelet count (/μL) | 190,000 (IQR: 144000–247000) | 193,000 (IQR: 145000–249000) | 171,500 (IQR: 133000–222000) | 0.019 |
| Fibrinogen (mg/dL) | 370 (IQR: 370–370) | 370 (IQR: 370–370) | 370(IQR: 370–370) | 0.868 |
| Creatinine (mg/dL) | 0.93 (IQR: 0.78–1.2) | 0.88 (IQR: 0.76–1.11) | 1.2 (IQR: 0.87–1.63) | 0.000 |
| D-Dimer (ng/dL) | 0.79 (IQR: 0.54–0.79) | 0.74 (IQR: 0.52–1.15) | 1.38 (IQR: 0.70–3.54) | 0.000 |
| hs-CRP (mg/L) | 98.7 (IQR: 42.7–166.4) | 90.3 (IQR: 38.3–154.0) | 137.0 (IQR: 70.2–206.9) | 0.000 |
| AST (U/L) | 35.0 (IQR: 24.0–52.8) | 36.0 (IQR: 25.0–52.0) | 33.5 (IQR: 20.0–58.3) | 0.546 |
| Ferritin (ng/mL) | 651.0 (IQR: 251.0–1218.0) | 528.5 (IQR: 221.5–1170.0) | 812.0 (IQR: 332.5–1708.5) | 0.067 |
| Lactate dehydrogenase (U/L) | 355.5 (IQR: 277.0–479.3) | 344.0 (IQR: 268.0–444.0) | 427 (IQR: 312.0–577.0) | 0.000 |
| High-sensitive troponin I (pg/mL) | 9.90 (IQR: 4.52–33.60) | 7.70 (IQR: 3.43–16.20) | 35.55 (IQR:12.25–140.15) | 0.000 |
ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker. BMI: body mass index. BUN: blood urea nitrogen. hs-CRP: high sensitivity C-reactive protein. q-SOFA: quick-Sequential Organ Failure score. SBP: systolic blood pressure. DBP: diastolic blood pressure.
Frequencies presented as % (n/total), medians followed by interquartile range (IQR).
The most frequent symptoms on admission were high temperature (77.8%) and cough (70.6%). Dyspnea was the most frequent symptom in nonsurvivors (82%). Cough, asthenia, arthralgia/myalgia, and dysgeusia/anosmia showed more frequently in survivors (p < 0.05). Deceased patients showed significantly poorer vital signs on admission (p < 0.001). Chest X-ray showed bilateral infiltration up to 73.9% of cases. In nonsurvivors, leukocyte count, creatinine, D-dimer, hs-CRP, LDH, alanine aminotransferase (ALT) and high-sensitive troponin I showed higher concentration on admission (p < 0.05) while hemoglobin and platelet count were lower than those in survivors (p < 0.05). Lymphocyte count had a median of 900/μL (IQR:600–1200), with almost half of the patients presenting a count under 800/μL (49.3%), which reached 68.8% in nonsurvivors.
Serum albumin concentration media on admission was 34.4 ± 4.0 g/L, being significantly lower in deceased patients (32.3 ± 4.1 g/L; p < 0.001). Hypoalbuminemia was frequent in our study (43.8%) and it was present in more than a half of nonsurvivors (65.6%, p < 0.001). Median days from admission to serum albumin assessment was 1 (IQR:1–1), with no difference between survivors and nonsurvivors (p = 0.258), meanwhile median days from symptoms onset to albumin assessment was 8 (IQR:5–11), which was significantly lower in nonsurvivors (median: 6, IQR: 2–9, p < 0.001).
The most frequent adverse events were bilateral pneumonia (82.1%) and acute respiratory failure (79.1%), which occurred in almost all deceased patients (96.9%). All adverse events were more frequent in deceased patients (p < 0.001) except for MAS and bleeding events. The main cause of death was ARDS (68.0%) followed by sepsis (23.4%). (Table 2 ).
Table 2.
