Abstract
Background
The neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) have drawn attention in recent years as novel non‐specific inflammatory markers; however, only a few studies have been conducted to investigate their value in RA.
Objective
To investigate the value of the neutrophil‐to‐lymphocyte ratio (NLR) and the platelet‐to‐lymphocyte ratio (PLR) as complementary diagnostic tools in rheumatoid arthritis (RA).
Method
This study included 1009 patients with RA, 170 patients with other rheumatic diseases, and 245 healthy individuals from four medical centers. The patients' general data, including complete blood count, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), and rheumatoid factor (RF), were retrospectively analyzed, and the NLR and PLR were calculated. Potential effective indicators were screened by logistic regression analysis, and a receiver operating characteristic (ROC) curve was plotted to evaluate their diagnostic value for RA.
Results
(a) The NLR and PLR were significantly higher in the RA group than in the non‐RA group and the control group (P < .05). (b) Spearman's Rho showed that the NLR was positively correlated with the PLR (r = .584, P < .05), RF (r = .167, P < .01), and CRP (r = .280, P < .01) but was not significantly correlated with ESR (r = .100, P > .05). The PLR was positively correlated with RF (r = .139, P < .01), CRP (r = .297, P < .01), and ESR (r = .262, P < .05). (c) Logistic analysis showed that RF, CRP, ESR, and the NLR had diagnostic value for RA. (d) For the NLR, the area under the curve (AUC) of the ROC curve was 0.831; at the cutoff value of 2.13, the diagnostic sensitivity, specificity, accuracy, and Youden index were 76.7%, 75.9%, 76.4%, and 0.5424, respectively.
Conclusion
The NLR was less effective than CRP and RF but was superior to ESR in the diagnosis of RA. The NLR can thus be used as a complementary diagnostic indicator in the diagnosis of RA.
Keywords: complementary diagnostic tools, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, rheumatoid arthritis
The ROC curve of the diagnostic value of the NLR for RA. At the cutoff value of 2.13 and an AUC of 0.831, the diagnostic sensitivity, specificity, and accuracy were 76.7%, 75.9%, and 76.4%, respectively, which were lower than the corresponding values for RF and CRP but higher than those for ESR. RF: rheumatoid factor; CRP: C‐reactive protein; ESR: erythrocyte sedimentation rate; NLR: neutrophil‐to‐lymphocyte ratio; Pre‐1: predicted probability‐1.
1. INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease, 1 which mainly affects synovial joints and even causes joint deformity and loss of function, 2 resulting in a severe impact on quality of life. 3 Synovitis is the major RA‐related pathological change. 4 No targeted treatment is available for RA, and the clinical goals involve achieving treatment targets. 5 The severity of inflammatory activity is a key measure of clinical efficacy and treatment endpoints. Although erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), and disease activity score (DAS) were currently used to estimate the RA, several studies have reported the limitations of these markers. 6 , 7
Neutrophils, lymphocytes, and platelets have been reported to play a role in the control of inflammation and are also associated with alterations in secondary to inflammation. 8 The neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) have drawn attention in recent years as novel non‐specific inflammatory markers. Previous studies have shown that the NLR and PLR are closely related to cardiovascular diseases 9 and malignant tumors. 10 , 11 To date, however, only a few studies have been performed to investigate their value in RA, and the sample size of those studies was small. In this study, we retrospectively analyzed the clinical data of RA patients from multiple centers to investigate the value of NLR and PLR as complementary diagnostic tools in the diagnosis of RA.
2. MATERIALS AND METHODS
2.1. Subjects
We selected 1179 rheumatic disease patients (clinic and hospital patients) with complete data who were treated at one of four medical centers between 2015 and 2019, including 432 patients (36.6%) from Wujin Hospital, Jiangsu University, 310 patients (26.3%) from Changzhou Second People's Hospital, Nanjing Medical University, 265 patients (22.5%) from the Third Affiliated Hospital of Soochow University, and 172 patients (14.6%) from Changzhou Traditional Chinese Hospital, Nanjing University of Traditional Chinese Medicine. The patients were divided into an RA group and a non‐RA group. The RA group contained 1009 patients, including 818 women and 191 men, whose ages ranged from 13 to 81 years. The non‐RA group contained 170 patients, including 84 patients with osteoarthritis, 51 patients with systemic connective tissue disease, and 35 patients with ankylosing spondylitis; of these, 138 were women and 32 were men, whose ages ranged from 19 to 85 years. The control group contained 245 healthy individuals, including 197 women and 48 men, whose ages ranged from 20 to 78 years.
