Abstract
Background
Patients diagnosed with the novel coronavirus disease (COVID‐19) who develop severe symptoms need to be determined in advance so that appropriate treatment strategies are in place.
Methods
To determine the clinic features of patients diagnosed definitely with COVID‐19 and evaluate risk factors for severe outcome, the medical records of hospitalized patients were reviewed retrospectively by us and data were compiled. Laboratory data from 90 cases were analyzed, and COVID‐19 patients were classified into two groups (severe and non‐severe) based on the severity.
Results
Severe COVID‐19 cases on admission had higher leukocyte and neutrophil counts, neutrophil‐to‐lymphocyte ratio (NLR), D‐dimer, fibrinogen, C‐reactive protein levels, and lower lymphocyte counts compared with those of non‐severe cases (p < 0.05). The area under the curve (AUC) for leukocyte counts, neutrophil counts, and levels of C‐reactive protein was 0.778, 0.831, and 0.800, respectively. The thresholds were 7.70 × 109/L for leukocyte counts, 5.93 × 10⁹/L for neutrophil counts, and 75.07 mg/L for C‐reactive protein, respectively. Logistic regression analyses indicated that higher white blood cell (WBC) counts (OR, 1.34; 95% CI, 1.05–1.71), neutrophil counts (OR, 1.35; 95% CI, 1.06–1.73), and C‐reactive protein levels (OR, 1.02; 95% CI, 1.0–1.04) were several predictive factors for severe outcome. Severe COVID‐19 patients had a reduction in WBC counts, D‐dimer, C‐reactive protein, and fibrinogen upon discharge from hospital, while lymphocyte counts increased (p < 0.05).
Conclusion
Counts of WBC, neutrophil, and lymphocyte, NLR, and levels of C‐reactive protein, D‐dimer, and fibrinogen are helpful for prediction of the deterioration trend in patients diagnosed with COVID‐19.
Keywords: Coronavirus disease 2019, prediction, review, risk factors, severity
The receiver operating characteristic (ROC) curve of white blood cell counts, neutrophil counts and C‐reactive protein. Receiver operating characteristic curve analysis suggested that WBC counts, neutrophils counts and C‐reactive protein could be used to assist the prediction of COVID‐19 severity on admission.

1. INTRODUCTION
The novel coronavirus disease 2019 (COVID‐19) since its outbreak in December 2019 has caused a pandemic worldwide. More than 19949122 cases were infected with COVID‐19 as of August 10, 2020, around more than 200 countries.
The rapid spread of COVID‐19 has significantly impacted human health. 1 , 2 , 3 , 4 To date, reports from the World Health Organization (WHO) have shown that COVID‐19 is still spreading rapidly resulting in severely burdening the healthcare systems of several countries. Countries around the world have paid a heavy price for prevention of the COVID‐19 spread and treatment of infected patients. 5 No specific drug has been developed for COVID‐19 at present. On August 4, Sarah Gilbert, professor of vaccines at Oxford University, said there were still many uncertainties regarding the successful development of a COVID‐19 vaccine and how it could be used on a large scale. Fever, fatigue, cough, and headaches are the common symptoms in COVID‐19 patients, and dyspnea in some severe cases. 6 , 7 , 8 , 9 There are no specific symptoms during the early stages; hence, it is hard to predict whether individuals will develop severe symptoms. Patients who develop severe symptoms need to be determined in advance so that appropriate treatment strategies are in place. Identifying laboratory predictors of severe and fatal type progression is essential. 10 These predictors will be used for risk stratification and treatment guidance of at‐risk patients. This is essential for the allocation of limited medical resources to those of greatest need. 10 Ruchong Chen et.al. have focused on the mortality‐associated risk factors in COVID‐19 patients. 11 Previous studies that have identified severe type predictors of COVID‐19 based on laboratory data during the initial stages of the disease derived from univariate analysis. This study compared predictors between COVID‐19 patients with severe and non‐severe symptoms on admission and those with severe COVID‐19 before and after treatment. Several predictive factors for severe outcomes were evaluated by logistic regression analyses. The optimal threshold for these predictors was identified by ROC curves. In the present study, we aimed to determine the clinic features of definitely diagnosed COVID‐19 patients and evaluate risk factors for severe outcomes.