Adverse events, causes of death and support treatment during hospitalization.
| Total (n = 609) | Alive (n = 481) | Deceased (n = 128) | p | |
|---|---|---|---|---|
| In-hospital adverse events | ||||
| Pneumonia | ||||
| Bilateral | 82.1% (499/608) | 79.4% (382/481) | 92.1% (117/127) | 0.000 |
| Unilateral | 10.4% (63/608) | 12.3% (59/481) | 3.1% (4/127) | 0.002 |
| Acute Respiratory failure | 79.1% (482/609) | 74.4% (358/481) | 96.9% (124/128) | 0.000 |
| MAS | 33.8% (206/609) | 32.2% (155/481) | 39.8% (51/128) | 0.105 |
| ARDS | 33.5% (107/609) | 47.5% (97/481) | 83.6% (107/128) | 0.000 |
| Acute Kidney Injury | 20.9% (127/609) | 13.9% (67/481) | 46.9% (60/128) | 0.000 |
| Acute Heart failure | 9.2% (56/609) | 7.3% (35/481) | 16.4% (21/128) | 0.001 |
| Sepsis | 6.9% (42/609) | 1.7% (8/481) | 26.6% (34/128) | 0.000 |
| Bleeding events | 4.1% (25/609) | 4.0% (19/481) | 4.7% (6/128) | 0.709 |
| Embolic events | 2.8% (17/609) | 2.1% (10/481) | 5.5% (7/128) | … |
| Cause of death | ||||
| ARDS | 67.97% (87/128) | |||
| Sepsis | 23.44% (30/128) | |||
| Suddden death | 3.12% (4/128) | |||
| Pulmonary embolism | 1.65% (2/128) | |||
| Other | 3.82% (5/128) | |||
| Support treatment | ||||
| Oxygen therapy | 85.2% (519/609) | 81.3% (391/481) | 100% (128/128) | 0.000 |
| High-flow oxygen therapy | 33.7% (205/609) | 19.3% (93/481) | 87.5% (112/128) | 0.000 |
| HFNC | 1.5% (9/609) | 0.6% (3/481) | 4.7% (6/128) | … |
| NIV | 2.1% (13/609) | 0.8% (4/481) | 7.0% (9/128) | … |
| IMV | 4.3% (26/609) | 0.8% (4/481) | 17.2% (22/128) | 0.000 |
| Prone ventilation | 15.6% (95/609) | 11.9% (57/481) | 9.4% (38/128) | 0.000 |
| Hydroxychloroquine | 90.8% (553/609) | 93.8% (451/481) | 79.7% (102/128) | 0.000 |
| Azithromycin | 83.3% (507/609) | 86.7% (417/481) | 70.3% (90/128) | 0.000 |
| Other antibiotics | 80.6% (491/609) | 78.0% (375/481) | 90.6% (116/128) | 0.001 |
| Corticoids | 43.2% (263/609) | 38.9% (187/481) | 59.4% (76/128) | 0.000 |
| Lopinavir/ritonavir | 22.2% (135/609) | 19.5% (94/481) | 32.0% (41/128) | 0.003 |
| Tocilizumab | 2.8% (17/609) | 2.3% (11/481) | 4.7% (6/128) | … |
| ACEI/ARB | 8.5% (52/609) | 8.3% (40/481) | 9.4% (12/128) | 0.666 |
| Anticoagulation | ||||
| Prohylactic LMWH | 70.3% (428/609) | 74.2% (357/481) | 55.5% (71/128) | 0.000 |
| Intermediate LMWH | 6.2% (38/609) | 6.0% (29/481) | 7.0% (9/128) | 0.674 |
| Anticoagulant LMWH | 14.0% (85/609) | 10.6% (51/481) | 26.6% (34/128) | 0.000 |
| Vitamin K antagonists | 1.5% (9/609) | 0.6% (3/481) | 4.7% (6/128) | … |
| Direct oral anticoagulants | 0.8% (5/609) | 0.8% (4/481) | 0.7% (1/128) | … |
ARDS: acute respiratory distress syndrome. ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker. HFNC: high flow nasal cannula. LMWH: low molecular weight heparin. MAS: macrophage activation syndrome. NIV: non invasive ventilation.
Mortality analysis: Charlson-age index adjusted associations
Given the fact that age and comorbidities have been shown to predict mortality in COVID-19, we decided to adjust associations with mortality to Charlson-Age Comorbidity Index. (Table 3 ). Details about association of specific comorbidities and previous treatments with mortality are summarized in Appendices Table 1.
Table 3.