Rheumatoid arthritis was diagnosed in accordance with the guidelines developed by the American Rheumatism Association (ARA) and the European League Against Rheumatism (EULAR) in 2010. 12 All patients had clinically active RA. The exclusion criteria were as follows: (a) tendency to develop allergies; (b) pregnant and nursing women; and (c) presence of severe primary or secondary diseases such as malignant tumors.
This study was approved by the Ethics Committee of Wujin Hospital, Jiangsu University (approval number: 2015‐03).
2.2. Methods
All patients were subjected to laboratory tests before treatment. Peripheral blood was used to determine complete blood count, and the numbers of neutrophils (NEUTs), lymphocytes (LYs), and platelets (PLTs) were recorded to calculate the NLR and PLR. The immunity transmission turbidimetric method was performed to analyze rheumatoid factor (RF), immunoscattering turbidimetry was performed to analyze C‐reactive protein (CRP), and the modified Westergren method was used to analyze the erythrocyte sedimentation rate (ESR).
2.3. Statistical analysis
SPSS 17.0 (IBM, Armonk, NY) and MedCalc (MedCalc Software bvba) were used for the statistical analyses. Normally distributed measurement data are expressed as the mean ± standard deviation (X ± S) and were analyzed using one‐way analysis of variance (ANOVA). Non‐normally distributed measurement data are expressed as M (P25‐P75) and were analyzed with the Kruskal‐Wallis H test. Spearman's rho was performed to evaluate whether the NLR and PLR were correlated with RF, CRP, or ESP; r ≥ .6 indicated a strong correlation, .4 ≤ r < .6 indicated a moderate correlation, and r < .4 indicated a weak correlation. Logistic regression analysis was performed to screen diagnostic indicators of RA. Receiver operating characteristic (ROC) curves were used to evaluate diagnostic sensitivity and specificity, and the optimal cutoff value was determined as the value corresponding to the maximum Youden index (sensitivity + specificity − 1). P < .05 was considered statistically significant.
3. RESULTS
3.1. Clinical data and laboratory tests
Table 1 contains the general data and laboratory tests of the included patients. In the RA group, the mean age was 64.52 ± 10.12, and 75.7% of the patients were women, while in the non‐RA group, the mean age was 68.26 ± 8.15, and 70.45% were women; in the control group, the mean age was 61.45 ± 12.50, and 77.5% of the patients were women. No significant among‐group differences were observed in age or sex composition. RF, CRP, NEUTs, NLR, and PLR were significantly higher in the RA group than in the non‐RA group and the control group, and the LY count was significantly lower in the RA group than in the non‐RA group and the control group (P < .05). The ESR was significantly higher in the RA group than in the control group (P < .05), but no significant difference was observed between the RA group and the non‐RA group (P > .05). No significant among‐group difference was observed in the PLT (P > .05).
Table 1.
Clinical data and laboratory tests
| Item | RA group | Other rheumatic diseases group | Control group |
|---|---|---|---|
| Number | 1009 | 170 | 245 |
| Age, years (mean ± SD, range) | 64.52 ± 10.12 | 68.26 ± 8.15 | 61.45 ± 12.50 |
| Gender (F/M) | 191/818 | 32/138 | 48/197 |
| RF (IU/mL) | 129.00 (41.85‐394.00) a , b | 59.52 (21.90‐136.50) b | 8.00 (4.40‐14.30) |
| CRP (mg/L) | 13.55 (10.37‐50.93) a , b | 8.16 (4.15‐29.87) b | 2.30 (1.50‐3.01) |
| ESR (mm/H) | 50.50 (28.25‐79.00) b | 33.5 (17‐61.75) b | 24.00 (17.00‐31.00) |
| NEUT (×109) | 4.65 (3.35‐6.36) a , b | 4.28 (3.11‐5.05) b | 3.50 (2.71‐4.25) |
| Ly (×109) | 1.41 (1.07‐1.91) a , b | 1.64 (1.23‐2.10) b | 2.17 (1.76‐2.70) |
| PLT | 244 (192‐312) | 224 (188‐266) | 255 (215‐296) |
| NLR | 3.23 (2.14‐4.80) a , b | 2.36 (1.78‐3.63) b | 1.54 (1.24‐2.05) |
| PLR | 168.68 (124.45‐239.57) a , b | 130.73 (95.83‐174.15) b | 113.77 (92.97‐144.95) |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; Ly, lymphocyte; NEUT, neutrophil; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet; RF, rheumatoid factor.