2. MATERIALS AND METHODS
2.1. Study design and participants
The medical records were retrospectively reviewed, and hospitalization patient data were compiled from two clinical centers, the Wuhan Red Cross Hospital and the Second People's Hospital of Yibin, West China Yibin Hospital, Sichuan University. All enrolled COVID‐19 inpatients in this study were confirmed with a positive result tested by high‐throughput sequencing or real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay of nasal and pharyngeal swab samples, anal swab samples, sputum samples, and stool samples. As of March 17, 2020, we collected 411 cases diagnosed definitely with COVID‐19 in our study. Ninety cases were included in this study, while the remaining cases were excluded due to incomplete medical records. COVID‐19 was diagnosed based on the 6th edition of guidelines released by the National Health Commission of China (in Chinese). COVID‐19 patients were classified into two groups (severe and non‐severe) based on the severity. Severe COVID 19 was diagnosed with at least one of the following conditions: (a) arterial oxygen saturation ≤93% at rest, (b) shortness of breath with respiratory rate (RR) ≥30 times/min, or (c) PaO2/FiO2 ≤300 mmHg. Non‐severe patients presented fever, symptoms of respiratory tract, and others with imaging sign of pneumonia. 12
The approval to conduct the study was voted by the Ethics Committee of Wuhan Red Cross Hospital and the Ethics Committee of the Second People's Hospital of Yibin, West China Yibin Hospital, Sichuan University. Considering the urgency to perform this study, waiver of written informed consent was conducted.
2.2. Data collection and definition
Clinical, epidemiological, and demographic information and laboratory test results including biochemical indices, blood routine results, and coagulation test were reviewed by accessing the medical records of COVID 19 inpatients on admission. Final laboratory test results before discharge or death were also collected. Clinical outcomes were followed until discharge or death.
2.3. Statistical analysis
Normally or non‐normally distributed continuous data were presented as mean ± SD and median (interquartile range), respectively. Numbers (percentages) were used for categorical variable presentation. Comparison between groups of non‐normally or normally distributed data was conducted by the Mann–Whitney U test or the Student t test. A paired t test or Wilcoxon's test was conducted for comparison of laboratory data on admission and before discharge or death based on whether the data were normally distributed. GraphPad Prism software version 8 was used to generate forest maps representing logistic regression analysis. Categorical data were analyzed by a chi‐square test. The dependent variable of the disease severity and the association between abnormal laboratory findings were analyzed by logistic regression analyses. All statistical analyses were carried out with SPSS statistical software, version 26.0 (IBM Corp.). p < 0.05 was considered of statistical significance.
3. RESULTS
3.1. Clinical characteristics
Ninety COVID‐19 patients were included in the present study for analyses. COVID‐19 patients were classified into the severe group (35 cases) and the non‐severe group (55 cases) to evaluate the characteristics of severe conditions. The average age of patients including 22 males and 13 females in the severe group was 66.00 ± 14.32 years, while the average age of patients in the non‐severe group (26 males and 29 females) was 49.13 ± 16.85 years. The mean age was significantly lower in non‐severe patients than severe patients (p < 0.001) (Table 1). Severe patients had more chance to present with fever, fatigue, asthma, dyspnea, chest distress, and loss of appetite during the early stages (p < 0.05).
TABLE 1.