Association of baseline characteristics, findings on admission and adverse events with mortality: crude OR and adjusted to Charlson-Age Index OR.
| OR | 95% CI | p | Adjusted OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age | 1.007 | 1.058–1.097 | 0.000 | |||
| Gender: male | 1.395 | 0.926–2.100 | 0.111 | 1.611 | 1.035–2.509 | 0.035 |
| BMI | 0.969 | 0.898–1.047 | 0.427 | 0.983 | 0.907–1.066 | 0.683 |
| Charlson Age Index | 1.477 | 1.343–1.625 | 0.000 | |||
| Symptoms on admission | ||||||
| Dyspnea | 2.706 | 1.662–4.406 | 0.000 | 2.651 | 1.582–4.442 | 0.000 |
| Dysgeusia/anosmia | 0.150 | 0.036–0.627 | 0.000 | 0.218 | 0.051–0.933 | 0.040 |
| Arthralgia/myalgia | 0.157 | 0.082–0.300 | 0.000 | 0.199 | 0.102–0.388 | 0.000 |
| Asthenia | 0.412 | 0.277–0.612 | 0.000 | 0.426 | 0.278–0.652 | 0.000 |
| Cough | 0.572 | 0.380–0.860 | 0.007 | 0.869 | 0.556–1.356 | 0.536 |
| High Temperature | 0.965 | 0.605–1.539 | 0.881 | 2.170 | 1.257–3.745 | 0.005 |
| Diarrhea | 0.662 | 0.396–1.107 | 0.116 | 0.735 | 0.422–1.281 | 0.278 |
| Vomiting | 0.829 | 0.418–1.644 | 0.591 | 0.925 | 0.436–1.965 | 0.840 |
| Vital signs on admission | ||||||
| Blood O2 saturation <92% | 5.615 | 3.671–8.587 | 0.000 | 5.359 | 3.395–8.461 | 0.000 |
| Tachypnea (>22 bpm) | 3.036 | 1.984–4.646 | 0.000 | 3.424 | 2.159–5.434 | 0.000 |
| Disorder of conciousness | 5.147 | 2.929–9.047 | 0.000 | 2.792 | 1.529–5.098 | 0.001 |
| SBP<90 or/and DBP<60 mmHg | 3.319 | 1.755–6.277 | 0.000 | 2.660 | 1.341–5.277 | 0.000 |
| q-SOFA≥2 | 5.611 | 2.964–10.624 | 0.000 | 3.153 | 1.595–6.234 | 0.001 |
| Chest X-ray on admission | ||||||
| Bilateral infiltration | 1.257 | 0.661–2.391 | 0.485 | 2.522 | 1.215–5.236 | 0.013 |
| Laboratory findings on admission | ||||||
| Albumin <34 g/L | 3.109 | 2.066–4.978 | 0.000 | 2.050 | 1.323–3.178 | 0.001 |
| Lymphocytes ≤800/μL | 2.781 | 1.837–4.211 | 0.000 | 2.566 | 1.645–4.004 | 0.000 |
| Creatinine | 2.137 | 1.550–2.945 | 0.000 | 1.372 | 1.044–1.802 | 0.023 |
| D-Dimer >0.49 ng/dL | 2.390 | 1.229–4.647 | 0.010 | 1.752 | 0.876–3.507 | 0.113 |
| hs-CRP >8 mg/L | 8.193 | 1.105–60.755 | 0.040 | 12.045 | 1.502–96.577 | 0.019 |
| Lactate dehydrogenase >250 U/L | 2.712 | 1.316–5.589 | 0.007 | 3.457 | 1.621–7.375 | 0.001 |
| Hemoglobin | 0.849 | 0.776–0.928 | 0.000 | 1.025 | 0.923–1.139 | 0.641 |
| Leucocyte count | 1.000 | 1.000–1.000 | 0.054 | 1.000 | 1.000–1.000 | 0.257 |
| Platelet count | 1.000 | 1.000–1.000 | 0.013 | 1.000 | 1.000–1.000 | 0.049 |
| Fibrinogen | 0.999 | 0.987–1.011 | 0.839 | 1.000 | 0.988–1.012 | 0.937 |
| Sodium | 1.019 | 0.971–1.070 | 0.437 | 1.009 | 0.964–1.055 | 0.711 |
| AST | 1.001 | 0.995–1.006 | 0.792 | 1.004 | 0.998–1.010 | 0.161 |
| Ferritin | 1.000 | 1.000–1.001 | 0.203 | 1.000 | 1.000–1.001 | 0.059 |
| Adverse events | ||||||
| ARDS | 20.717 | 12.015–33.864 | 0.000 | 31.699 | 16.843–59.660 | 0.000 |
| Sepsis | 21.386 | 9.596–47.659 | 0.000 | 20.994 | 9.049–48.710 | 0.000 |
| Acute Respiratory Failure | 10.651 | 3.854–29.434 | 0.000 | 12.349 | 4.280–35.631 | 0.000 |
| Bilateral Pneumonia | 1.861 | 1.186–2.921 | 0.007 | 4.244 | 1.610–4.257 | 0.003 |
| Acute Kidney Injury | 5.452 | 3.538–8.403 | 0.000 | 2.970 | 1.838–4.800 | 0.000 |
| MAS | 1.396 | 0.932–2.083 | 0.106 | 2.121 | 1.354–3.322 | 0.001 |
| Acute Heart Failure | 2.501 | 1.399–4.470 | 0.002 | 0.895 | 0.459–1.744 | 0.743 |
| Bleeding events | 1.196 | 0.467–3.059 | 0.709 | 0.678 | 0.245–1.880 | 0.455 |
| Embolic events | 2.725 | 1.016–7.306 | 0.046 | 2.906 | 0.981–8.609 | 0.054 |
ARDS: acute respiratory distress syndrome. BMI: body mass index. hs-CRP: high sensiticity C-reactive protein. MAS: macrophage activation syndrome. DBP: diastolic blood pressure. q-SOFA: quick-Sequential Organ Failure score. SBP: systolic blood pressure.