P < .05 vs rheumatic diseases group.
P < .05 vs control group.
3.2. Correlation between the NLR and PLR and laboratory indicators in the RA group
Spearman's rho was performed to analyze how the NLR and PLR were correlated with RF, CRP, and ESR in the RA group (Table 2). The results showed that the NLR was positively correlated with the PLR (r = .584, P < .05), RF (r = .167, P < .01), and CRP (r = .280, P < .01) but that it was unrelated to ESR (r = .100, P > .05). The PLR was positively correlated with RF (r = .139, P < .01), CRP (r = .297, P < .01), and ESR (r = .262, P < .05).
Table 2.
Correlation between NLR and PLR and laboratory indicators in the RA group
| ESR | CRP | NLR | PLR | |
|---|---|---|---|---|
| RF | 0.183** | 0.054 | 0.167** | 0.139* |
| ESR | 0.289** | 0.100 | 0.262** | |
| CRP | 0.280** | 0.297** | ||
| NLR | 0.584** |
Abbreviations; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; RF, rheumatoid factor.
P < .05.
P < .01.
3.3. Logistic regression analysis
The significant indicators (RF, CRP, ESR, NLR, PLR) shown in Table 1 were used as independent variables, and the clinical diagnosis was used as the dependent variable for logistic regression analysis. The PLR (P = .807) was excluded due to its P value (P = .807). The remaining independent variables, including RF (X1), CRP (X2), ESR (X3), and NLR (X4), were included in the following formula: LogitP = −6.506 + 0.163 X1 + 0.076 X2 + 0.014 X3 + 0.514X4. The result showed that RF, CRP, ESR, and NLR were diagnostic indicators of RA (Table 3). In addition, logistic regression analysis was used to obtain the combined predicted probability‐1 (Pre‐1) of RF, CRP, ESR, and NLR. Hosmer‐Lemeshow tests indicated that P > .05, which suggests a good fit.
Table 3.
Logistic analysis results
| Independent variable | B | Standard error | Wals | P value | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|
| RF | 0.163 | .023 | 50.644 | <.01 | 1.177 | 1.125 ~ 1.231 |
| ESR | 0.014 | .007 | 4.029 | .045 | 1.014 | 1.000 ~ 1.029 |
| CRP | 0.076 | .015 | 27.272 | <.01 | 1.079 | 1.049 ~ 1.111 |
| NLR | 0.514 | .197 | 6.828 | .009 | 1.672 | 1.137 ~ 2.459 |
| Normal | −6.506 | .766 | 72.113 | <.01 | 0.001 |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NLR, neutrophil‐to‐lymphocyte ratio; RF, rheumatoid factor. Wals is a statistic.
3.4. Evaluation of the diagnostic efficacy of the NLR for RA using an ROC curve
RF, CRP, ESR, NLR, and Pre‐1 served as test variables, while the clinical diagnosis served as the state variable for the ROC analysis. The area under the curve (AUC) was obtained for each test variable, and the diagnostic cutoff value for RA was determined as the value corresponding to the maximum Youden index. The results showed that when RF = 19.0 IU/mL and the AUC = 0.974, the diagnostic sensitivity was 87.5%, the specificity was 91.0%, and the accuracy was 89.0%; when CRP = 3.2 and the AUC = 0.938, the corresponding values were 77.0%, 89.4%, and 82.2%, respectively; when ESR = 34.0 and the AUC = 0.751, the corresponding values were 74.1%, 73.5%, and 73.9%, respectively; when the NLR = 2.13 and the AUC = 0.831, the values were 76.7%, 75.9%, and 76.4%, respectively; and when Pre‐1 = 0.61 and the AUC = 0.988, the values were 93.0%, 95.1%, and 93.9%, respectively (Table 4, Figure 1).
Table 4.
