Abstract
Objectives
To determine factors associated with COVID-19-related death in people with rheumatic diseases.
Methods
Physician-reported registry of adults with rheumatic disease and confirmed or presumptive COVID-19 (from 24 March to 1 July 2020). The primary outcome was COVID-19-related death. Age, sex, smoking status, comorbidities, rheumatic disease diagnosis, disease activity and medications were included as covariates in multivariable logistic regression models. Analyses were further stratified according to rheumatic disease category.
Results
Of 3729 patients (mean age 57 years, 68% female), 390 (10.5%) died. Independent factors associated with COVID-19-related death were age (66–75 years: OR 3.00, 95% CI 2.13 to 4.22; >75 years: 6.18, 4.47 to 8.53; both vs ≤65 years), male sex (1.46, 1.11 to 1.91), hypertension combined with cardiovascular disease (1.89, 1.31 to 2.73), chronic lung disease (1.68, 1.26 to 2.25) and prednisolone-equivalent dosage >10 mg/day (1.69, 1.18 to 2.41; vs no glucocorticoid intake). Moderate/high disease activity (vs remission/low disease activity) was associated with higher odds of death (1.87, 1.27 to 2.77). Rituximab (4.04, 2.32 to 7.03), sulfasalazine (3.60, 1.66 to 7.78), immunosuppressants (azathioprine, cyclophosphamide, ciclosporin, mycophenolate or tacrolimus: 2.22, 1.43 to 3.46) and not receiving any disease-modifying anti-rheumatic drug (DMARD) (2.11, 1.48 to 3.01) were associated with higher odds of death, compared with methotrexate monotherapy. Other synthetic/biological DMARDs were not associated with COVID-19-related death.
Conclusion
Among people with rheumatic disease, COVID-19-related death was associated with known general factors (older age, male sex and specific comorbidities) and disease-specific factors (disease activity and specific medications). The association with moderate/high disease activity highlights the importance of adequate disease control with DMARDs, preferably without increasing glucocorticoid dosages. Caution may be required with rituximab, sulfasalazine and some immunosuppressants.
Keywords: antirheumatic agents, autoimmune diseases, epidemiology, glucocorticoids, outcome assessment, health care
Key messages.
What is already known about this subject?
To date, most available data on outcomes for people with rheumatic diseases infected with SARS-CoV-2 come from single centre or single country case series or from one large international registry; the COVID-19 Global Rheumatology Alliance (GRA) physician registry.
The first GRA publication identified factors associated with higher odds of COVID-19 hospitalisation, including older age, presence of comorbidities and higher dosages of glucocorticoids (≥10 mg/day of prednisolone equivalent).
Clinical outcome information on patients with COVID-19 who have rheumatic disease therefore remains limited, particularly with regard to factors associated with COVID-19-related death.
What does this study add?
In this analysis of 3729 patients with rheumatic diseases, older age, male sex, and cardiovascular and chronic lung disease were associated with COVID-19-related death.
Disease-specific factors, namely, moderate/high disease activity and certain medications (rituximab, sulfasalazine and immunosuppressants (as opposed to immunomodulators like disease-modifying anti-rheumatic drugs (DMARDs)) were also associated with COVID-19-related death.
How might this impact on clinical practice or future developments?
There is differential risk of COVID-19-related death according to disease activity and treatments in patients with rheumatic disease, highlighting the need for adequate disease control with DMARDs, preferably without increasing the glucocorticoid dosage.
Key messages.
What is already known about this subject?
To date, most available data on outcomes for people with rheumatic diseases infected with SARS-CoV-2 come from single centre or single country case series or from one large international registry; the COVID-19 Global Rheumatology Alliance (GRA) physician registry.
The first GRA publication identified factors associated with higher odds of COVID-19 hospitalisation, including older age, presence of comorbidities and higher dosages of glucocorticoids (≥10 mg/day of prednisolone equivalent).
Clinical outcome information on patients with COVID-19 who have rheumatic disease therefore remains limited, particularly with regard to factors associated with COVID-19-related death.
What does this study add?
In this analysis of 3729 patients with rheumatic diseases, older age, male sex, and cardiovascular and chronic lung disease were associated with COVID-19-related death.
Disease-specific factors, namely, moderate/high disease activity and certain medications (rituximab, sulfasalazine and immunosuppressants (as opposed to immunomodulators like disease-modifying anti-rheumatic drugs (DMARDs)) were also associated with COVID-19-related death.
How might this impact on clinical practice or future developments?
There is differential risk of COVID-19-related death according to disease activity and treatments in patients with rheumatic disease, highlighting the need for adequate disease control with DMARDs, preferably without increasing the glucocorticoid dosage.
Introduction
There is a lack of robust data to inform our understanding of outcomes following SARS-CoV-2 infection in patients with inflammatory rheumatic diseases, leading to uncertainties regarding chronic disease management, especially for those taking immunosuppressant or immunomodulatory drugs.1–3
Whether people with rheumatic diseases belong to a vulnerable, higher risk population for SARS-CoV-2 infection and have poorer outcomes is unclear.1–8 In general, this population seems to have similar or only slightly poorer outcomes compared with those without rheumatic disease.7–9 However, important confounding disease-related factors, such as disease activity or treatments, have previously not been addressed.
Medications commonly used to treat rheumatic diseases have been used or are being tested for the prevention and/or treatment of COVID-19 and its complications,10 raising questions about the impact of these treatments on the outcomes of SARS-CoV-2 infection. Continuation of immunomodulatory or immunosuppressive therapy is essential for controlling rheumatic disease activity, avoiding disease progression and preventing joint or organ-damage related to sustained inflammation. Withdrawal of effective treatments should be based on sound evidence, even during a pandemic.
To generate more granular data relevant to rheumatic diseases, a global network of rheumatologists, data scientists and patients developed a COVID-19 physician-reported case registry in March 2020.11 12 Analysis of the first 600 patients revealed that older age and comorbidities were associated with hospitalisation,13 similar to results in the general population.8 14 More robust data on the risk of poor outcomes, in particular risk of death, are required.
The aim of this study was to investigate factors associated with COVID-19-related death in patients with rheumatic diseases and to analyse these associations by disease group.
Methods
Data source
The COVID-19 Global Rheumatology Alliance (C19-GRA) physician-reported registry is an observational registry launched on 24 March 2020. Data are entered voluntarily by rheumatologists or under supervision of rheumatologists; patients are eligible for inclusion if they have a pre-existing rheumatic disease and a COVID-19 diagnosis. Data are entered either directly into the global or European data entry systems or transferred from national registries (France, Germany, Italy, Portugal and Sweden).
We used data collected on or before 1 July 2020. Further details of this registry have been described elsewhere.11–13 Countries were assigned to the six WHO regions (www.who.int); the ‘Americas’ was further divided into north and south. Given the registry collects anonymous data, the UK Health Research Authority and the University of California San Francisco Institutional Review Board considered it exempt from patient consent.
Patient stratification into diagnostic groups
Rheumatic diseases differ regarding the disease-modifying antirheumatic drugs (DMARDs) approved for their treatment. To minimise the impact of this heterogeneity on the associations of interest, in addition to the main analysis with all patients, diagnostic categories were defined (figure 1) and stratified analyses were undertaken for patients with (1) inflammatory joint diseases (IJD), (2) rheumatoid arthritis (a subset of the IJD subgroup) and (3) connective tissue diseases (CTD)/vasculitis.
Figure 1.
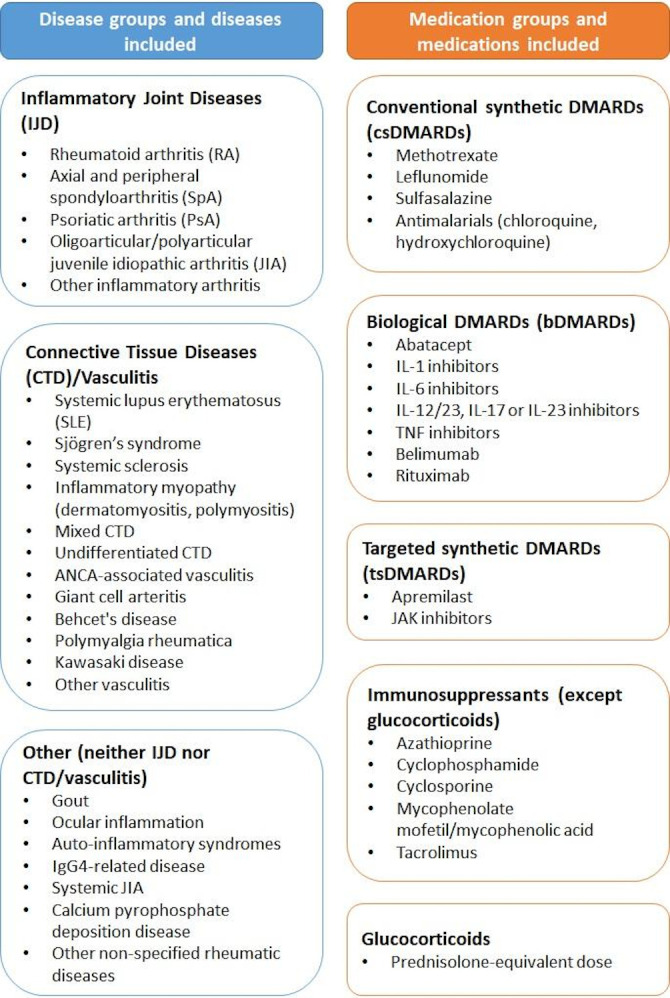
Disease and medication groups. ANCA, anti-neutrophil cytoplasm antibodies; DMARD, disease-modifying antirheumatic drugs; IgG, immunoglobulin; IL, interleukin; JAK, Janus kinase; TNF, tumour necrosis factor.
COVID-19 reporting and outcome
Both confirmed and presumptive cases of COVID-19 were reported. The method of COVID-19 diagnosis was specified: PCR, CT scan, metagenomic testing, laboratory assays or based on symptoms only.
For analysis, patients were subsequently categorised into (1) confirmed or high likelihood of COVID-19 (chest imaging (CT or chest X-ray) showing bilateral infiltrates and/or symptoms after close contact with a known laboratory-confirmed COVID-19 positive patient) or (2) presumptive cases based on symptoms alone.
The primary outcome was COVID-19-related death.
Treatment prior to COVID-19
Antirheumatic medications used prior to COVID-19 diagnosis were categorised into groups shown in figure 1. Immunomodulatory drugs (conventional synthetic (cs)/biological (b)/targeted synthetic (ts) DMARDs) were distinguished from immunosuppressive drugs (azathioprine, cyclophosphamide, ciclosporin, mycophenolate mofetil/mycophenolic acid, tacrolimus) as recommended by Isaacs and Burmester15; glucocorticoids are also immunosuppressive but they were examined separately and categorised by prednisolone-equivalent dosage (1–10 mg/day and >10 mg/day). Methotrexate monotherapy was adopted as the medication reference group; methotrexate is the anchor drug in multiple rheumatic diseases16 and it represents the largest medication category in the registry.