Male gender was associated with mortality (adjusted OR of 1.611, 95%CI: 1.035–2.509, p = 0.035). Dyspnea and high temperature on admission were related to poorer outcome, whereas dysgeusia/anosmia, arthralgia/myalgia, and asthenia were related to better outcome. Poor vital signs on admission were strong predictors of mortality, especially blood saturation <92%. A q-SOFA≥2 on admission increased the risk of death threefold (adjusted OR 3.153, 95%CI: 1.595–6.234, p = 0.001). For chest X-ray, bilateral infiltration on admission was associated with worse outcome (adjusted OR 2.522, 95%CI: 1.215–5.236, p < 0.05). Lymphocyte count <800/μL (adjusted OR 2.566, 95%CI: 1.645–4.004, p < 0.001), creatinine (adjusted OR 1.372, 95%CI: 1.044–1.802, p = 0.023), hs-CRP >8 mg/L (adjusted OR 12.045, 95%CI: 1.502–96.577, p = 0.019) and LDH >250 U/L (adjusted OR 3.457, 95%CI: 1.621–7.375, p = 0.001) were significant predictors of mortality. Hypoalbuminemia on admission was associated with a twofold risk of in-hospital mortality (adjusted OR 2.050, 95%CI: 1.323–3.178, p = 0.001).
Adverse events during hospitalization were related to mortality, except for bleeding and embolic events, possibly due to the low number of these events in our cohort. MAS was only associated with increased risk of mortality when adjusted to Charlson-Age Index. Patients who developed MAS were younger (median age 67, IQR: 57–80, p = 0.025) and had fewer comorbidities (median Charlson Index 3, IQR 2–5, p < 0.001) than those who did not.
Hypoalbuminemia as predictor of adverse outcomes
Hypoalbuminemia on admission predicted adverse outcomes in our study. (Table 4 ). It increased the risk of intensive care unit (ICU) admission and invasive mechanic ventilation (IMV), sepsis, MAS, acute heart failure, ARDS, acute respiratory failure and acute kidney injury, independently of Charlson-Age Index. Survivors with hypoalbuminemia on admission had longer hospital stays than those with normal concentration (median 8 days, IQR: 6–12, p = 0.020). Patients with q-SOFA ≥2 on admission showed hypoalbuminemia in 79.1% of cases (24/43), p < 0.001.
Table 4.
Association of hypoalbuminemia (albumin <34 g/) with adverse events: crude OR and adjusted to Charlson-Age Index OR.
| Dependent variable | OR for albumin <34 g/L | 95% CI | p | Adjusted OR for albumin <34 g/L | 95% CI | p |
|---|---|---|---|---|---|---|
| Acute respiratory failure | 2.025 | 1.334–3.074 | 0.001 | 1.944 | 1.253–3.015 | 0.003 |
| Acute heart failure | 3.955 | 2.138–7.317 | 0.000 | 2.338 | 1.206–4.533 | 0.012 |
| Acute kidney injury | 3.055 | 2.029–4.599 | 0.000 | 1.863 | 1.186–2.926 | 0.007 |
| Sepsis | 4.521 | 2.180–9.376 | 0.000 | 3.719 | 1.747–7.913 | 0.001 |
| MAS | 2.151 | 1.529–3.026 | 0.000 | 3.165 | 2.152–4.653 | 0.000 |
| ARDS | 2.357 | 1.672–3.324 | 0.000 | 2.146 | 1.495–3.079 | 0.000 |
| Bleeding events | 2.359 | 1.025–5.425 | 0.043 | 1.696 | 0.711–4.041 | 0.233 |
| Embolic events | 1.862 | 0.699–4.959 | 0.213 | 1.820 | 0.647–5.118 | 0.257 |
| ICU admission | 3.018 | 1.291–7.053 | 0.011 | 4.643 | 1.867–11.546 | 0.001 |
| IMV | 3.666 | 1.518–8.857 | 0.004 | 5.505 | 2.148–14.113 | 0.000 |
ARDS: acute respiratory distress syndrome. ICU: intensive care unit. IMV: invasive mechanical ventilation. MAS: macrophage activation syndrome.