Assessment of the ability of CRP, RF, ESR, NLR, and Pre‐1 in the diagnosis of RA
| Item | AUC | Cutoff | Sensitivity (%) | Specificity (%) | Maximum Youden index | Negative likelihood ratio | Positive likelihood ratio | Diagnosis accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| CRP | 0.938 | 3.20 | 77.0 | 89.4 | 0.8064 | 0.0072 | 5.30 | 82.20 |
| RF | 0.974 | 19.0 | 87.5 | 91.0 | 0.8352 | 0.074 | 9.53 | 89.00 |
| ESR | 0.751 | 34.0 | 74.1 | 73.5 | 0.5222 | 0.37 | 3.98 | 73.90 |
| NLR | 0.831 | 2.13 | 76.7 | 75.9 | 0.5424 | 0.31 | 3.51 | 76.40 |
| Pre‐1 | 0.988 | 0.61 | 93.0 | 95.1 | 0.8958 | 0.078 | 32.35 | 93.90 |
Abbreviations: AUC, area under the curve; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NLR, neutrophil‐to‐lymphocyte ratio; Pre‐1, predicted probability‐1; RF, rheumatoid factor.
Figure 1.
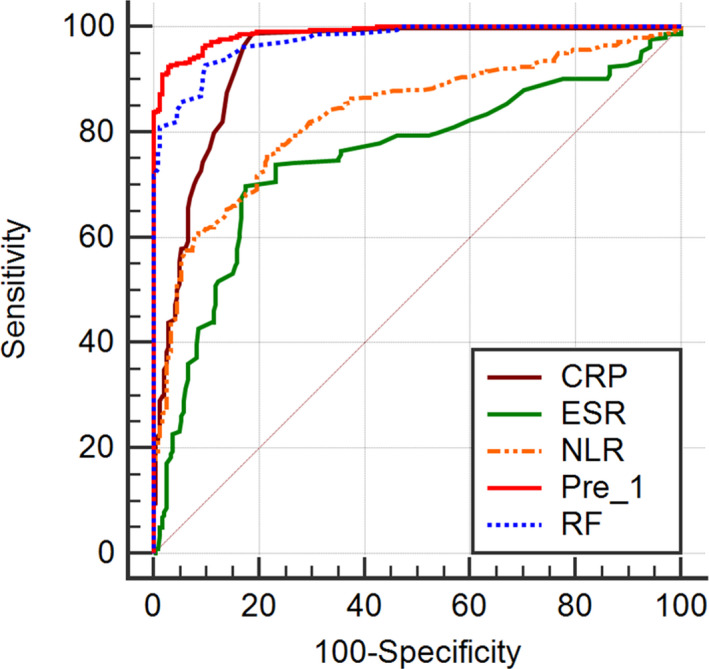
Receiver operating characteristic curve of CRP, RF, ESR, NLR, and pre‐1 diagnosis of RA. CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NLR, neutrophil‐to lymphocyte ratio; Pre‐1, predicted probability‐1; RF, rheumatoid factor. The ROC curve of the diagnostic value of the NLR for RA. At the cutoff value of 2.13 and an AUC of 0.831, the diagnostic sensitivity, specificity, and accuracy were 76.7%, 75.9%, and 76.4%, respectively, which were lower than the corresponding values for RF and CRP but higher than those for ESR
4. DISCUSSION
The inflammatory response promotes pannus formation over the joint, which is the major cause of joint damage. 13 In recent years, researchers have developed a deeper understanding of RA thanks to more in‐depth studies. 14 , 15 However, the evaluation of the severity of inflammatory activity in RA patients is still challenging. 16 Some RA patients do not present typical symptoms, which increases the difficulty in diagnosis. Common RA assessments have some limitations. For example, in patients with low disease activity, ESR, CRP, RF, the disease activity score (DAS), and the clinical disease activity index (CDAI) are at cutoff thresholds and are often overlooked; however, patients still have synovial inflammation and progressive joint damage. Previous study showed that even in clinical remission, bone and joint damage continued to progress in some patients due to persistent synovial inflammation. 17 Therefore, it is critical to accurately assess the severity of inflammation.