Statistical analyses
Descriptive tables were produced for the whole cohort and then by diagnostic group, country (for the six countries with the highest number of cases: France, Germany, Italy, Spain, UK and USA) and medication. Independent associations between demographic and disease features and COVID-19-related death were estimated using multivariable logistic regression and reported as OR and 95% CI. Covariates included in the model were age, sex, key comorbidities (hypertension alone or cardiovascular disease (CVD) alone, hypertension combined with CVD, chronic lung disease, chronic kidney disease (CKD) and diabetes), smoking status (ever vs never), rheumatic disease diagnostic group, disease activity as per the physician’s global assessment (severe/high or moderate disease activity vs minimal/low disease activity or remission), rheumatic disease treatment prior to COVID-19 diagnosis and prednisolone-equivalent glucocorticoid use.
All patients with confirmed or presumptive COVID-19 were included in the main analyses. Patients with missing primary outcome (N=82) or missing values for age, sex and DMARD (N=19) were excluded from analysis. Missing values for comorbidities, smoking status, glucocorticoid therapy and disease activity were derived by multiple imputation using full conditional specification.17 Results of the logistic regression analyses for 10 imputed datasets were pooled by Rubin’s rules. As disease activity was missing for all French patients, country-level life expectancy was used in the imputation model to explain potential structural differences in disease activity between countries not accounted for in the patient-level data (data from 2018, source: http://hdr.undp.org/).
To account for pronounced heterogeneity between participating countries regarding both healthcare systems and infection dynamics, countries were implicitly considered as data clusters in the regression analysis by assuming that the data arose from a cluster sample design; this was done by applying a Taylor series linearisation in the variance estimation.18
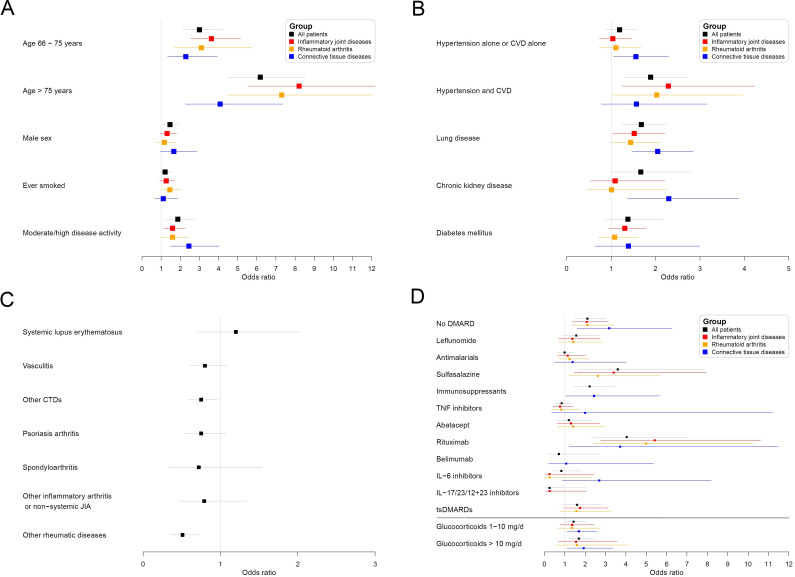
For patients listed as having more than one rheumatic disease or being treated with more than one of the medications of interest, we created a hierarchy based on clinical expertise to categorise patients. This process creates disjoint categories, allowing a clear reference group for interpretation of the regression models and avoiding collinearities. Patients with more than one of the following diseases were grouped according to the following hierarchy: systemic lupus erythematosus (SLE)>vasculitis>other CTD>RA>psoriatic arthritis (PsA)>(other) spondyloarthritis (SpA)>other IJD>other non-IJD/non-CTD rheumatic disease. Patients receiving multiple csDMARDs or immunosuppressants (except glucocorticoids) were grouped according to the following hierarchy: immunosuppressants>sulfasalazine>antimalarials>leflunomide>methotrexate. Patients receiving a b/tsDMARD were considered solely in the b/tsDMARD group. Patients treated with more than one b/tsDMARD (N=4), patients receiving IL-1 inhibitors (N=20) and patients receiving DMARDs atypical for their disease subgroup (N=48) were excluded from analysis due to very low numbers (figure 2). Patients were excluded from a particular analysis if the medication they received provided ≤20 patients for that analysis or if there were no deaths reported for that specific medication.
Figure 2.
Patient flowchart. Some patients had diagnoses in multiple groups; as a result, the sum of patients in each group is greater than the total number of patients. (*) Patients belonging to more than one diagnosic group: IJD and CTD: N=78 (10 deaths); IJD and other: N=70 (12 deaths); CTD and other: N=50 (13 deaths); IJD and CTD and other: N=5 (2 deaths). (§) Patients belonging to more than one diagnosic group: IJD and CTD: N=77 (10 deaths); IJD and other: N=70 (12 deaths); CTD and other: N=49 (12 deaths); IJD and CTD and other: N=5 (2 deaths). (#) Patients belonging to more than one diagnosic group: IJD and CTD: N=59 (7 deaths). (**) Non-typical DMARDs for IJD and RA: immunosuppressants and belimumab; non-typical DMARDs for RA: IL-17/IL-23/IL-12+23 inhibitors. (***) Non-typical DMARDs for CTD: abatacept, IL-17/IL-23/IL-12+23 inhibitors, sulfasalazine, leflunomide and tsDMARDs. b/tsDMARDs, biological/targeted synthetic disease-modifying antirheumatic drugs; CTD, connective tissue disease/vasculitis; DMARDs, disease-modifying anti-rheumatic drugs; IJD, inflammatory joint disease; IL, interleukin; RA, rheumatoid arthritis.
The following sensitivity analyses were performed to examine the robustness of our findings to procedures for handling missing data: (1) excluding patients from France (no disease activity data available); (2) complete case analysis. Further sensitivity analyses were conducted to assess the stability of the results: (1) limited to patients with confirmed or highly likely COVID-19; (2) using the alternative outcome ‘death or invasive ventilation’; (3) using a reduced number of covariates to assess the risk of overfitting; (4) analysis explicitly controlling for country, using data from the top six reporting countries; (5) analysis stratified for several binary key variables (age >65 or not, sex, ever smoked vs not, high/moderate/severe disease activity vs remission/low disease activity, CVD, chronic lung disease, glucocorticoid use) to assess the possibility of interactions.
Data were considered statistically significant for p values <0.05. All analyses were conducted in SAS (V.9.4) and R (V.3.6.3).
Results
As of 1 July 2020, 3830 patients were in the registry, of whom 3729 had no missing values for death, age, sex and DMARD therapy (table 1, results for all patients; online supplemental table 1, results stratified by diagnostic subgroup; online supplemental table 2, results stratified by country; online supplemental table 3, results stratified by medication of interest).
Table 1.
Patient demographic and clinical characteristics
| Parameter | Not deceased | Deceased | Total |
| N | 3339 | 390 | 3729 |
| General | |||
| Age (years) | 55.5 (15.2) | 69.7 (14.6) | 57.0 (15.7) |
| ≤30 | 197 (5.9) | 9 (2.3) | 206 (5.5) |
| 31–50 | 1012 (30.3) | 31 (7.9) | 1043 (28) |
| 51–65 | 1255 (37.6) | 82 (21) | 1337 (35.9) |
| 66–75 | 536 (16.1) | 109 (27.9) | 645 (17.3) |
| >75 | 339 (10.2) | 159 (40.8) | 498 (13.4) |
| Male sex | 1031 (30.9) | 164 (42.1) | 1195 (32) |
| Ever smoker | 664 (23.3) (N=2854) (Missing=485) |
112 (36.1) (N=310) (Missing=80) |
776 (24.5) (N=3164) (Missing=565) |
| Regions | |||
| African region | 14 (0.4) | 2 (0.5) | 16 (0.4) |
| Eastern Mediterranean region | 83 (2.5) | 11 (2.8) | 94 (2.5) |
| European region | 2040 (61.1) | 275 (70.5) | 2315 (62.1) |
| North American region | 1024 (30.7) | 81 (20.8) | 1105 (29.6) |
| South American region | 112 (3.4) | 10 (2.6) | 122 (3.3) |
| South-East Asian region | 11 (0.3) | 0 | 11 (0.3) |
| Western Pacific region | 55 (1.6) | 11 (2.8) | 66 (1.8) |
| Inflammatory joint diseases | |||
| Rheumatoid arthritis | 1224 (36.7) | 170 (43.6) | 1394 (37.4) |
| Spondyloarthritis | 416 (12.5) | 15 (3.8) | 431 (11.6) |
| Psoriatic arthritis | 420 (12.6) | 20 (5.1) | 440 (11.8) |
| Juvenile idiopathic arthritis (poly, oligo, not systemic) | 21 (0.6) | 4 (1) | 25 (0.7) |
| Other inflammatory arthritis | 90 (2.7) | 8 (2.1) | 98 (2.6) |
| Total Inflammatory joint diseases | 2158 (64.6) | 215 (55.1) | 2373 (63.6) |
| Connective tissue diseases/Vasculitis | |||
| Systemic lupus erythematosus | 355 (10.6) | 36 (9.2) | 391 (10.5) |
| Connective tissue diseases (other than SLE) | 473 (14.2) | 60 (15.4) | 533 (14.3) |
| Vasculitis | 258 (7.7) | 68 (17.4) | 326 (8.7) |
| Total CTD | 1035 (31) | 158 (40.5) | 1193 (32.0) |
| Other RMDs | |||
| Total | 306 (9.2) | 50 (12.8) | 356 (9.5) |
| Disease activity |
N=2464
(Missing=875) |
N=294
(Missing=96) |
N=2758
(Missing=971) |
| Remission | 799 (32.4) | 94 (32) | 893 (32.4) |
| Minimal/low disease activity | 1202 (48.8) | 107 (36.4) | 1309 (47.5) |
| Moderate disease activity | 388 (15.7) | 60 (20.4) | 448 (16.2) |
| Severe/high disease activity | 75 (3) | 33 (11.2) | 108 (3.9) |
| Other outcomes | |||
| Hospitalised | 1368 (43.3) (N=3162) (Missing=177) |
371 (96.6) (N=384) (Missing=6) |
1739 (49) (N=3546) (Missing=183) |
| Invasive ventilation | 67 (2.5) (N=2701) (Missing=638) |
120 (40.8) (N=294) (Missing=96) |
187 (6.2) (N=2995) (Missing=734) |
| Comorbidities |
N=3314
(Missing=25) |
N=386
(Missing=4) |
N=3700
(Missing=29) |
| Hypertension | 1095 (33) | 212 (54.9) | 1307 (35.3) |
| Cardiovascular disease | 318 (9.6) | 124 (32.1) | 442 (11.9) |
| Cerebrovascular disease | 89 (2.7) | 20 (5.2) | 109 (2.9) |
| Chronic lung disease | 581 (17.5) | 138 (35.8) | 719 (19.4) |
| Chronic kidney disease | 181 (5.5) | 77 (19.9) | 258 (7) |
| Obesity (BMI ≥30) | 539 (16.3) | 58 (15) | 597 (16.1) |