On the other hand, serum albumin concentration showed linear correlation with highest D-dimer (r = −0.387, p < 0.001), highest high-sensitivity troponin I (r = −0.365, p < 0.001), highest hs-CRP (r = −0.306, p < 0.001), highest procalcitonin (r = −0.286, p < 0.001) and lowest lymphocyte count (r = 0.264, p < 0.001).
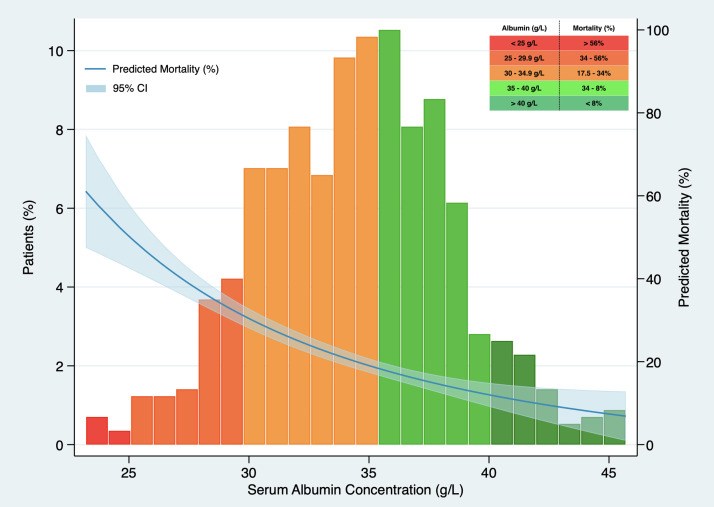
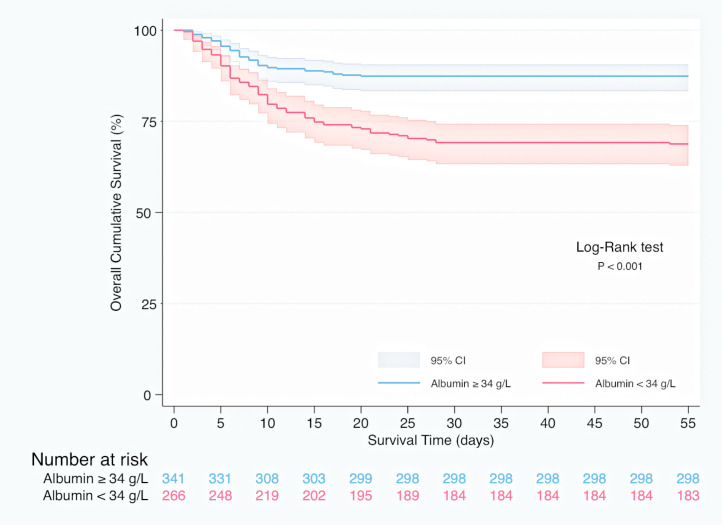
In Kaplan–Meier survival analysis (Supplementary material, Figure 1), patients with hypoalbuminemia on admission showed a reduced cumulative overall survival compared to those with serum albumin concentration over 34 g/L (Log-Rank test; p < 0.001). Hypoalbuminemia was a predictor of mortality in multivariable Cox regression analysis (HR 1.537, 95% CI 1.050–2.250, p = 0.027), independently of Charlson-Age Index, gender, lymphocyte count <800/μL, creatinine, hs-CRP >8 mg/L, LDH >250 U/L, bilateral infiltration on chest X-ray and q-SOFA ≥2. (Table 5 ). Association of serum albumin as a continuous variable with predicted mortality after adjustment to variables entered in Cox regression model is displayed graphically in Fig. 1 .
Table 5.
Cox regression analysis regarding variables on admission associated with in-hospital death.
| Variable | p | HR | 95% CI |
|---|---|---|---|
| Charlson-Age Index | 0.000 | 1.276 | 1.183–1.377 |
| Albumin <34 g/L | 0.039 | 1.534 | 1.021–2.303 |
| Lymphocytes ≤800/μL | 0.001 | 1.961 | 1.321–2.910 |
| Lactate dehydrogenase >250 U/L | 0.010 | 2.507 | 1.245–5.046 |
| Creatinine | 0.044 | 1.196 | 1.005–1.423 |
| q-SOFA ≥2 | 0.004 | 2.123 | 1.272–3.542 |
hs-CRP: high sensitivity C-reactive protein. q-SOFA: quick-Sequential Organ Failure score. HR: Hazard Ratio.