Clinical characteristics of the patients with severe or non‐severe conditions
| Severe group, n = 35 | Non‐severe group, n = 55 | p‐Value | |
|---|---|---|---|
| Age (years) | 66.00 ± 14.32 | 49.13 ± 16.85 | <0.001 |
| Gender | |||
| Female n (%) | 13 (37.14%) | 29 (52.73%) | 0.149 |
| Male, n (%) | 22 (62.86%) | 26 (47.27%) | |
| Symptoms | |||
| Fever, n (%) | 31 (88.57%) | 37 (67.27%) | 0.022 |
| Headache, n (%) | 3 (8.57%) | 1 (1.82%) | 0.295 |
| Fatigue, n (%) | 23 (65.71%) | 17 (30.91%) | 0.001 |
| Cough, n (%) | 25 (71.43%) | 33 (60.00%) | 0.270 |
| Expectoration, n (%) | 12 (34.29%) | 12 (21.82%) | 0.192 |
| Asthma, n (%) | 18 (51.43%) | 13 (23.64%) | 0.007 |
| Nasal obstruction, n (%) | 1 (2.86%) | 2 (3.64%) | 1.000 |
| Runny nose, n (%) | 1 (2.86%) | 1 (1.82%) | 1.000 |
| Pharyngalgia, n (%) | 1 (2.86%) | 4 (7.27%) | 0.645 |
| Dyspnea, n (%) | 16 (45.71%) | 3 (5.45%) | <0.001 |
| Chest distress, n (%) | 22 (62.86%) | 17 (30.91%) | 0.003 |
| Dizziness, n (%) | 1 (2.86%) | 2 (3.64%) | 1.000 |
| Diarrhea, n (%) | 7 (20.00%) | 13 (23.64%) | 0.686 |
| Poor appetite, n (%) | 20 (57.14%) | 7 (12.73%) | <0.001 |
3.2. Laboratory findings on admission
Compared with non‐severe patients, the white blood cell counts of severe patients were higher (8.25 vs. 5.29 × 10⁹/L) (p < 0.001). Neutrophil counts were higher in severe patients compared with those of non‐severe patients (7.54 vs. 3.25 × 10⁹/L) (p < 0.001). However, lymphocyte counts of severe patients were lower compared with those of non‐severe patients (0.74 vs. 1.29 × 10⁹/L) (p < 0.001). C‐reactive protein was significantly higher in severe patients than non‐severe patients (86.41 vs. 6.24 mg/L) (p < 0.001). D‐dimer (1.80 vs. 0.63 mg/L, p < 0.001) and fibrinogen (3.89 vs. 2.78 g/L, p < 0.001) in severe patients were higher compared with those in non‐severe patients. Neutrophil‐to‐lymphocyte ratio (NLR) in severe group was significantly higher than that in non‐severe group (9.95 vs. 2.24) (p < 0.001). No difference in red blood cell counts, hemoglobin, platelet counts, partial thromboplastin time, and prothrombin time was found between patients with different severity(p > 0.05) (Table 2).
TABLE 2.