| Morbid obesity (BMI ≥40) | 106 (3.2) | 16 (4.1) | 122 (3.3) |
| Diabetes | 410 (12.4) | 95 (24.6) | 505 (13.6) |
| Cancer | 165 (5) | 49 (12.7) | 214 (5.8) |
| Other comorbidities | 771 (23.3) | 126 (32.6) | 897 (24.2) |
| Number of comorbities | 1.3 (1.3) | 2.5 (1.6) | 1.4 (1.3) |
| No comorbidity | 1090 (32.9) | 28 (7.3) | 1118 (30.2) |
| One comorbidity | 1032 (31.1) | 83 (21.5) | 1115 (30.1) |
| Two comorbidities | 597 (18) | 110 (28.5) | 707 (19.1) |
| ≥3 comorbidites | 595 (18) | 165 (42.7) | 760 (20.5) |
| DMARD therapies | |||
| csDMARDs monotherapy | 592 (17.7) | 59 (15.1) | 651 (17.5) |
| csDMARDs combination therapy | 692 (20.7) | 61 (15.6) | 753 (20.2) |
| Methotrexate monotherapy | 531 (15.9) | 47 (12.1) | 578 (15.5) |
| Methotrexate combination therapy | 607 (18.2) | 52 (13.3) | 659 (17.7) |
| Leflunomide monotherapy | 61 (1.8) | 12 (3.1) | 73 (2) |
| Leflunomide combination therapy | 120 (3.6) | 10 (2.6) | 130 (3.5) |
| Sulfasalazine monotherapy | 51 (1.5) | 16 (4.1) | 67 (1.8) |
| Sulfasalazine combination therapy | 129 (3.9) | 26 (6.7) | 155 (4.2) |
| Antimalarial monotherapy | 287 (8.6) | 17 (4.4) | 304 (8.2) |
| Antimalarial combination therapy | 322 (9.6) | 39 (10) | 361 (9.7) |
| Immunosuppressants monotherapy | 149 (4.5) | 26 (6.7) | 175 (4.7) |
| Immunosuppressants combination therapy | 147 (4.4) | 21 (5.4) | 168 (4.5) |
| Mycophenolate mofetil monotherapy | 68 (2) | 14 (3.6) | 82 (2.2) |
| Mycophenolate mofetil combination therapy | 81 (2.4) | 15 (3.8) | 96 (2.6) |
| Azathioprine monotherapy | 63 (1.9) | 7 (1.8) | 70 (1.9) |
| Azathioprine combination therapy | 51 (1.5) | 3 (0.8) | 54 (1.4) |
| Cyclophosphamide monotherapy | 10 (0.3) | 3 (0.8) | 13 (0.3) |
| Cyclophosphamide combination therapy | 5 (0.1) | 5 (1.3) | 10 (0.3) |
| Tacrolimus monotherapy | 5 (0.1) | 2 (0.5) | 7 (0.2) |
| Tacrolimus combination therapy | 11 (0.3) | 0 | 11 (0.3) |
| Ciclosporin monotherapy | 3 (0.1) | 0 | 3 (0.1) |
| Ciclosporin combination therapy | 11 (0.3) | 1 (0.3) | 12 (0.3) |
| bDMARDs monotherapy | 675 (20.2) | 48 (12.3) | 723 (19.4) |
| bDMARDs combination therapy | 562 (16.8) | 46 (11.8) | 608 (16.3) |
| TNF inhibitors monotherapy | 434 (13) | 13 (3.3) | 447 (12) |
| TNF inhibitors combination therapy | 340 (10.2) | 17 (4.4) | 357 (9.6) |
| Abatacept monotherapy | 28 (0.8) | 4 (1) | 32 (0.9) |
| Abatacept combination therapy | 46 (1.4) | 5 (1.3) | 51 (1.4) |
| B-cell-targeted bDMARDs monotherapy | 71 (2.1) | 25 (6.4) | 96 (2.6) |
| B-cell-targeted bDMARDs combination therapy | 106 (3.2) | 18 (4.6) | 124 (3.3) |
| Rituximab monotherapy | 66 (2) | 25 (6.4) | 91 (2.4) |
| Rituximab combination therapy | 85 (2.5) | 17 (4.4) | 102 (2.7) |
| Belimumab monotherapy | 5 (0.1) | 0 | 5 (0.1) |
| Belimumab combination therapy | 22 (0.7) | 1 (0.3) | 23 (0.6) |
| IL-6 inhibitors monotherapy | 51 (1.5) | 3 (0.8) | 54 (1.4) |
| IL-6 inhibitors combination therapy | 34 (1) | 2 (0.5) | 36 (1) |
| IL-1 inhibitors monotherapy | 10 (0.3) | 2 (0.5) | 12 (0.3) |
| IL-1 inhibitors combination therapy | 4 (0.1) | 4 (1) | 8 (0.2) |
| IL-17, IL-23, IL-12/23 inhibitors monotherapy | 79 (2.4) | 1 (0.3) | 80 (2.1) |
| IL-17, IL-23, IL-12/23 inhibitors combination therapy | 36 (1.1) | 0 | 36 (1) |
| tsDMARDs monotherapy | 61 (1.8) | 5 (1.3) | 66 (1.8) |
| tsDMARDs (*) combination therapy | 71 (2.1) | 10 (2.6) | 81 (2.2) |
| JAK inhibitors monotherapy | 54 (1.6) | 4 (1) | 58 (1.6) |
| JAK inhibitors combination therapy | 67 (2) | 9 (2.3) | 76 (2) |
| Apremilast monotherapy | 7 (0.2) | 1 (0.3) | 8 (0.2) |
| Apremilast combination therapy | 3 (0.1) | 1 (0.3) | 4 (0.1) |
| No DMARD therapies | 615 (18.4) | 124 (31.8) | 739 (19.8) |
| Further therapies | |||
| Glucocorticoids (#) | 1056 (32) (N=3302) (Missing=37) |
217 (57.1) (N=380) (Missing=10) |
1273 (34.6) (N=3682) (Missing=47) |
| Glucocorticoids 1–10 mg/day | 833 (25.6) (N=3254) (Missing=85) |
150 (41.3) (N=363) (Missing=27) |
983 (27.2) (N=3617) (Missing=112) |
| Glucocorticoids>10 mg/day | 171 (5.3) (N=3254) (Missing=85) |
49 (13.5) (N=363) (Missing=27) |
220 (6.1) (N=3617) (Missing=112) |
| NSAIDs | 600 (19.3) (N=3103) (Missing=236) |
38 (11.0) (N=345) (Missing=45) |
638 (18.5) (N=3448) (Missing=281) |
Data are N (column %) for categorical variables or mean (SD) for continuous variables. The table includes all patients with a non-missing outcome and non-missing values for age, sex and disease-modifying anti-rheumatic drugs (DMARDs) (101 patients excluded). Data refer to patients with non-missing values for the respective variable; total N for patients with non-missing values is given in parentheses for variables with missing values; the total number of missing values is also given in parenthesis, for the applicable variables. (*) Includes one patient on a study medication (Lenabasum). (#) Includes patients with a missing glucocorticoid dosage.
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CTD, connective tissue diseases; DMARD, disease-modifying antirheumatic drug; IL, interleukin; JAK, Janus kinase; JIA, juvenile idiopathic arthritis; N, number; NSAID, non-steroidal anti-inflammatory drugs; SLE, systemic lupus erythematosus; TNF, tumour necrosis factor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
annrheumdis-2020-219498supp001.pdf (659.3KB, pdf)
Patient characteristics and outcomes of COVID-19
Mean age was 57 (15.7) years and most patients were ≤65 years (2586/3729, 69.3%) and female (2534/3729, 68%). The most common disease was RA (1394/3729, 37.4%), followed by CTDs other than SLE (533/3729, 14.3%), SLE (391/3729, 10.5%), PsA (440/3729, 11.8%) and other SpA (431/3729, 11.6%).
Patients were primarily from Europe (2315/3729, 62.1%) or North America (1105/3729, 29.6%). Nearly half (1309/2758, 47.5%) had minimal or low disease activity and one-third (893/2758, 32.4%) were in remission before COVID-19. One-quarter of all patients (776/3164, 24.5%) were ever smokers.
Most patients had a laboratory-confirmed diagnosis of COVID-19 (2897/3729, 77.7%); 2.4% (91/3729) had a high likelihood of infection based on imaging or confirmed COVID-19 contacts.
Death occurred in 10.5% (390/3729) of patients; 68.7% (268/390) of those who died were >65 years. Nearly half of all patients (1739/3546; 49.0%) were hospitalised. Invasive ventilation was reported in 6.2% (187/2995) of patients, but in 40.8% (120/294) of those who died.
Comorbidities
Most patients (2582/3700, 69.8%) had at least one comorbidity, and 20.5% (760/3700) had more than three. The most frequent were hypertension (1307/3700, 35.3%), chronic lung disease (719/3700, 19.4%), obesity (BMI ≥30; 597/3700, 16.1%), diabetes (505/3700, 13.6%), other CVD (442/3700, 11.9%) and CKD (258/3700, 7.0%). Among deceased patients, the proportion of those with comorbidities was higher, with 42.7% (165/386) having ≥3 comorbidities, namely, 54.9% (212/386) with hypertension, 35.8% (138/386) with chronic lung disease, 24.6% (95/386) with diabetes, 32.1% (124/386) with other CVD and 19.9% (77/386) with CKD.
Treatments
At the time of COVID-19 diagnosis, 40.6% (1514/3729) of patients were treated only with csDMARDs, immunosuppressants or combinations of these; 35.7% (1331/3729) received bDMARDs and 3.9% (147/3729) received tsDMARDs. One-fifth (739/3729, 19.8%) were not receiving any DMARD/immunosuppressive treatment (except glucocorticoids), and this proportion was higher among deceased patients (124/390, 31.8%).
Among the patients not receiving any DMARD/immunosuppressive treatment, 39.8% (290/729) received glucocorticoids, 9.8% (70/712) with a prednisolone-equivalent dosage of >10 mg/day; the most frequent diagnostic categories being other non-specified rheumatic diseases (173/739, 23.4%), vasculitis (161/739, 21.8%), CTD other than SLE (156/739, 21.1%) and RA (110/739, 14.9%).
Country-specific differences
The majority of cases (2993/3729, 80.3%) were reported from six countries with considerable differences in reported percentages of death (online supplemental table 2). Overall, 10.5% (390/3729) of patients died, with highest proportions in the UK (91/435, 20.9%) and Italy (53/315, 16.8%). Death was reported in lower proportions in the USA (70/1005, 7.0%), Germany (15/198, 7.6%), France (62/793, 7.8%) and Spain (21/247, 8.5%). Other major differences between the countries were the distribution of rheumatic diseases and the distribution and frequency of comorbidities.
Factors associated with death
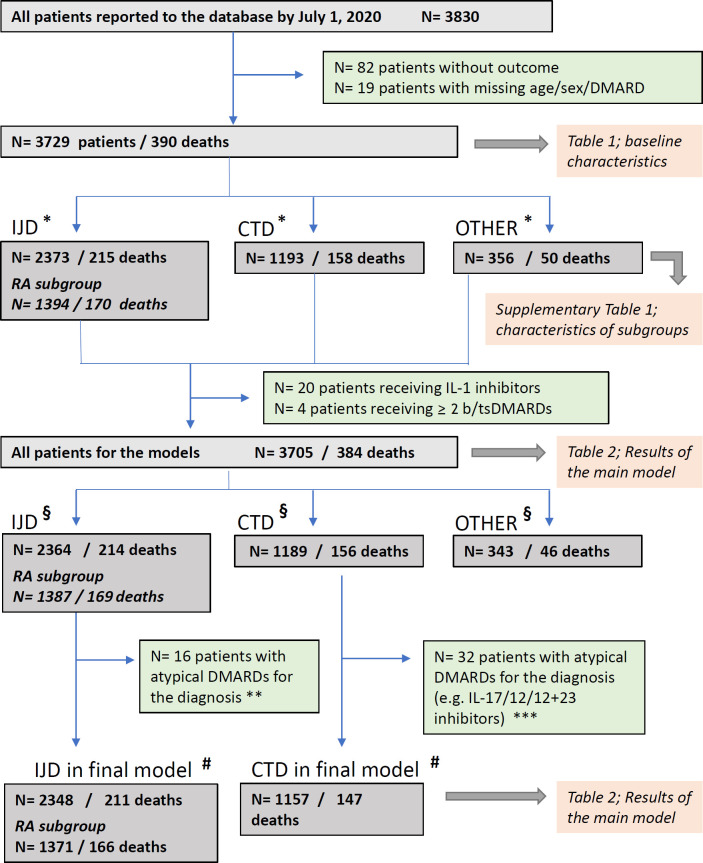
In multivariable analyses (table 2, figure 3), patients between 66 and 75 years of age were more likely to have died (OR 3.00, 95% CI 2.13 to 4.22) than those ≤65 years. The association was even more pronounced in patients over 75 years (6.18, 4.47 to 8.53; vs ≤65 years). Male sex was also associated with higher odds of death (1.46, 1.11 to 1.91). Current or former smoking was only associated with death in the RA subgroup (1.45, 1.02 to 2.04).