Variables such as CRP >8 mg/L (p = 0.232), male gender (p = 0.210) and bilateral infiltration (p = 0.086) were not independent predictors of mortality when entered in Cox regression model.
Fig. 1.
Association between serum albumin concentration on admission and predicted mortality. The predicted probability of all-cause death (blue line) is shown, together with 95% confidence interval, after adjustment to Charlson-Age Index, gender, lymphocyte count <800/μL, creatinine, hs-CRP >8 mg/L, lactate dehydrogenase >250 U/L, bilateral infiltration on chest X-ray and q-SOFA ≥2. Histograms show the population distribution according to serum albumin concentration on admission.
Discussion
In our study, hypoalbuminemia, defined as serum concentration <34 g/L, was an independent early predictor of in-hospital mortality regardless of age, comorbidities, gender, lymphocyte count, hs-CRP, creatinine and LDH. Besides, we found significant association of hypoalbuminemia with the development of ARDS, MAS and other adverse events, independently of age and comorbidities, assessed by the Charlson-Age Index. Our results support the clinical usefulness of serum albumin determination on emergency room or early during patient's hospital admission, as it may help to improve risk stratification in SARS-CoV-2 infection.
Albumin has important physiological functions which include maintenance of colloidal osmotic pressure, binding different compounds and plasma antioxidant activity. Serum albumin has been shown to decrease in many acute and chronic diseases related to the magnitude of the inflammatory response they generate; in fact, it has been termed as a “negative acute phase reactant”.12 Several mechanisms have been described to explain this fact. First, capillary leakage triggered by release of cytokines and chemokines, which distributes great amounts of albumin to the interstitial space, where it acts as an antioxidative agent and a source of amino-acids for cell and matrix synthesis. Second, there is an increased degradation and a decreased albumin synthesis during inflammatory response due to a cytokine-mediated reduced gene transcription, mainly mediated by interleukin (IL)-6 and tumor necrosis factor (TNF)-α.13
Hypoalbuminemia has been associated with short- and long-term mortality in hospitalized patients for numerous medical and surgical settings. Studies in patients with SARS-CoV-2 infection have also confirmed the significant association of serum albumin concentration with mortality.14 Violi and coworkers15 found that the lowest tertile of albumin concentration (<32 g/L) predicted mortality (HR 2.48, 95%CI: 1.44–4.26; p = 0.001), after adjusting to age, ICU admission, gender, heart failure, chronic obstructive pulmonary disease and hs-CRP. Huang et al.16 reported that hypoalbuminemia (<35 g/L), lymphopenia (<1000/μL) and the presence of at least one comorbidity were independent predictors of death. Gong et al.17 designed a nomogram for early identification of patients at high risk of progression to severe COVID-19 infection including age, LDH, CRP, coefficient of variation of red blood cell distribution width, direct bilirubin, blood urea nitrogen and albumin.
Although pathophysiology behind the relationship between albumin and poor outcomes is unclear, albumin is a nonspecific biomarker of disease severity that has been reported to be decreased in elderly patients and in numerous acute and chronic diseases, including inflammatory states such as malnourishment. Due to this significant relationship between serum albumin, chronic diseases and age, we accomplished an exhaustive compilation of comorbidities using Charlson Comorbidity Index, which has been recently reported as a mortality risk predictor in SARS-CoV-2 infection.18 Hypoalbuminemia was a significant predictor of in-hospital mortality in our study even after adjustment to age and multimorbidity assessed by the Charlson-Age Index.
In our cohort, hypoalbuminemia was also significantly associated with the development of adverse events including sepsis, MAS, acute heart failure, ARDS and acute kidney injury, even after adjustment to Charlson-Age Index. Hypoalbuminemia on admission has been related to the development of acute respiratory failure and acute kidney injury in hospitalized patients for any cause, probably due to changes in osmotic pressure and a reduced ability to combat oxidative stress.19 It has also been associated to the occurrence of heart failure and other cardiovascular diseases given the physiological properties of serum albumin such as anti-inflammatory, antioxidant, anticoagulant and antiplatelet aggregation activity as well as colloid osmotic effect.20 We have not found other studies analyzing the association of hypoalbuminemia and adverse events in COVID-19 except for Wu and coworkers,21 who reported inverse association between serum albumin concentration and ARDS. In our study, a significant inverse correlation was found between serum albumin on admission and peak hs-CRP during hospital stay, which supports the role of albumin in inflammatory response. We also found a significant inverse correlation between serum albumin and D-dimer, which has been related to embolic and bleeding events in COVID-19 due to disseminated coagulopathy.22 Albumin encompasses anticoagulant properties and has been described to have a heparin-like action, probably due to the similarity of both molecules.23 Nevertheless, hypoalbuminemia was not a significant predictor of embolic and bleeding events, probably related to the low incidence or infra-diagnosis of these events in our cohort.