Laboratory findings on patient admission
| Laboratory findings | Severe group, n = 35 | Non‐severe group, n = 55 | p‐Value |
|---|---|---|---|
| WBC (10⁹/L) | 8.25 (1.82–13.96) | 5.29 (2.82–16.83) (n = 54) | <0.001 |
| Neutrophils count (10⁹/L) | 7.54 (1.01–12.84) | 3.25 (1.30–14.85) (n = 54) | <0.001 |
| Lymphocyte count (10⁹/L) | 0.74 (0.26–2.01) | 1.29 (0.31–3.47) (n = 54) | <0.001 |
| RBC (1012/L) | 4.24 (2.57–5.17) | 4.18 (2.79–5.53) | 0.700 |
| Hemoglobin (g/L) | 133.00 (70.00–169.00) | 132.00 (69.00–164.00) | 0.562 |
| CRP (mg/L) | 86.41 (0.87–328.45) (n = 30) | 6.24 (0.00–141.83) (n = 47) | <0.001 |
| Platelet count (10⁹/L) | 181.00 (51.00–469.00) | 229.00 (75.00–446.00) | 0.093 |
| APTT (s) | 27.90 (22.40–56.90) | 28.20 (16.40–60.60) (n = 52) | 0.597 |
| TT (s) | 17.60 (14.60–23.90) (n = 34) | 18.40 (15.30–24.80) (n = 52) | 0.171 |
| PT (s) | 12.80 (10.80–19.70) (n = 34) | 12.95 (10.10–18.60) (n = 52) | 0.584 |
| FIB (g/L) | 3.89 (1.15–6.50) (n = 34) | 2.78 (1.15–5.00) (n = 52) | 0.001 |
| D‐dimer (mg/L) | 1.80 (0.16–140.94) (n = 34) | 0.63 (0.11–21.20) (n = 53) | <0.001 |
| NLR | 9.95 (1.35–36.13) | 2.24 (0.84–15.99) (n = 54) | <0.001 |
Abbreviations: APTT, partial thromboplastin time; CRP, C‐reactive protein; FIB, fibrinogen; NLR, neutrophil‐to‐lymphocyte ratio; PT, prothrombin time; RBC, red blood cell; TT, thrombin time; WBC, white blood cell.
ROC curves with a single predictor showed that WBC and neutrophil counts, and levels of C‐reactive protein in COVID‐19 patients were valuable predictors for severe conditions. The area under the curve (AUC) was 0.778, 0.831, and 0.800, respectively, for these predictors. The thresholds were 7.70 × 10⁹/L for WBC counts, 5.93 × 10⁹/L for neutrophil counts, and 75.07 mg/L for C‐reactive protein (Figure 1).
FIGURE 1.
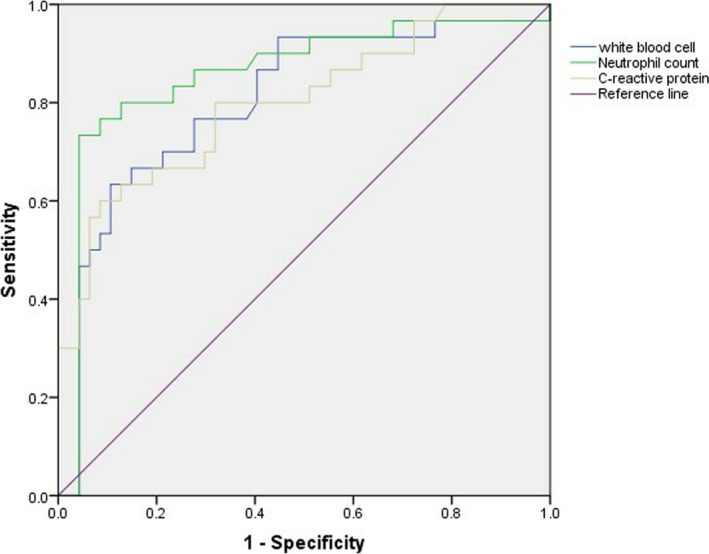
The receiver operating characteristic (ROC) curve of white blood cell counts, neutrophil counts and C‐reactive protein. Receiver operating characteristic curve analysis suggested that WBC counts, neutrophils counts and C‐reactive protein could be used to assist the prediction of COVID‐19 severity on admission
3.3. Risk factors for patients with severe symptoms
Logistic regression analyses identified several predictive factors for severe outcomes. Variables such as higher counts of WBC (OR, 1.34; 95% CI, 1.05–1.71), neutrophil (OR, 1.35; 95% CI, 1.06–1.73), and levels of C‐reactive protein (OR, 1.02; 95% CI, 1.0–1.04) were significantly related to patients with severe COVID‐19 (Figure 2).
FIGURE 2.