Table 2.
Multivariable logistic regression analysis of factors associated with COVID-19-related death in patients with rheumatic diseases (all patients)
| N deaths/patients (%) | All | Patients with inflammatory joint diseases (IJDs) | Only patients with rheumatoid arthritis | Patients with connective tissue diseases (CTDs) or vasculitis | ||||||||
| 384/3705 (10.4%) | 211/2348 (9.0%) | 166/1371 (12.1%) | 147/1157 (12.7%) | |||||||||
| N deaths/patients | OR | 95% CI | N deaths/patients | OR | 95% CI | N deaths/patients | OR | 95% CI | N deaths/patients | OR | 95% CI | |
| Age, years | ||||||||||||
| Age≤65 | 118/2565 | 1 | Reference | 55/1657 | 1 | Reference | 40/840 | 1 | Reference | 56/779 | 1 | Reference |
| 65 years<Age≤75 | 109/644 | 3 | 2.13 to 4.22 | 71/426 | 3.63 | 2.55 to 5.15 | 55/314 | 3.10 | 1.68 to 5.72 | 33/187 | 2.29 | 1.34 to 3.93 |
| Age>75 | 157/496 | 6.18 | 4.47 to 8.53 | 85/265 | 8.21 | 5.54 to 12.18 | 71/217 | 7.30 | 4.42 to 12.06 | 58/191 | 4.08 | 2.27 to 7.36 |
| Male sex (vs female) | 161/1188 | 1.46 | 1.11 to 1.91 | 82/788 | 1.31 | 0.95 to 1.8 | 55/345 | 1.17 | 0.78 to 1.76 | 63/296 | 1.66 | 0.96 to 2.86 |
| Ever smoked (vs never) | 140/922 | 1.21 | 0.94 to 1.57 | 84/607 | 1.26 | 0.93 to 1.72 | 71/385 | 1.45 | 1.02 to 2.04 | 42/248 | 1.11 | 0.67 to 1.86 |
| Comorbidities | ||||||||||||
| Hypertension alone or CVD alone | 155/1150 | 1.19 | 0.89 to 1.59 | 79/690 | 1.04 | 0.74 to 1.46 | 66/454 | 1.11 | 0.74 to 1.67 | 69/406 | 1.56 | 1.06 to 2.29 |
| Hypertension and CVD | 89/301 | 1.89 | 1.31 to 2.73 | 53/168 | 2.29 | 1.25 to 4.23 | 38/118 | 2.03 | 1.03 to 3.97 | 28/106 | 1.57 | 0.78 to 3.16 |
| Chronic lung disease | 136/721 | 1.68 | 1.26 to 2.25 | 76/406 | 1.52 | 1.04 to 2.21 | 63/293 | 1.44 | 0.99 to 2.09 | 54/285 | 2.05 | 1.47 to 2.85 |
| Chronic kidney disease | 76/259 | 1.67 | 0.99 to 2.8 | 27/111 | 1.09 | 0.54 to 2.21 | 21/83 | 1.01 | 0.46 to 2.24 | 41/124 | 2.30 | 1.37 to 3.88 |
| Diabetes mellitus | 96/508 | 1.38 | 0.88 to 2.17 | 55/313 | 1.31 | 0.95 to 1.79 | 39/213 | 1.08 | 0.72 to 1.61 | 32/154 | 1.39 | 0.64 to 3 |
| Rheumatic disease | ||||||||||||
| Rheumatoid arthritis | 160/1326 | 1 | Reference | 166/1373 | 1 | Reference | n.a. | n.a. | ||||
| Systemic lupus erythematosus | 36/391 | 1.2 | 0.70 to 2.04 | n.a. | n.a. | 32/378 | 1 | Reference | ||||
| Vasculitis | 67/325 | 0.8 | 0.60 to 1.08 | n.a. | n.a. | 64/318 | 0.81 | 0.49 to 1.33 | ||||
| Other connective tissue diseases | 53/473 | 0.75 | 0.58 to 0.97 | n.a. | n.a. | 51/461 | 0.78 | 0.39 to 1.54 | ||||
| Psoriasis arthritis | 19/429 | 0.75 | 0.53 to 1.07 | 19/437 | 0.82 | 0.55 to 1.22 | n.a. | n.a. | ||||
| Spondyloarthritis | 15/423 | 0.72 | 0.34 to 1.54 | 15/424 | 0.82 | 0.4 to 1.69 | n.a. | n.a. | ||||
| Other inflammatory arthritis or non-systemic JIA | 10/109 | 0.79 | 0.46 to 1.34 | 11/114 | 0.76 | 0.43 to 1.36 | n.a. | n.a. | ||||
| Other rheumatic diseases (not IJDs/CTDs/vasculitis) | 24/229 | 0.51 | 0.35 to 0.73 | n.a. | n.a. | n.a. | ||||||
| High/moderate/severe disease activity (DA) vs remission/low DA | 109/722 | 1.87 | 1.27 to 2.77 | 54/453 | 1.6 | 1.13 to 2.26 | 44/274 | 1.60 | 1.03 to 2.47 | 51/230 | 2.45 | 1.49 to 4.02 |
| Medication | ||||||||||||
| Methotrexate | 47/595 | 1 | Reference | 41/487 | 1 | Reference | 34/354 | 1 | Reference | 6/94 | 1 | Reference |
| No DMARD therapy | 124/739 | 2.11 | 1.48 to 3.01 | 38/239 | 2.08 | 1.38 to 3.14 | 25/110 | 2.12 | 1.34 to 3.37 | 67/353 | 3.18 | 1.61 to 6.27 |
| Leflunomide | 12/90 | 1.56 | 0.9 to 2.7 | 10/83 | 1.37 | 0.69 to 2.73 | 9/68 | 1.43 | 0.71 to 2.86 | n.a. | ||
| Antimalarials | 27/426 | 0.99 | 0.66 to 1.48 | 17/167 | 1.14 | 0.65 to 2 | 17/141 | 1.24 | 0.7 to 2.19 | 11/271 | 1.38 | 0.48 to 4.02 |
| Sulfasalazine | 33/144 | 3.6 | 1.66 to 7.78 | 31/137 | 3.40 | 1.46 to 7.93 | 21/85 | 2.62 | 1.21 to 5.68 | n.a. | ||
| Immunosuppressants | 38/276 | 2.22 | 1.43 to 3.46 | n.a. | n.a. | 32/247 | 2.44 | 1.06 to 5.65 | ||||
| TNF inhibitors | 30/803 | 0.85 | 0.52 to 1.36 | 26/764 | 0.77 | 0.42 to 1.41 | 16/292 | 0.82 | 0.39 to 1.76 | 4/39 | 2.00 | 0.36 to 11.2 |
| Abatacept | 9/81 | 1.20 | 0.61 to 2.34 | 9/75 | 1.3 | 0.62 to 2.71 | 9/68 | 1.4 | 0.65 to 2.99 | n.a. | ||
| Rituximab | 42/192 | 4.04 | 2.32 to 7.03 | 22/90 | 5.42 | 2.77 to 10.61 | 21/86 | 4.99 | 2.43 to 10.26 | 22/104 | 3.72 | 1.21 to 11.48 |
| Belimumab | 1/27 | 0.71 | 0.19 to 2.68 | n.a. | n.a. | 1/27 | 1.07 | 0.21 to 5.37 | ||||
| IL-6 inhibitors | 5/90 | 0.83 | 0.38 to 1.84 | 1/68 | 0.25 | 0.03 to 2.43 | 1/63 | 0.25 | 0.03 to 2.33 | 4/23 | 2.69 | 0.88 to 8.19 |
| IL-17/IL-23/IL-12+23 inhibitors | 1/115 | 0.25 | 0.03 to 2.04 | 1/112 | 0.26 | 0.03 to 2.06 | n.a. | n.a. | ||||
| tsDMARDs | 15/145 | 1.60 | 0.91 to 2.8 | 15/142 | 1.75 | 0.99 to 3.12 | 13/118 | 1.57 | 0.75 to 3.27 | n.a. | ||
| Glucocorticoids (GCs) | ||||||||||||
| No GCs | 165/2417 | 1 | Reference | 109/1721 | 1 | Reference | 78/863 | 1 | Reference | 38/551 | 1 | Reference |
| GCs 1–10 mg/day | 170/1062 | 1.43 | 0.98 to 2.09 | 89/567 | 1.36 | 0.76 to 2.45 | 78/464 | 1.34 | 0.66 to 2.74 | 75/469 | 1.69 | 1.11 to 2.57 |
| GCs>10 mg/day | 49/226 | 1.69 | 1.18 to 2.41 | 12/60 | 1.55 | 0.67 to 3.57 | 10/44 | 1.59 | 0.6 to 4.18 | 34/137 | 1.93 | 1.11 to 3.36 |
Missing values were imputed via multiple imputation, patient numbers may thus be rounded. Effects significant at level α=0.05 are marked in bold. Patients were excluded from a particular analysis if the medication they received provided ≤20 patients for that analysis or if there were no deaths reported for that specific medication.
TNF; tumour necrosis factor; CTD, connective tissue diseases; CVD, cardiovascular duisease; DMARD, disease-modifying antirheumatic drugs; GC, glucocorticoids; IL, interleukin; JIA, juvenile idiopathic arthritis; N, number; n.a., not applicable; tsDMARD, targeted synthetic disease-modifying antirheumatic drugs.
Figure 3.
Results of the main logistic regression analysis. Shown are multivariable-adjusted ORs for the outcome COVID-19-related death with 95% CIs, assessing the association with (A) general patient characteristics, (B) comorbidities, (C) rheumatic disease diagnoses (RMD) and (D) rheumatic disease medications. ORs are shown for four groups: all patients (black), patients with inflammatory joint disease (red), patients with rheumatoid arthritis (orange), and patients with a connective tissue disease or vasculitis (blue). For (C), only ORs for all patients are shown. The reference categories are as follows: (A) ≤65 years, females, never smoked, remission or low disease activity; (B) the non-presence of the specific comorbidities (for all effects); (C) rheumatoid arthritis (for all effects); (D) methotrexate monotherapy (for all effects except for glucocorticoids), no glucocorticoids (for glucocorticoid dosage groups). Patients receiving multiple csDMARDs or immunosuppressants (except glucocorticoids) were grouped according to the following hierarchy: immunosuppressants>sulfasalazine>antimalarials>leflunomide>methotrexate; patients receiving a b/tsDMARD were considered solely in the b/tsDMARD group; glucocorticoids were examined separately and categorised by prednisolone-equivalent dosage (1–10 mg/day and >10 mg/day). bDMARD, biological disease-modifying anti-rheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CTD, connective tissue diseases; CVD, cardiovascular disease; JIA, juvenile idiopathic arthritis; tsDMARD, targeted synthetic disease-modifying anti-rheumatic drug.