Our results coincide with a recent study by Huang and coworkers,16 that showed a significant correlation between serum albumin and inflammatory indicators (hs-CRP, white blood cell count and neutrophil-to-lymphocyte ratio). These same authors reported that hypoalbuminemia in SARS-CoV-2 infection may be produced by an inflammatory-mediated capillary leakage and a decreased albumin synthesis in hepatocytes.24 Therefore, it may be argued that hypoalbuminemia is just a biomarker for the underlying disease severity and not a direct pathogenic mediator implicated in COVID-19 adverse outcomes. However, hypoalbuminemia was significantly associated with mortality regardless of hs-CRP in our study. Hence, a direct pathogenic role of hypoalbuminemia in COVID-19 adverse events cannot be ruled out. It has been hypothesized that SARS-CoV2 virions bind competitively to serum albumin diminishing its normal transport function. As infection continues, release of virions into the bloodstream prevents albumin from transporting nutrients into the cells, which results in unstable cells vulnerable to apoptosis.25
Different trials have shown no evidence for albumin administration to reduce mortality in critically ill patients,26 suggesting that hypoalbuminemia is just an epiphenomenon rather than a pathogenic mediator related directly to poor outcomes. However, exogen albumin administration has been reported to improve oxygenation in ARDS.27 Whether albumin administration in COVID-19 patients with severe hypoalbuminemia translates in net clinical benefit deserves further research.
As previously recommended, albumin assay should be clearly specified when reporting biochemical data regarding serum albumin measurement, taken into account the significant differences of results between chromogenic BCG and bromocresol purple (BCP) assays that may even affect clinical decision-making.28 Serum albumin concentration is an average of 5 g/L higher using BCG as a colorant than those obtained with the BCP assay. This serum albumin overestimation with dye-binding BCG assay has been reported to be mediated by a positive interference from acute phase α-1 and α-2 globulins, especially at low serum albumin concentration. In fact, it has been suggested that BCP assay should be used instead of BCG method to assess albumin concentration, particularly in patients with inflammation.29 We used BCG assay to determine serum albumin concentration. Given the inflammatory nature of SARS-CoV-2 infection, the BCG method may overestimate albuminemia in our patients and therefore underestimate mortality risk. However, this potential bias of BCG assay reinforces the concept that even a slight decrease in serum albumin concentration may have a significant impact in adverse outcomes. As shown in our study, albuminemia below lower limit of normal range (<34 g/L) was significantly associated with in-hospital mortality.
Our study has several limitations. First, its observational nature makes it impossible to draw any conclusions on causality. However, despite the relatively small sample size, our study encompasses consecutive hospitalized patients in a single center with institutional medical protocols which reflects a real-life scenario of SARS-CoV-2 clinical management. Secondly, we used dye-binding BCG colorimetric assay to determine serum albumin, which may overestimate serum albumin concentration. Using a bromocresol purple method or a more specific immunoassay may help to improve accuracy of risk stratification models based on serum albumin concentration. Third, we did not have information regarding the nutritional status of the patients, other than body mass index in a relatively small subgroup of patients. Therefore, to avoid potential confounding factors implicated in the association between albuminemia and adverse outcomes, we adjusted these associations by using the validated Charlson-Age Comorbidity Index. Fourth, time to serum albumin measurement may modify its prognostic impact. It has been described that serum albumin decreases progressively from early in the course of clinical illness and it does not increase again until the recovery phase.30 In our study median days from admission to albumin assessment did not differ between survivors and nonsurvivors and in fact, median days from symptoms onset to albumin assessment were even lower in nonsurvivors. This supports our hypothesis of albumin being an early marker of poor prognosis. Fifth, due to clinical similarity of MAS and secondary hemophagocytic lymphohistiocytosis, diagnosis of MAS was made according to a modified HLH-2004 diagnostic criteria10 based on a multidisciplinary consensus of our institution, which may affect diagnosis accuracy. However, differentiation of MAS from an underlying inflammatory disease remains challenging and there are no uniform diagnostic criteria recommended so far.