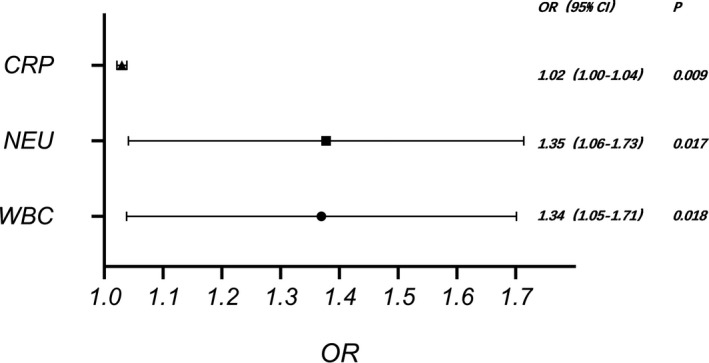
The effects of various potential risk factors on severe COVID‐19 patients on admission. Logistic regression analyses showed several predictive factors for severe outcome, such as higher WBC counts (OR, 1.34; 95% CI, 1.05–1.71), neutrophil counts (OR, 1.35; 95% CI, 1.06–1.73), and C‐reactive protein levels (OR, 1.02; 95% CI, 1.0–1.04)
3.4. Laboratory findings before discharge
Comparing laboratory tests on admission and before discharge, only 19 discharged patients were included in the severe group due to patient death, and 36 cases were included in the non‐severe group due to incomplete data. The white blood cell counts of severe patients before discharge were lower compared with those on admission (5.96 vs. 7.37 × 10⁹/L) (p = 0.040). Lymphocyte counts of severe patients were higher before discharge than on admission (1.38 vs. 0.75 × 10⁹/L) (p = 0.020). Neutrophil counts before discharge were lower than on admission in the severe group (3.94 vs. 5.98 × 10⁹/L) (p = 0.080); however, the difference was not statistically different. C‐reactive protein of severe patients before discharge was significantly lower than that on admission (3.26 vs. 22.38 mg/L) (p < 0.001). However, the differences in thrombin time, fibrinogen, and D‐dimer before discharge and on admission were not statistically significant in severe patients (p > 0.05). The differences in counts of WBC, neutrophil, and lymphocyte, levels of C‐reactive protein, fibrinogen, and D‐dimer, and thrombin time of non‐severe patients before discharge and on admission were not statistically significant (p > 0.05) (Table 3).
TABLE 3.
Laboratory findings before patient discharge
| Laboratory findings | Severe group, n = 19 | p‐Value | Non‐severe group, n = 36 | p‐Value | ||
|---|---|---|---|---|---|---|
| On admission | Before discharge | On admission | Before discharge | |||
| WBC (10⁹/L) | 7.37 (1.82–13.59) | 5.96 (2.67–11.72) | 0.04 | 5.07 (2.82–16.83) | 4.97 (3.14–9.62) | 0.96 |
| Neutrophil count (10⁹/L) | 5.98 (1.01–12.72) | 3.94 (1.05–10.5) | 0.08 | 3.44 (1.30–14.85) | 3.11 (1.57–7.74) | 0.48 |
| Lymphocyte count (10⁹/L) | 0.75 (0.40–2.01) | 1.38 (0.36–2.81) | 0.02 | 1.18 (0.31–2.73) | 1.37 (0.56–3.01) | 0.16 |
| CRP (mg/L) | 22.38 (1.97–328.45) (n = 18) | 3.26 (0.4–97.44) (n = 18) | 0.001 | 9.68 (0.19–141.83) (n = 24) | 4.63 (0.36–85.39) (n = 24) | 0.05 |
| TT (s) | 18.45 (16.50–23.90) (n = 18) | 19.45 (17.10–21.90) (n = 18) | 0.10 | 18.50 (16.40–24.80) (n = 27) | 18.90 (16.40–23.10) (n = 27) | 0.53 |
| FIB (g/L) | 3.91 (1.15–5.21) (n = 18) | 2.88 (1.44–5.95) (n = 18) | 0.25 | 2.84 (1.15–4.54) (n = 27) | 2.79 (1.42–4.97) (n = 27) | 0.84 |
| D‐dimer (mg/L) | 1.80 (0.16–35.20) (n = 18) | 1.63 (0.24–6.41) (n = 18) | 0.13 | 0.97 (0.11–21.20) (n = 28) | 0.57 (0.15–20.85) (n = 27) | 0.72 |
Abbreviations: CRP, C‐reactive protein; FIB, fibrinogen; TT, thrombin time; WBC, white blood cell.