Other factors associated with death included chronic lung disease (1.68, 1.26 to 2.25) and CVD combined with hypertension (1.89, 1.31 to 2.73), whereas hypertension or CVD alone did not show a significant association. CKD was significantly associated with death in patients with CTD or vasculitis (2.30, 1.37 to 3.88) but not in other disease subgroups.
Across all diagnostic groups, treatments with leflunomide, antimalarials, TNF inhibitors, abatacept, belimumab, IL-6 inhibitors, IL-17/IL-23/IL-12+23 inhibitors and tsDMARDs were not associated with death, as compared with methotrexate monotherapy. In the overall model, not receiving DMARD treatment was associated with death (2.11, 1.48 to 3.01) compared with methotrexate monotherapy. This was also seen in the IJD, RA and CTD subgroups.
Compared with methotrexate monotherapy, treatments associated with a higher odds of death were rituximab (4.04, 2.32 to 7.03, in the overall model; 5.42, 2.77 to 10.61, in the IJD subgroup; 4.99, 2.43 to 10.26, in the RA subgroup; 3.72, 1.21 to 11.48, in the CTD/vasculitis subgroup), sulfasalazine (3.60, 1.66 to 7.78, in the overall model and consistent across all subgroups) and immunosuppressants (azathioprine, cyclophosphamide, ciclosporin, mycophenolate or tacrolimus: 2.22, 1.43 to 3.46, in the overall model; 2.44, 1.06 to 5.65, in the CTD/vasculitis subgroup; not applicable to other subgroups).
An additional analysis indicated that the association of sulfasalazine with an increased odds for death was mainly driven by the larger group of sulfasalazine monotherapy and persisted even when sulfasalazine combination treatment (plus either antimalarials, leflunomide or methotrexate) was considered separately (data not shown).
Treatment with higher dosages of glucocorticoids (>10 mg/day prednisolone-equivalent dose vs no use) was also found to be associated with death (1.69, 1.18 to 2.41), particularly in the CTD/vasculitis subgroup (1.93, 1.11 to 3.36).
Higher disease activity at COVID-19 diagnosis was consistently associated with death across all disease groups. Patients with high/moderate/severe disease activity had higher odds of death (1.87, 1.27 to 2.77) than patients with low disease activity or in remission (overall model and consistent across all subgroups).
Sensitivity analyses
Results were largely consistent in our sensitivity analyses (online supplemental tables 4–9). In the complete case analysis (online supplemental table 5), the association between sulfasalazine and death was no longer statistically significant. In stratified analyses (online supplemental tables 10–16), sulfasalazine use was not associated with death among patients that never smoked, with the OR among ever smokers being almost threefold than among non-smokers (online supplemental table 12).
Discussion
With global cooperation, the C19-GRA physician-reported registry is the largest collection to date of patients with rheumatic diseases and COVID-19. We found that moderate/high disease activity was significantly associated with COVID-19-related death, confirming recent recommendations regarding the importance of disease control in rheumatic diseases in the COVID-19 era.1 Other factors associated with death were older age, male sex and the presence of comorbidities, which is consistent with reports from the general population.8 Overall, compared with methotrexate monotherapy, most DMARDs were not associated with higher odds of death, although rituximab and sulfasalazine were notable exceptions. Prednisolone-equivalent dosages >10 mg/day and other immunosuppressive drugs (as opposed to immunomodulatory DMARDs) were also associated with COVID-19-related death.
In this cohort of patients with underlying rheumatic diseases, the COVID-19-related death rate was 10.5%, clearly higher than that reported in the general population in most countries. However, this study was not designed to calculate a precise point estimate for mortality. Reporting biases and population-related factors, including COVID-19 testing rates, could explain this figure and, importantly, it should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19.
The association of rituximab with poorer COVID-19-related outcomes is a previously unreported finding outside of case reports. Rituximab binds to CD20 on the surface of B-cells, effectively depleting this cell type, and interferes with antibody development. Therefore, B-cell depletion could potentially compromise antiviral immunity, including the development of SARS-CoV-2 antibodies.19 With our data, it was not possible to determine the exact timing of infection following rituximab infusion, although all patients were clinically judged by their rheumatologist to have been exposed to the immunological effects of the drug at the time of COVID-19 diagnosis. The association between rituximab and COVID-19-related death could have also been influenced by the typical coadministration of methylprednisolone with rituximab.
A finding that merits further research is the higher odds of death found with sulfasalazine treatment. This association has also been reported in results from an international registry of patients with inflammatory bowel disease and COVID-19, where sulfasalazine or 5-aminosalicylate (5-ASA) use was associated with severe COVID-19 (adjusted OR of 3.1 (1.3 to 7.7)).20 This finding is surprising as sulfasalazine is usually considered to have a low immunosuppressive effect. Prior research supports an immune regulatory effect driven by sulfasalazine or its metabolite 5-ASA against other RNA viruses.21–24 However, causal interpretation of the association between sulfasalazine and COVID-19-related death should not be made. The perceived low immunosuppressive effect of sulfasalazine may have led rheumatologists to prescribe preferentially sulfasalazine over methotrexate in patients who were perceived to be at higher risk, for example, patients with pulmonary disease, smoking or recurrent chest infections. In an observational study like ours, this could lead to unmeasured confounding. A salient difference in sulfasalazine users in our study was a higher proportion of current or former smokers, compared with non-users. In the stratified analyses for chronic lung disease, the association between death and sulfasalazine was significant in both subgroups with and without chronic lung disease, while in the stratified analyses for smoking, the association between death and sulfasalazine was limited to ever smokers, so the factor ‘smoking’ could potentially be an effect modifier. Another potential explanation for this finding could be the merging of sulfasalazine combination therapy (with other csDMARDs) with sulfasalazine monotherapy; however, the increased odds for death persisted in the sulfasalazine monotherapy group and was not driven by the combination treatment (data not shown).
Despite the large overall sample size, for some therapies (eg, IL-6 and IL-17/IL-23/IL-12+23 inhibitors) the number of users was low and no firm conclusions could be made. IL-6 inhibitors have been used to counteract the hyperinflammatory state produced by COVID-19, with mostly disappointing randomised trial results.25 26 Their efficacy is still being investigated in ongoing trials, but it is reassuring that they were not associated with COVID-19-related death in our analyses. Previous studies had shown an association between TNF inhibitors and a decreased risk of sepsis and mortality in patients with RA after serious infection compared with csDMARDs.27 28 We could not confirm such an association after stratification by disease and adjustment for disease activity. However, the data indicate that some associations may exist among patients diagnosed with IJD other than RA (a subgroup comprising predominantly patients with axial SpA and PsA), in whom male sex and diabetes mellitus were associated with a higher odds of death, and TNF inhibitor use was associated with a lower odds of death (univariable analysis, data not shown). Due to a small number of deceased patients in this subgroup with non-RA subtypes of IJD (n=37 deaths), these effects could not be assessed in a multivariable model and this should be investigated in the future when higher case numbers allow a more stable assessment.
This study has limitations. As a cross-sectional, case-reporting registry, it may be subject to selection bias if more severe cases are more likely to come to the rheumatologists’ attention and therefore to be reported. There is an absence of a population-based comparator, and we are unable to make comparisons between those with and without COVID-19. Moreover, we caution against interpreting our estimates causally. There is likely unmeasured confounding dependent on the particularities of health systems and case reporting differences. We tried to address this by limiting the research questions to those that could be answered with this dataset and by accounting for potential confounders in our analyses. The high number of variables compared with outcome events in the subgroup models may result in biased estimates.29 30 However, the consistency between the main model and the sensitivity analyses (including using a lower number of variables) do not indicate an issue with overfitting.
In conclusion, people with rheumatic diseases with higher disease activity have higher odds of COVID-19-related death, highlighting the importance of disease control, preferably by managing DMARDs effectively without increasing glucocorticoids. Future studies should address the observed association of rituximab and sulfasalazine with poor outcomes. Finally, as in the general population, older age, male sex and/or the presence of comorbidities increase the odds of COVID-19-related death.
Acknowledgments
We wish to thank all rheumatology providers who entered data into the registry.
Footnotes
Handling editor: Josef S Smolen
Twitter: @rheum_cat, @rthritis, @emilysirotich, @zach_wallace_md, @carmona_loreto, @hausmannmd, @philipcrobinson, @pedrommcmachado
AS and MS contributed equally.
PCR, JY and PMM contributed equally.
Correction notice: This article has been corrected since it published Online First. The collaborator names have been updated.