In conclusion, hypoalbuminemia was an independent and early predictor of in-hospital mortality in SARS-CoV-2 infection regardless of age, comorbidity, and inflammatory markers. Hypoalbuminemia was also significantly associated with severe adverse events, regardless of Charlson-Age Comorbidity Index. Our results suggest that serum albumin concentration may help to identify patients at high risk of developing potential life-threatening conditions and death, improving risk stratification in patients with SARS-Cov-2 infection.
Funding
His research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
María C. Viana-Llamas and Ramón Arroyo-Esliguero conceived and designed the study. María C. Viana-Llamas, Ramón Arroyo-Espliguero, Alberto Silva-Obregón, Giovanna Uribe-Heredia, Belén García-Magallón, Claudio G. Torán-Martínez, Alicia Castillo-Sandoval, Eva Díaz-Caraballo and Itsaso Rodríguez-Guinea performed data collection. María C. Viana-Llamas and Ramón Arroyo-Esliguero oversaw data collection. Jesús Dómínguez-López gave advice for laboratory variables and completed data collection. María Viana-Llamas, Ramón Arroyo-Espliguero, Alberto Silva-Obregón and Iván J. Núñez-Gil performed data analysis. All authors contributed to revision and final approval of the article.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.medcli.2020.12.018.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., et al. Epidemiology genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erstad B.L. Albumin disposition in critically Ill patients. J Clin Pharm Ther. 2018;43:746–751. doi: 10.1111/jcpt.12742. [DOI] [PubMed] [Google Scholar]
- 6.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.Y., Chen Y.X., Guo S.B., Mei X., Yang P. Predictive performance of quick sepsis-related organ failure assessment for mortality and ICU admission in patients with infection at the ED. Am J Emerg Med. 2016;34:1788–1793. doi: 10.1016/j.ajem.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henter J.I., Horne A., Aricó M., Egeler R.M., Filipovich A.H., Imashuku S., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 12.Goldwasser P., Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 13.Soeters P.B., Wolfe R.R., Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43:181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz M., Fatima R., Lee-Smith W., Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violi F., Cangemi R., Romiti G.F., Ceccarelli G., Oliva A., Alessandri F., et al. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal. 2020:22. doi: 10.1089/ars.2020.8142. Epub ahead of print. PMID: 32524832. [DOI] [PubMed] [Google Scholar]
- 16.Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y., et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020 doi: 10.1002/jmv.26003. Epub ahead of print. PMID: 32406952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen D.M., Strange J.E., Gislason G., Torp-Pedersen C., Gerds T., Fosbøl E., et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med. 2020;35:2801–2803. doi: 10.1007/s11606-020-05991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thongprayoon C., Cheungpasitporn W., Chewcharat A., Mao M.A., Thirunavukkarasu S., Kashani K.B. Risk of acute respiratory failure among hospitalized patients with various admission serum albumin levels: a cohort study. Medicine (Baltimore) 2020;99:e19352. doi: 10.1097/MD.0000000000019352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jøorgensen K.A., Stoffersen E. Heparin like activity of albumin. Thromb Res. 1979;16:569–574. doi: 10.1016/0049-3848(79)90105-1. [DOI] [PubMed] [Google Scholar]
- 24.Huang W., Li C., Wang Z., Wang H., Zhou N., Jiang J., et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A.S., Fatemi R., Winlow W. SARS-CoV-2 bound human serum albumin and systemic septic shock. Front Cardiovasc Med. 2020;7:153. doi: 10.3389/fcvm.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cochrane Injuries Group Albumin Reviewers Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhlig C., Silva P.L., Deckert S., Schmitt J., de Abreu M.G. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014;18:R10. doi: 10.1186/cc13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Logt A.E., Rijpma S.R., Vink C.H., Prudon-Rosmulder E., Wetzels J.F., van Berkel M. The bias between different albumin assays may affect clinical decision-making. Kidney Int. 2019;95:1514–1517. doi: 10.1016/j.kint.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Moreira V., Beridze Vaktangova N., Martinez Gago M.D., Laborda Gonzalez B., Garcia Alonso S., Fernandez Rodriguez E. Overestimation of albumin measured by bromocresol green vs bromocresol purple method: influence of acute-phase globulins. Lab Med. 2018;49:355–361. doi: 10.1093/labmed/lmy020. [DOI] [PubMed] [Google Scholar]
- 30.Levitt D.G., Levitt M.D. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.