3.5. Laboratory findings before the death of patients in the severe group
Sixteen of the 35 severe patients died shortly after admission. Several of the laboratory indicators were not re‐tested due to patient death. The WBC counts of severe non‐survivors were significantly higher prior to death than that on admission (13.36 ± 5.22 vs. 8.97 ± 2.85 × 10⁹/L) (p = 0.006). In addition, neutrophil counts measured before death was higher than that on admission (11.07 ± 5.49 vs. 7.85 ± 2.95 × 10⁹/L) (p = 0.040). No significant difference in lymphocyte counts was existed between patients who died versus those who survived (p > 0.05) (Table 4).
TABLE 4.
Laboratory findings before death in the severe non‐survivors
| Laboratory findings | Non‐survivors in the severe group (n = 16) | p‐Value | |
|---|---|---|---|
| On admission | Before death | ||
| WBC (10⁹/L) | 8.97 ± 2.85 | 13.36 ± 5.22 | 0.006 |
| Neutrophil count (10⁹/L) | 7.85 ± 2.95 | 11.07 ± 5.49 | 0.04 |
| Lymphocyte count (10⁹/L) | 0.74 ± 0.40 | 0.92 ± 0.53 | 0.30 |
Abbreviation: WBC, white blood cell.
4. DISCUSSION
Since the outbreak of COVID‐19, the healthcare system, especially in countries with severe outbreaks, has been exhausted. 13 It is particularly important to manage patients with different symptoms and to identify patients who may progress to severe symptoms. Our study determined the clinic features of definitely diagnosed COVID‐19 patients and evaluated risk factors for severe outcomes. Severe COVID‐19 symptoms increased with age. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Our study demonstrated that the age was significantly higher in patients with severe symptoms compared to those with non‐severe symptoms. This may be because older patients have lower immunity and are more likely to have underlying diseases. Hence, more intensive surveillance is needed when treating elderly patients. Severe patients had more chances to have a fever, fatigue, asthma, dyspnea, chest distress, and loss of appetite at an early stage. We demonstrated that the counts of white blood cell and neutrophil in severe patients were higher compared to patients with non‐severe symptoms. Previous studies have reached similar conclusions. 22 , 23 , 24 This study also demonstrated that the counts of white blood cell in severe patients with COVID‐19 before discharge were lower compared with those on admission. In addition, an increase in leukocyte and neutrophil counts were severe COVID‐19 risk factors evaluated by logistic regression analyses. The threshold values for leukocyte and neutrophil counts were obtained using ROC curves in this study. However, our study and previous studies have shown that the increase in WBC and neutrophil counts were not very obvious during the early stages of COVID‐19, even sometimes within the normal range. 18 Given this, we do not recommend the use of WBC and neutrophil counts alone as indicators of possible severe deterioration of COVID‐19 patients.
In the present study, we found that lymphocyte counts were significantly lower in severe patients on admission compared with those in non‐severe patients and were higher before discharge than on admission. Our study also showed that lymphocyte counts remained reduced in COVID‐19 patients. Hence, lymphocyte depletion could be a good indicator of disease deterioration. 25
In addition, we demonstrated that C‐reactive protein was significantly higher in severe COVID‐19 patients compared with that in non‐severe patients and was significantly lower when they were discharged from the hospital after treatment than on admission. We found that increase in C‐reactive protein was a risk factor for severe patients with COVID‐19 using logistic regression analyses. The study performed by L.Wang et. al. declared that the levels of C‐reactive protein were positively associated with lung lesions in COVID‐19 patients and could reflect disease severity during the early stages. 26 Elevated C‐reactive protein may be served as a better marker for COVID‐19 progression.