Collaborators: COVID-19 Global Rheumatology Alliance Consortium: Brahim Dahou (Association Rhumatologues Algériens Privés; Algeria), Marcelo Pinheiro (Universidade Federal De São Paulo Escola Paulista de Medicina e Escola Paulista de Enfermagem; Brazil), Francinne M Ribeiro (Hospital Universitário Pedro Ernesto Universidade do Estado do Rio de Janeiro; Brazil), Anne-Marie Chassin-Trubert (Complejo Hospitalario San José; Chile), Sebastián Ibáñez (Clínica Alemana de Santiago, Chile), Lingli Dong (Tongji Hospital, China)
Lui Cajas (Clinica Universitaria Colombia - Centro Medico Providencia Sanitas; Colombia), Hesham Hamoud (Al Azhar University Hospitals; Egypt), Jérôme Avouac (Rheumatology A Department, Cochin University Hospitals Paris-Centre, AP-HP; France), Véronique Belin (Department of Rheumatology, Hospital Center of Thonon-les-Bains; France), Raphaël Borie (Department of Pneumology, Bicha Hospital, AP-HP; France), Pascal Chazerain (Department of Rheumatology and Internal Medicine, Diaconesses Croix Saint Simon Hospital, Paris; France), Xavier Chevalier (Department of Rheumatology, Henri Mondor University Hospitals, AP-HP, Créteil; France), Pascal Claudepierre (Department of Rheumatology, Henri Mondor University Hospitals, AP-HP, Créteil; France), Gaëlle Clavel (Department of Internal Medicine, Rothschild Foundation, Paris; France), Marie-Eve Colette-Cedoz (Nord-Isère Rheumatology practice, Bourgoin-Jallieu; France), Bernard Combe (Department of Rheumatology, Lapeyronie University Hospital of Montpellier France), Elodie Constant (Department of Rheumatology, Hospital Center of Valence; France), Nathalie Costedoat-Chalumeau (Department of Internal Medicine, Cochin University Hospitals Paris-Centre, AP-HP; France), Marie Desmurs (Department of Rheumatology, Mulhouse-South Alsace hospital group; France), Valérie Devauchelle-Pensec (Rheumatology Department, Cavale Blanche Hospital and Brest Occidentale University; France), Mathilde Devaux (Department of Internal Medicine, Intercommunal Hospital Center of Poissy-Saint Germain; France), Robin Dhote (Department of Internal Medicine, Avicenne University Hospital, AP-HP, Paris; France), Yannick Dieudonné (Department of Internal Medicine and Clinical Immunology, Strasbourg University Hospital; France), Fanny Domont (Department of Internal Medicine and Clinical Immunology, Pitié-Salpêtrière Hospital, AP-HP; France), Pierre-Marie Duret (Department of Rheumatology, Colmar Civil Hospitals; France), Mikaël Ebbo (Department of Internal Medicine, La Timone Hospital, Aix-Marseille Univerity, AP-HM; France), Esther Ebstein (Department of Rheumatology, Bicha Hospital, AP-HP; France), Soumaya El Mahou (Department of Rheumatology and Internal Medicine, Hospital Center of Tourcoing; France), Bruno Fautrel (Department of Rheumatology, University Hospital Pitie Salpetriere, AP-HP, Paris; France), Renaud Felten (Department of Rheumatology, University Hospital of Strasbourg; France), René-Marc Flipo (Department of Rheumatology, University Hospital of Lille; France), Violaine Foltz (Department of Rheumatology, University Hospital Pitie Salpetriere, AP-HP; France), Antoine Froissart (Department of Internal Medicine, Intercommunal Hospital Center of Créteil; France), Joris Galland (Department of Internal Medicine, Lariboisière University Hospital, AP-HP, Paris; France), Véronique Gaud-Listrat (Rheumatology practice, Saint-Michel-sur-Orge; France), Sophie Georgin-Lavialle (Department of Internal Medicine, Tenon Hospital, AP-HP; France), Aude Giraud-Morelet (Val d'Ouest Clinical Medicine Center; France), Jeanine S Giraudet-Le Quitrec (Rheumatology A Department, Cochin University Hospitals Paris-Centre, AP-HP; France), Philippe Goupille (Department of Rheumatology, University Hospital of Tours; France), Sophie Govindaraju-Audouard (Rheumatology practice Les Haberges, Vesoul; France), Franck Grados (Department of Rheumatology, Amiens University Hospital; France), Séverine Guillaume-Czitrom (Department of Adolescent Medicine, University Hospital Paris-Sud, AP-HP, Le Kremlin-Bicêtre; France), Marion Hermet (Department of Internal Medicine, Hospital Center of Vichy; France), Ambre Hittinger-Roux (Department of Rheumatology, University Hospital of Reims; France), Christophe Hudry (Institute of Rheumatology Paris 8; France), Isabelle Kone-Paut (Department of Paediatric Rheumatology, University Hospital Paris-Sud, AP-HP, Le Kremlin-Bicêtre; France), Sylvain La Batide Alanore (Rheumatology practice, Paris; France), Pierre Lafforgue (Department of Rheumatology, Sainte-Marguerite Hospital, Aix-Marseille Univerity, AP-HM; France), Sophie Lahalle (Department of Rheumatology and Internal Medicine, Diaconesses Croix Saint Simon Hospital, Paris; France), Isabelle Lambrecht (Department of Rheumatology, Maison-Blanche Hospital, Reims University Hospitals; France), Vincent Langlois (Department of Infectious Diseases and Internal Medicine, Jacques Monod Hospital, Le Havre; France), Jean-Paul Larbre (Department of Rheumatology, Lyon-Sud Hospital, Hospices Civils Lyon; France), Emmanuel Ledoult (Department of Internal Medicine, Hospital Center of Tourcoing; France), Christophe Leroux (Department of Polyvalent Medicine, Dreux Hospital center; France), Frédéric Liote (Department of Rheumatology, Lariboisière University Hospital, AP-HP, Paris; France), Alexandre TJ Maria (Department of Internal Medicine and Multiorganic Diseases, Saint-Eloi University Hospital of Montpellier; France), Hubert Marotte (Department of Rheumatology, Saint-Etienne University Hospital; France), Arsène Mekinian (Department of Internal Medicine, Saint-Antoine Hospital, AP-HP, Paris; France), Isabelle Melki (Paediatric Hematology-Immunology and Rheumatology Department, Necker-Enfants-Malades University Hospital, AP-HP;France), Laurent Messer (Department of Rheumatology, Colmar Civil Hospitals; France), Catherine Michel (Department of Dermatology, Mulhouse-South Alsace hospital group; France), Gauthier Morel (Department of Rheumatology, Hospital Center of Valencienne; France), Jacques Morel (Department of Rheumatology, Lapeyronie University Hospital of Montpellier; France), Marie-Noelle Paris-Havard (Department of Rheumatology, Hospital Center of Argenteuil; France), Edouard Pertuiset (Department of Rheumatology, René Dubos Hospital Center, Pontoise; France), Thao Pham (Department of Rheumatology, Sainte-Marguerite Hospital, Aix-Marseille Univerity, AP-HM; France), Myriam Renard (Department of Rheumatology, Hospital Center of Aix-les-Bains, France), Sabine Revuz (Department of Internal Medicine and Clinical Immunology, Metz private Hospitals; France), Sébastien Rivière (Department of Internal Medicine and Inflammation-Immunopathology-Biotherapy, Saint-Antoine Hospital, AP-HP, Paris; France), Clémentine Rousselin (Department of Internal Medicine and Nephrology, Hospital Center of Valencienne; France), Christian Roux (Department of Rheumatology, Pasteur 2 University Hospital of Nice Sophia-Antipolis; France), Diane Rouzaud (Department of Internal Medicine, Bicha Hospital, AP-HP; France), Jérémie Sellam (Department of Rheumatology, Saint-Antoine Hospital, AP-HP, Paris; France), Raphaele Seror (Department of Rheumatology, University Hospitals Paris-Sud, AP-HP, Le Kremlin-Bicêtre; France), Amelie Servettaz (Department of Internal Medicine, University Hospital of Reims; France), Vincent Sobanski (Department of Internal Medicine and Clinical Immunology, University Hospital of Lille; France), Christelle Sordet (Department of Rheumatology, University Hospital of Strasbourg; France), Lionnel Spielmann (Department of Rheumatology, Colmar Civil Hospitals; France), Nathalie Tieulié (Department of Rheumatology, Pasteur 2 University Hospital of Nice Sophia-Antipolis; France), Alice Tison (Department of Rheumatology, University Hospital of Bordeaux; France), Sophie Trijau (Department of Rheumatology, Sainte-Marguerite Hospital, Aix-Marseille Univerity, AP-HM; France), Alexandre Virone (Department of Rheumatology, University Hospitals Paris-Sud, AP-HP, Le Kremlin-Bicêtre; France), Ursula Warzocha (Department of Internal Medicine, Avicenne University Hospital, AP-HP, Paris; France), Daniel Wendling (Department of Rheumatology, University Hospital of Besançon; France), Frederik N Albach (Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin; Germany), Peer Aries (Rheumatology Struensee-Haus, Hamburg; Germany), Elvira Decker (Medical Care Center, Alsfeld; Germany), Urs Hartmann (Private Practice, Mainz; Germany), Joerg Henes (Department of Internal Medicine II, University of Tubingen; Germany), Bimba F Hoyer (University Hospital Schleswig-Holstein - Campus Kiel, Department of Rheumatology and Clinical Immunology, Clinic for Internal Medicine I; Germany), Andreas Krause (Immanuel Hospital Berlin, Department of Rheumatology, Clinical Immunology and Osteology; Germany), Klaus Krüger (Private Practice, Munich; Germany), Hanns-Martin Lorenz (University of Heidelberg, Department of Rheumatology; Germany), Ulf Müller-Ladner (Campus Kerckhoff, Justus-Liebig-University Giessen, Department of Rheumatology and Clinical Immunology; Germany), Alexander Pfeil (Department of Internal Medicine III, University Hospital Jena; Germany), Anne Regierer (German Rheumatism Research Center Berlin; Germany), Jutta G Richter (Heinrich-Heine-University, Medical Faculty, Department of Rheumatology and Hiller Research Unit; Germany), Markus Rihl (Private Practice, Traunstein; Germany), Tim Schmeiser (Private Practice, Cologne; Germany), Hendrik Schulze-Koops (University of Munich, Division of Rheumatology and Clinical Immunology, Department of Internal Medicine IV; Germany), Christof Specker (KEM Kliniken Essen-Mitte, Department of Rheumatology and Clinical Immunology; Germany), Reinhard E Voll (University Medical Center Freiburg, Department of Rheumatology and Clinical Immunology; Germany), Stephanie Werner (RHIO, Dusseldorf; Germany), Gabriela