The NLR and PLR have continued to draw attention as novel non‐specific inflammatory markers. Uslu et al showed that it was more economical to use NLR (rather than CRP or ESR) as an inflammatory marker of RA. 18 The NLR represents the balance between neutrophils (inflammatory activators) and lymphocytes (inflammatory regulators). A higher NLR is associated with more severe imbalance and inflammation. 19 The PLR reflects the state of coagulation activation and the inflammatory response. 20 This study showed that the NLR and PLR were significantly higher in the RA group than in the non‐RA group and were significantly higher in the non‐RA group than in the control group, which were consistent with the findings of Erre et al 21 A correlation analysis demonstrated that both the NLR and PLR were positively correlated with RF, an important inflammatory marker of RA, which suggests that the NLR and PLR may aid in the initial diagnosis and the differential diagnosis of RA. Moreover, this study showed that the NLR and PLR were weakly correlated with RF and CRP, which was consistent with the results reported by chandrashekara et al 22 NLR is a cheap and readily available marker for the assessment of disease activity in RA. 23 A recent meta‐analysis of 16 studies showed that the NLR and PLR were significantly elevated in RA patients and were positively and weakly correlated with RA activity. 24
To further analyze the diagnostic value of the NLR, PLR, RF, CRP, and ESR in RA, we performed a logistic regression analysis to analyze these independent variables in RA patients and found that the NLR, RF, CRP, and ESR were related to the diagnosis of RA, while the PLR was unrelated to the diagnosis. Again, these results were consistent with those of previous reports. Boulos et al showed that the NLR is an objective inflammatory marker of RA that can be tested in a cost‐effective and reproducible manner and that an NLR > 2.7 is an independent predictor of RA triple therapy failure; however, they also found that the PLR was not an independent predictor of triple therapy failure. 25 The study of Zengin et al 26 found that NLR is a new inflammatory marker to assist the diagnosis of early rheumatoid arthritis (ERA), but PLR is not applicable to the diagnosis of ERA.
Receiver operating characteristic analysis showed that RF was still the best laboratory diagnostic indicator for RA, with an AUC of 0.974, a diagnostic sensitivity of 87.5%, a specificity of 91.0%, and an accuracy of 89.0%. For the NLR, the sensitivity, specificity, and accuracy were 76.7%, 75.9%, and 76.4%, respectively, which are all lower than the corresponding values for RF and CRP but higher than those for ESR. These data indicate that while the NLR is less valuable than CRP and RF for the diagnosis of RA, it is more valuable than ESR, which is an inflammatory marker commonly used to assess RA‐related inflammation activity. The sensitivity, specificity, and accuracy of Pre‐1 for the diagnosis of RA were higher than those of any individual marker, such as NLR, RF, CRP, and ESR, which indicates that although these indicators can be used alone or in combination to aid in RA diagnosis, a combination of these indicators is more effective.
In summary, the NLR has limited value as an independent diagnostic marker for RA. However, the NLR can be obtained via complete blood count, which is convenient, inexpensive, and fast. Therefore, the NLR is especially suitable for primary care centers and can serve as an additional useful marker for the diagnosis of RA. The NLR has important value in the assessment of RA‐related inflammatory activity and may be used as a complementary diagnostic indicator in the diagnosis of RA.
AUTHOR CONTRIBUTIONS
Zihan Jin, Gaojun Cai, and Ping Zhang performed the experiments, analyzed the data, and wrote the article. Xiaohong Li, Shuang Yao, Lin Zhuang, and Min Ren were involved in performing the experiments. Xiaolong Yu and Qiang Wang conceived the study and assumed overall responsibility for this work. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication.
Jin Z, Cai G, Zhang P, et al. The value of the neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: A multicenter retrospective study. J Clin Lab Anal.2021;35:e23569 10.1002/jcla.23569
Zihan Jin, Gaojun Cai, and Ping Zhang contributed equally to this work and should be considered co‐first authors.
Funding information
This study was funded by Young Talent Development Plan of Changzhou Health Commission (CZQM2020120)and Medical Scientific Research Project of Jiangsu Provincial Health Commission in 2019 (H2019080).