The NLR has been recently identified as an inflammatory biomarker and a reliable predictor of different acute medical conditions, including not only infections but also cerebral hemorrhage and tumor. 27 , 28 , 29 , 30 In this study, the univariate analysis of NLR in severe group and non‐severe group was carried out. It was found that NLR in severe group was higher than that in non‐severe group. NLR was a simple, inexpensive, and easily available composite index.
Several limitations were existed in the current study, which may have resulted in some potential bias. First, the study was retrospective, and only two centers and a small patient cohort were analyzed. Second, there were missing data due to patient deaths and incomplete data for some patients.
In conclusion, counts of WBC, neutrophil, and lymphocyte, neutrophil‐to‐lymphocyte ratio, and levels of D‐dimer, C‐reactive protein, and fibrinogen are helpful to predict the deterioration trend of COVID‐19 patients. Close attention needs to be paid to patients with decreased lymphocyte counts, elevated C‐reactive protein levels, WBC counts, and neutrophil counts during the initial stages of the disease.
CONFLICT OF INTEREST
None to declare.
ACKNOWLEDGMENTS
Shaorui Shi and Bin Nie accessed data of included patients and were responsible for the integrity and accuracy. Shaorui Shi, Bin Nie, and Xinzu Chen were involved in the conception of this study. Shaorui Shi, Qiang Cai, Chunxin Lin, Guangda Zhao, and Xingying Zhang selected the patient cohort. Shaorui Shi and Bin Nie performed data analysis. Shaorui Shi and Bin Nie wrote and revised the article. The final article was approved by all authors for publication.
Contributor Information
Shaorui Shi, Email: 774516315@qq.com.
Bin Nie, Email: 2176863599@qq.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID‐19: why children fare better than adults? Indian J Pediatr. 2020;87:537‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu B. Free DNA, a reason for severe COVID‐19 infection? Med Hypotheses. 2020;142:109812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubey S, Biswas P, Ghosh R, et al. Psychosocial impact of COVID‐19. Diabetes Metab Syndr. 2020;14:779‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID‐19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets. 2020;31:674‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Chen L, Deng Q, et al. The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. J Med Virol. 2020;92:833‐840. [DOI] [PubMed] [Google Scholar]
- 9. Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID‐19. Int J Antimicrob Agents. 2020;55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 11. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2019;2020(158):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gong J, Ou J, Qiu X, et al. A tool to early predict severe Corona Virus Disease 2019 (COVID‐19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92:568‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID‐19 and intensive care unit admission: a systematic review and meta‐analysis. Int J Public Health. 2020;65:533‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID‐19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gemes K, Talback M, Modig K, et al. Burden and prevalence of prognostic factors for severe COVID‐19 in Sweden. Eur J Epidemiol. 2020;35:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID‐19 infection. Ann Acad Med Singap. 2020;49:108‐118. [PubMed] [Google Scholar]
- 20. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Team CC‐R . <Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID‐19)—United States, February 12‐March 16, 2020.pdf>. Morb Mortal Wkly Rep. 2020;69:343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid‐19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velavan TP, Meyer CG. Mild versus severe COVID‐19: laboratory markers. Int J Infect Dis. 2020;95:304‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98‐102. [DOI] [PubMed] [Google Scholar]
- 28. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8(34):57489‐57494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Świtońska M, Piekuś‐Słomka N, Słomka A, Sokal P, Żekanowska E, Lattanzi S. Neutrophil‐to‐lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sci. 2020;10(11):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta‐analysis. Clin Biochem. 2018;52:131‐136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