MG Melgar (Hospital del Valle; Honduras), Mahdi Vojdanian (Iran Rheumatology Center; Iran), Laura Andreoli (UO di Reumatologia ed Immunologia Clinica, Spedali Civili di Brescia; Italy), Elena Bartoloni-Bocci (University of Perugia; Italy), Maurizio Benucci (Ospedale civile San Giovanni di Dio; Italy), Francesco Campanaro (ASST Sette Laghi, Varese; Italy), Marta Caprioli (Rheumatology, Humanitas Clinical and Research Center – IRCCS; Italy), Davide Carboni (Azienda ospedaliero-universitaria Careggi; Italy), Greta Carrara (Italian Society for Rheumatology; Italy), Edoardo Cipolletta (Università Politecnica delle Marche; Italy), Chiara Crotti (ASST Gaetano Pini; Italy), Gloria Dallagiacoma (Ospedale di Brunico; Italy), Paola Faggioli (ASST ovest milanese legnano; Italy), Rosario Foti (Policlinico-Vitt. Emanuele; Policinico San Marco; Italy), Franco Franceschini (UO di Reumatologia ed Immunologia Clinica, Spedali Civili di Brescia Italy), Micaela Fredi (UO di Reumatologia ed Immunologia Clinica, Spedali Civili di Brescia; Italy), Giacomo Guidelli (Rheumatology, Humanitas Clinical and Research Center – IRCCS; Italy), Florenzo Iannone (University of Bari; Italy), Gianpiero Landolfi (Italian Society for Rheumatology; Italy), Caludia Lomater (Azienda Ospedaliera Ordine Mauriziano di Torino; Italy), Ceciclia Nalli (UO di Reumatologia ed Immunologia Clinica, Spedali Civili di Brescia; Italy), Simone Parisi (AOU Città della Salute e della Scienza; Italy), Luca Quartuccio (Università degli Studi di Udine; Italy), Bernd Raffeiner (Ospedale di Bolzano; Italy), Rossella Reggia (Ospedale Maggiore di Cremona; Italy), Marta Riva (Ospedale San Gerardo di Monza; Italy), Nicoletta Romeo (Azienda Ospedaliera Santa Croce e Carle di Cuneo; Italy), Cinzia Rotondo (Azienda Ospedaliero-Universitaria "Ospedali Riuniti" di Foggia; Italy), Ettore Silvagni (University of Ferrara; Italy), Luigi Sinigaglia (Italian Society for Rheumatology; Italy), Ilaria Tinazzi (Ospedale Sacro Cuore don Calabria di Negrar a Verona; Italy), Anna Zanetti (Italian Society for Rheumatology; Italy), Giovanni Zanframundo (Fondazione IRCCS Policlinico San Matteo di Pavia; Italy), Fatemah Abutiban (Kuwait Rheumatology Association; Kuwait), Deshiré Alpízar-Rodríguez (Mexican College of Rheumatology; Mexico), Marina Rull-Gabayet (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; Mexico), Fedra Irazoque (Private Practice; Mexico), Xochitl Jimenez (Centro Medico Naval; Mexico), Eduardo Martín-Nares (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; Mexico), Angel Castillo-Ortiz (Centro Medico Las Americas; Mexico), Tatiana S Rodriguez-Reyna (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; Mexico), Diana C Rosete (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; Mexico), Erick A Zamora-Tehozol (Centro Medico Pensiones; Mexico), David Vega-Morales (Hospital General de Zona #17; Mexico), Beatriz Elena Zaueta-Montiel (Centro Medico del Angel; Mexico), Rebecca Grainger (University of Otago, Wellington; New Zealand), Nasra Al-Adhoubi (Royal Hospital; Oman), Babur Salim (Fauji Foundation Hospital; Pakistan), Enrique Giraldo (Complejo Hospitalario; Panama), Ariel Salinas (Hospital Essalud Alberto Sabogal Sologuren; Peru), Manuel Ugarte-Gil (Universidad Científica del Sur-Hospital Guillermo Almenara Irigoyen; Peru), Diogo Almeida (Rheumatology Department, Unidade Local de Saúde do Alto Minho, Ponte de Lima; Portugal), Miguel Bernardes (Rheumatology Department, Centro Hopitalar São João, Porto; Portugal), Rita C Machado (Rheumatology and Metabolic Bone Diseases Department, Hospital de Santa Maria, CHLN, Lisbon Academic Medical Centre, Lisboa; Portugal), Maria Rato (Rheumatology Department, Centro Hopitalar São João, Porto; Portugal), Samar Al-Emadi (Hamad Medical Corporation; Qatar), Richard Conway (St. James's Hospital; Republic of Ireland), Rachael Flood (Tallaght University Hospital; Republic of Ireland), Juan J Alegre-Sancho (Hospital Universitari Dr Peset; Spain), Montserrat C Coro (complejo asistencial de Ávila; Spain), Natalia de la Torre-Rubio (Hospital Universitario Puerta de Hierro Majadahonda; Spain), Jose C Esteban (Hospital Universitario Puerta de Hierro; Spain), Maria del Carmen T Martin (HOSPITAL NUESTRA SEÑORA SONSOLES; Spain), Jose G Puerta (Hospital Clinic; Spain), Johan Back (Uppsala University Hospital, Uppsala; Sweden), Maryam Dastmalchi (Karolinska University Hospital, Stockholm; Sweden), Brigitte Dupré (Academic Specialist Center, Stockholm; Sweden), Emma Grenholm (Falu Lasarett, Region Dalarna, Falun; Sweden), Aase Hensvold (Academic Specialist Center, Stockholm; Sweden), Ann Knight (Uppsala University Hospital, Uppsala; Sweden), Servet Akar (Izmir Katip Celebi University Atatürk Training and Research Hospital; Turkey), Ozan C Icacan (Bakırköy Dr. Sadi Konuk Research and Training Hospital; Turkey), Laura Chadwick (St Helens & Knowsley NHS Trust; UK), Kirsty Devine (York Hospital; UK), Sasha Dunt (Countess of Chester NHS Foundation Trust; UK), Lucia Fusi (King's College Hospital; UK), Caroline M Jones (Llandudno Hospital; UK), Elizabeth Macphie (Lancashire and South Cumbria NHS Foundation Trust; UK), Elena Nikiphorou (King's College Hospital; UK), Diana O'Kane (Royal National Hospital For Rheumatic Diseases at Royal United Hospital; UK), Sheila O'Reilly (Royal Derby Hospital; UK), Samir Patel (Queen Elizabeth hospital; UK), Rosaria Salerno (King's College Hospital; UK), Lucy Thornton (Bradford Royal Infirmary; UK), Jenny Tyler (Royal United Hospital, Bath; UK), Claire Vandevelde (Leeds Teaching Hospitals Trust; UK), Elizabeth Warner (Lister Hospital; UK), Su-Ann Yeoh (University College London Hospitals NHS Foundation Trust; UK), Sara Baig (Arthritis and Rheumatology Consultants, PA; USA), Hammad Bajwa (Arthritis and Rheumatology Consultants, PA; USA), Byung Ban (Medstar Georgetown University Hospital; USA), Vernon Berglund (Arthritis and Rheumatology Consultants, PA; USA), Cassandra Calabrese (Cleveland Clinic; USA), Kristin D'Silva (Brigham and Women's Hospital; USA), Angela Dahle (Arthritis and Rheumatology Consultants, PA; USA), Kathryn Dao (UT Southwestern Medical Center; USA), Nicole Daver (Institute of Rheumatic and Autoimmune Diseases; USA), William Davis (Ochsner Medical Center Rheumatology Department; USA), Walter Dorman (Arthritis and Rheumatology Consultants, PA; USA), Ezzati Fatemeh (UT Southwestern Medical Center; USA), Theodore Fields (Hospital for Special Surgery; USA), Jody Hargrove (Arthritis and Rheumatology Consultants, PA; USA), Melissa Harvey (Institute of Rheumatic and Autoimmune Diseases; USA), Maren Hilton (Arthritis and Rheumatology Consultants, PA; USA), Tiffany Hsu (Brigham and Women's Hospital; USA), Zara Izadi (University of California, San Francisco, CA; USA), Arundathi Jayatilleke (Temple University Hospital; USA), David Karp (UT Southwestern Medical Center; USA), Gilbert Kepecs (Private Practice; USA), Neil Kramer (Institute of Rheumatic and Autoimmune Diseases; USA), Concetta Lamore (Institute of Rheumatic and Autoimmune Diseases; USA), Nicholas Lebedoff (Arthritis and Rheumatology Consultants, PA; USA), Susan Leonard (Arthritis and Rheumatology Consultants, PA;USA), Sushama Mody (Riverside Medical Group; USA), Jennifer Morgan (Arthritis and Rheumatology Consultants, PA; USA), Emily Pfeifer (Arthritis and Rheumatology Consultants, PA; USA), Guillermo Quiceno (UT Southwestern Medical Center; USA), Robert Quinet (Ochsner Medical Center Rheumatology Department; USA), Elliot Rosenstein (Institute of Rheumatic and Autoimmune Diseases; USA), Eric Ruderman (Northwestern Memorial; USA), Evangeline Scopelitis (Ochsner Medical Center Rheumatology Department; USA), Naomi Serling-Boyd (Brigham and Women's Hospital; USA), Faizah Siddique (Loyola University Medical Center; USA), Archibald Skemp (Arthritis and Rheumatology Consultants, PA; USA), Derrick Todd (Brigham and Women's Hospital; USA), Karen T Toribio (Ochsner Medical Center Rheumatology Department; USA), Rachel Wallwork (Brigham and Women's Hospital; USA), Tameka Webb-Detiege (Ochsner Medical Center Rheumatology Department; USA), Douglas White (Gundersen Health System; USA), Jeffrey Wilson (Arthritis and Rheumatology Consultants, PA; USA), Melanie Winter (Gundersen Health System; USA), Leanna Wise (Los Angeles County + USC Medical Center; USA), Anne Wolff (Arthritis and Rheumatology Consultants, PA; USA), Kristen Young (UT Southwestern Medical Center; USA), Jerald Zakem (Ochsner Medical Center Rheumatology Department; USA), JoAnn Zell (University of Colorado; USA), Kurt Zimmerman (Arthritis and Rheumatology Consultants, PA; USA).
Contributors: AS, MS and PMM had access to the study data, developed the figures and tables, and vouch for the data and analyses. MS performed the statistical analyses and contributed to data quality control, data analysis and interpretation of the data. AS, MG, SL-T, JL, LL, CR, MJS, GS, CAS, SA-A, JB-C, LC, RC, LG, EH, RH, KLH, ZI, PK and LK-F, contributed to data collection, data quality control, data analysis and interpretation of the data. EFM, JS, ES, PS, LT, ZSW, SB, WC, RG, JH and LJ contributed to data collection, data analysis and interpretation of the data. PCR, JY and PMM, directed the work, designed the data collection methods, contributed to data collection, data analysis and interpretation of the data, and had final responsibility for the decision to submit for publication. All authors contributed intellectual content during the drafting and revision of the work and approved the final version to be published.
Funding: Financial support from the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR).
Disclaimer: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology (ACR), the European League Against Rheumatism (EULAR), the (UK) National Health Service (NHS), the National Institute for Health Research (NIHR), or the (UK) Department of Health, or any other organisation.