REFERENCES
- 1. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338‐2348. [DOI] [PubMed] [Google Scholar]
- 2. Lin YJ, Anzaghe M, Schulke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9(4):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou H, Hu B, Zhaopeng Z, et al. Elevated circulating T cell subsets and cytokines expression in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38(7):1831‐1839. [DOI] [PubMed] [Google Scholar]
- 4. Satis H, Tufan A. Telescoping fingers in rheumatoid arthritis. N Engl J Med. 2019;381(24):e41. [DOI] [PubMed] [Google Scholar]
- 5. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orr CK, Najm A, Young F, et al. The utility and limitations of CRP, ESR and DAS28‐CRP in appraising disease activity in rheumatoid arthritis. Front Med. 2018;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):ITC1‐ITC16. [DOI] [PubMed] [Google Scholar]
- 8. Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pourafkari L, Choi C, Garajehdaghi R, Tajlil A, Dosluoglu HH, Nader ND. Neutrophil‐lymphocyte ratio is a marker of survival and cardiac complications rather than patency following revascularization of lower extremities. Vasc Med. 2018;23(5):437‐444. [DOI] [PubMed] [Google Scholar]
- 10. Lee HN, Kim YK, Kim GT, et al. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio as predictors of 12‐week treatment response and drug persistence of anti‐tumor necrosis factor‐alpha agents in patients with rheumatoid arthritis: a retrospective chart review analysis. Rheumatol Int. 2019;39(5):859‐868. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Zou J, Liu C, et al. Baseline derived neutrophil‐to‐lymphocyte ratio as a prognostic biomarker for non‐colorectal gastrointestinal cancer patients treated with immune checkpoint blockade. Clin Immunol. 2020;212:e108345. [DOI] [PubMed] [Google Scholar]
- 12. Villeneuve E, Nam J, Emery P. 2010 ACR‐EULAR classification criteria for rheumatoid arthritis. Revista Brasileira de Reumatologia. 2010;50(5):481‐483. [PubMed] [Google Scholar]
- 13. Hammer HB, Kvien TK, Terslev L. Ultrasound of the hand is sufficient to detect subclinical inflammation in rheumatoid arthritis remission: a post hoc longitudinal study. Arthritis Res Ther. 2017;19(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328‐2337. [DOI] [PubMed] [Google Scholar]
- 15. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coutant F, Miossec P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr Opin Rheumatol. 2020;32(1):57‐63. [DOI] [PubMed] [Google Scholar]
- 17. Yu X, Li Z, Ren M, Xi J, Wu J, Ji Y. Superb microvascular imaging (SMI) for evaluating hand joint lesions in patients with rheumatoid arthritis in clinical remission. Rheumatol Int. 2018;38(10):1885‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uslu AU, Kucuk A, Sahin A, et al. Two new inflammatory markers associated with Disease Activity Score‐28 in patients with rheumatoid arthritis: neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio. Int J Rheum Dis. 2015;18(7):731‐735. [DOI] [PubMed] [Google Scholar]
- 19. Ku JY, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Prognostic value of neutrophil‐to‐lymphocyte ratio in older patients with head and neck cancer. J Geriatr Oncol. 2020;11(3):417‐422. [DOI] [PubMed] [Google Scholar]
- 20. Peng YF, Cao L, Zeng YH, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in patients with rheumatoid arthritis. Open Med. 2015;10(1):249‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erre GL, Paliogiannis P, Castagna F, et al. Meta‐analysis of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49(1):e13037. [DOI] [PubMed] [Google Scholar]
- 22. Chandrashekara S, Mukhtar Ahmad M, Renuka P, Anupama KR, Renuka K. Characterization of neutrophil‐to‐lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis. 2017;20(10):1457‐1467. [DOI] [PubMed] [Google Scholar]
- 23. Mercan R, Bitik B, Tufan A, et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal. 2016;30(5):597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee YH. Association between the neutrophil‐to‐lymphocyte ratio, and platelet‐to‐lymphocyte ratio and rheumatoid arthritis and their correlations with the disease activity: a meta‐analysis. J Rheum Dis. 2018;25(3):169. [Google Scholar]
- 25. Boulos D, Proudman SM, Metcalf RG, McWilliams L, Hall C, Wicks IP. The neutrophil‐lymphocyte ratio in early rheumatoid arthritis and its ability to predict subsequent failure of triple therapy. Semin Arthritis Rheum. 2019;49(3):373‐376. [DOI] [PubMed] [Google Scholar]
- 26. Zengin O, Onder ME, Kalem A, et al. New inflammatory markers in early rheumatoid arthritis. Z Rheumatol. 2018;77(2):144‐150. [DOI] [PubMed] [Google Scholar]