Competing interests: AS reports personal fees from lectures for AbbVie, MSD, Roche, BMS and Pfizer, all outside the submitted work. MG reports grants from National Institutes of Health, NIAMS, outside the submitted work. JL reports a research grant from Pfizer, outside of the submitted work. EFM reports that LPCDR received support for specific activities: grants from Abbvie, Novartis, Janssen-Cilag, Lilly Portugal, Sanofi, Grünenthal S.A., MSD, Celgene, Medac, Pharmakern and GAfPA; grants and non-financial support from Pfizer; non-financial support from Grünenthal GmbH, outside the submitted work. CR has received consulting/speaker’s fees from Abbvie, Amgen, AstraZeneca, BMS, Biogen, Eli Lilly, Glenmark, GSK, MSD, Mylan and Pfizer, and grants from Biogen, Lilly and Nordic Pharma, all unrelated to this manuscript. MJS is supported by unrestricted grants from AbbVie, Biogen, Gilead, Lilly, MSD, Novartis and Pfizer. Her work is supported by grants from the National Institutes of Health and Agency for Healthcare Research and Quality. She leads the Data Analytic Center for the American College of Rheumatology, which is unrelated to this work. ES reports non-financial support from Canadian Arthritis Patient Alliance, outside the submitted work. JS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253 and P30 AR072577), the Rheumatology Research Foundation (R Bridge Award), the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. He has received research support from Amgen and Bristol-Myers Squibb and performed consultancy for Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum and Pfizer unrelated to this work. PS reports personal fees from American College of Rheumatology/Wiley Publishing, outside the submitted work. TT reports personal fees for lectures and expertises from Amgen, Arrow, Biogen, BMS, Chugai, Expanscience, Gilead, Grunenthal, LCA, Lilly, Medac, MSD, Nordic, Novartis, Pfizer, Sandoz, Sanofi, Theramex, Thuasne, TEVA and UCB, and reports financial support or fees for research activities from Amgen, Bone Therapeutics, Chugai, MSD, Novartis, Pfizer and UCB, all unrelated to this manuscript. ZSW reports grant support from Bristol-Myers Squibb and consulting fees from Viela Bio. JB-C has received consulting/speaker’s fees from Abbvie, MSD, BMS and Roche, and grants from Pfizer, all unrelated to this manuscript. He reports non-branded marketing campaigns for Novartis. PC has received consulting and lecturing fees from Abbvie, AstraZeneca, Bristol-Myers Squibb, Gilead, Glaxo Smith Kline, Innotech, Janssen, Merck Sharp Dohme, Roche, Servier and Vifor. LC has not received fees or personal grants from any laboratory, but her institute works by contract for laboratories among other institutions, such as Abbvie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España, S.A., Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal GmbH and UCB Pharma. LG reports personal consultant fees from AbbVie, Amgen, BMS, Biogen, Celgene, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis and UCB, and grants from Amgen, Lilly, Janssen, Pfizer, Sandoz, Sanofi and Galapagos, all unrelated to this manuscript. RG reports non-financial support from Pfizer Australia, personal fees from Pfizer Australia, personal fees from Cornerstones, personal fees from Janssen New Zealand, non-financial support from Janssen Australia, personal fees from Novartis, outside the submitted work. EH reports personal consultant fees from Actelion, Sanofi-Genzyme and GSK, and grants from GSK, all unrelated to this manuscript. RH reports research grant from Pfizer and personal fees from AbbVie, Pfizer, Novartis, Amgen, Mylan, Gilead, Medac and Takeda, all outside the submitted work. JH reports grants from Rheumatology Research Foundation, grants from Childhood Arthritis and Rheumatology Research Alliance (CARRA), personal fees from Novartis, outside the submitted work. KLH reports she has received non-personal speaker’s fees from Abbvie and grant income from BMS, UCB and Pfizer, all unrelated to this manuscript. KLH is supported by the NIHR Manchester Biomedical Research Centre. PCR reports personal fees from Abbvie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche and UCB, non-financial support from BMS, research funding from Janssen, Novartis, Pfizer and UCB, all outside the submitted work. JY reports consulting fees from AstraZeneca and Eli Lilly, and grants from Pfizer, outside the submitted work. PMM has received consulting/speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript, and is supported by the National Institute for Health Research (NIHR), University College London Hospitals (UCLH) and Biomedical Research Centre (BRC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
COVID-19 Global Rheumatology Alliance:
Brahim Dahou, Marcelo Pinheiro, Francinne M Ribeiro, Anne-Marie Chassin-Trubert, Sebastián Ibáñez, Lingli Dong, Lui Cajas, Hesham Hamoud, Jérôme Avouac, Véronique Belin, Raphaël Borie, Pascal Chazerain, Xavier Chevalier, Pascal Claudepierre, Gaëlle Clavel, Marie-Eve Colette-Cedoz, Bernard Combe, Elodie Constant, Nathalie Costedoat-Chalumeau, Marie Desmurs, Valérie Devauchelle-Pensec, Mathilde Devaux, Robin Dhote, Yannick Dieudonné, Fanny Domont, Pierre-Marie Duret, Mikaël Ebbo, Esther Ebstein, Soumaya El Mahou, Bruno Fautrel, Renaud Felten, René-Marc Flipo, Violaine Foltz, Antoine Froissart, Joris Galland, Véronique Gaud-Listrat, Sophie Georgin-Lavialle, Aude Giraud-Morelet, Jeanine S Giraudet-Le Quitrec, Philippe Goupille, Sophie Govindaraju-Audouard, Franck Grados, Séverine Guillaume-Czitrom, Marion Hermet, Ambre Hittinger-Roux, Christophe Hudry, Isabelle Kone-Paut, Sylvain La Batide Alanore, Pierre Lafforgue, Sophie Lahalle, Isabelle Lambrecht, Vincent Langlois, Jean-Paul Larbre, Emmanuel Ledoult, Christophe Leroux, Frédéric Liote, Alexandre TJ Maria, Hubert Marotte, Arsène Mekinian, Isabelle Melki, Laurent Messer, Catherine Michel, Gauthier Morel, Jacques Morel, Marie-Noelle Paris-Havard, Edouard Pertuiset, Thao Pham, Myriam Renard, Sabine Revuz, Sébastien Rivière, Clémentine Rousselin, Christian Roux, Diane Rouzaud, Jérémie Sellam, Raphaele Seror, Amelie Servettaz, Vincent Sobanski, Christelle Sordet, Lionnel Spielmann, Nathalie Tieulié, Alice Tison, Sophie Trijau, Alexandre Virone, Ursula Warzocha, Daniel Wendling, Frederik N Albach, Peer Aries, Elvira Decker, Urs Hartmann, Joerg Henes, Bimba F Hoyer, Andreas Krause, Klaus Krüger, Hanns-Martin Lorenz, Ulf Müller-Ladner, Alexander Pfeil, Anne Regierer, Jutta G Richter, Markus Rihl, Tim Schmeiser, Hendrik Schulze-Koops, Christof Specker, Reinhard E Voll, Stephanie Werner, Gabriela MG Melgar, Mahdi Vojdanian, Laura Andreoli, Elena Bartoloni-Bocci, Maurizio Benucci, Francesco Campanaro, Marta Caprioli, Davide Carboni, Greta Carrara, Edoardo Cipolletta, Chiara Crotti, Gloria Dallagiacoma, Paola Faggioli, Rosario Foti, Franco Franceschini, Micaela Fredi, Giacomo Guidelli, Florenzo Iannone, Gianpiero Landolfi, Caludia Lomater, Ceciclia Nalli, Simone Parisi, Luca Quartuccio, Bernd Raffeiner, Rossella Reggia, Marta Riva, Nicoletta Romeo, Cinzia Rotondo, Ettore Silvagni, Luigi Sinigaglia, Ilaria Tinazzi, Anna Zanetti, Giovanni Zanframundo, Fatemah Abutiban, Deshiré Alpízar-Rodríguez, Marina Rull-Gabayet, Fedra Irazoque, Xochitl Jimenez, Eduardo Martín-Nares, Angel Castillo-Ortiz, Tatiana S Rodriguez-Reyna, Diana C Rosete, Erick A Zamora-Tehozol, David Vega-Morales, Beatriz Elena Zaueta-Montiel, Nasra Al-Adhoubi, Babur Salim, Enrique Giraldo, Ariel Salinas, Manuel Ugarte-Gil, Diogo Almeida, Miguel Bernardes, Rita C Machado, Maria Rato, Samar Al-Emadi, Richard Conway, Rachael Flood, Juan J Alegre-Sancho, Montserrat C Coro, Natalia de la Torre-Rubio, Jose C Esteban, Maria del Martin, Jose G Puerta, Johan Back, Maryam Dastmalchi, Brigitte Dupré, Emma Grenholm, Aase Hensvold, Ann Knight, Servet Akar, Ozan C Icacan, Laura Chadwick, Kirsty Devine, Sasha Dunt, Lucia Fusi, Caroline M Jones, Elizabeth Macphie, Elena Nikiphorou, Diana O'Kane, Sheila O'Reilly, Samir Patel, Rosaria Salerno, Lucy Thornton, Jenny Tyler, Claire Vandevelde, Elizabeth Warner, Su-Ann Yeoh, Sara Baig, Hammad Bajwa, Byung Ban, Vernon Berglund, Cassandra Calabrese, Kristin D'Silva, Angela Dahle, Kathryn Dao, Nicole Daver, William Davis, Walter Dorman, Ezzati Fatemeh, Theodore Fields, Jody Hargrove, Melissa Harvey, Maren Hilton, Tiffany Hsu, Zara Izadi, Arundathi Jayatilleke, David Karp, Gilbert Kepecs, Neil Kramer, Concetta Lamore, Nicholas Lebedoff, Susan Leonard, Sushama Mody, Jennifer Morgan, Emily Pfeifer, Guillermo Quiceno, Robert Quinet, Elliot Rosenstein, Eric Ruderman, Evangeline Scopelitis, Naomi Serling-Boyd, Faizah Siddique, Archibald Skemp, Jeffrey Sparks, Derrick Todd, Karen T Toribio, Rachel Wallwork, Tameka Webb-Detiege, Douglas White, Jeffrey Wilson, Melanie Winter, Leanna Wise, Anne Wolff, Kristen Young, Jerald Zakem, JoAnn Zell, and Kurt Zimmerman
Data availability statement
Data are available upon reasonable request. Applications to access the data should be made to the C19-GRA Steering Committee.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The C19-GRA physician-reported registry was determined “not human subjects’ research” by the UK Health Research Authority and the University of Manchester, as well as under United States Federal Guidelines assessed by the University of California San Francisco Institutional Review Board.
References
- 1. Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis 2020;79:851–8. 10.1136/annrheumdis-2020-217877 [DOI] [PubMed] [Google Scholar]
- 2. Mikuls TR, Johnson SR, Fraenkel L, et al. American College of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 2. Arthritis Rheumatol 2020;72:e1–12. 10.1002/art.41437 [DOI] [PubMed] [Google Scholar]
- 3. Mikuls TR, Johnson SR, Fraenkel L, et al. American College of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol 2020;72:1241–51. 10.1002/art.41301 [DOI] [PubMed] [Google Scholar]
- 4. Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei Province, China: a multicentre retrospective observational study. Lancet Rheumatol 2020;2:e557–64. 10.1016/S2665-9913(20)30227-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Favalli EG, Monti S, Ingegnoli F, et al. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol 2020;72:1600–6. 10.1002/art.41388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2020;50:564–70. 10.1016/j.semarthrit.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 2020;79:1170–3. 10.1136/annrheumdis-2020-217763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021;80:384–91. 10.1136/annrheumdis-2020-218946 [DOI] [PubMed] [Google Scholar]
- 10. Putman M, Chock YPE, Tam H. Antirheumatic disease therapies for the treatment of COVID-19: a systematic review and meta-analysis. Arthritis Rheumatol 2020. 10.1002/art.41469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet Rheumatol 2020;2:e250–3. 10.1016/S2665-9913(20)30095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liew JW, Bhana S, Costello W. The COVID-19 global rheumatology alliance: evaluating the rapid design and implementation of an international registry against best practice. Rheumatology 2021;60:353–8. 10.1093/rheumatology/keaa483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isaacs JD, Burmester GR. Smart battles: immunosuppression versus immunomodulation in the inflammatory RMDs. Ann Rheum Dis 2020;79:991–3. 10.1136/annrheumdis-2020-218019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bedoui Y, Guillot X, Sélambarom J, et al. Methotrexate an old drug with new tricks. Int J Mol Sci 2019;20. 10.3390/ijms20205023. [Epub ahead of print: 10 Oct 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 18. Morel JG. Logistic regression under complex survey designs. Surv Methodol 1989;15:203–23. [Google Scholar]
- 19. Mehta P, Porter JC, Chambers RC, et al. B-Cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol 2020;2:e589–90. 10.1016/S2665-9913(20)30270-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell LA, Richie CT, Zhang Y, et al. In vitro modeling of HIV proviral activity in microglia. Febs J 2017;284:4096–114. 10.1111/febs.14293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feria-Garzón MG, Rugeles MT, Hernandez JC, et al. Sulfasalazine as an immunomodulator of the inflammatory process during HIV-1 infection. Int J Mol Sci 2019;20. 10.3390/ijms20184476. [Epub ahead of print: 11 Sep 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hui DS, Lee N, Chan PK, et al. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res 2018;150:202–16. 10.1016/j.antiviral.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pandrea I, Xu C, Stock JL, et al. Antibiotic and antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-Infected Pigtailed macaques. PLoS Pathog 2016;12:e1005384. 10.1371/journal.ppat.1005384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furlow B. COVACTA trial raises questions about tocilizumab's benefit in COVID-19. Lancet Rheumatol 2020;2:e592. 10.1016/S2665-9913(20)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winthrop KL, Mariette X. To immunosuppress: whom, when and how? that is the question with COVID-19. Ann Rheum Dis 2020;79:1129–31. 10.1136/annrheumdis-2020-218694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galloway JB, Hyrich KL, Mercer LK, et al. Anti-Tnf therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for rheumatology biologics register with special emphasis on risks in the elderly. Rheumatology 2011;50:124–31. 10.1093/rheumatology/keq242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richter A, Listing J, Schneider M, et al. Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1667–73. 10.1136/annrheumdis-2015-207838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 30. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and COX regression. Am J Epidemiol 2007;165:710–8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219498supp001.pdf (659.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Applications to access the data should be made to the C19-GRA Steering Committee.