Abstract
Background
The emergence and rapid spread of the deadly novel coronavirus disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a swiftly evolving public health crisis worldwide. SARS‐CoV‐2 infection is characterized by the development and progression of inflammatory responses. Hematological parameters, such as white blood cells (WBCs) and their subpopulations, red cell distribution width, platelet count, mean platelet volume, plateletcrit, and derived markers such as neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and lymphocyte‐to‐monocyte ratio, are established biomarkers of inflammatory responses. We aimed to investigate associations between hematological parameters and disease severity in patients with SARS‐CoV‐2 infection.
Methods
We retrospectively analyzed data from 68 patients with confirmed SARS‐CoV‐2 infection. Twenty‐two patients had mild illness, and 46 had moderate or severe illness at the time of admission. Univariate and multivariate regression analyses were used to identify correlates of disease severity. The areas under receiver operating characteristic curves were calculated to estimate and compare the predictive values of different diagnostic markers.
Results
Mean lymphocyte and monocyte counts were lower while WBC counts, neutrophil counts, NLR, and PLR were higher in patients with severe disease compared with those with mild disease (all P < .01). Univariate analysis revealed that older age, high WBC counts, high neutrophil counts, high NLR, high PLR, low monocyte counts, and low lymphocyte counts were independent correlates of severe illness. Multivariate analysis identified high NLR as the only independent correlate of severe illness. Receiver operating characteristic curve analysis showed that NLR had the highest area under curve of all hematological parameters.
Conclusion
Among hematological parameters, the NLR showed superior prediction of disease severity in patients with SARS‐CoV‐2 infection. Thus, the NLR could be a valuable parameter to complement conventional measures for identification of patients at high risk for severe disease.
Keywords: disease severity, hematological parameters, SARS‐CoV‐2
ROC curves showing the relative diagnostic performance of age and hematological parameters for prediction of disease severity in patients with SARS‐CoV‐2 infection. Abbreviations: WBC, white blood cell; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; LMR, lymphocyte‐to‐monocyte ratio; PSI, Pneumonia Severity Index.
1. INTRODUCTION
The emergence and rapid spread of the deadly novel coronavirus disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a swiftly evolving public health crisis worldwide, with over 130,000 confirmed cases. 1 , 2 , 3 In most patients infected with SARS‐CoV‐2, symptoms are mild or moderate and include dyspnea occurring one week after infection. However, some patients experience severe illness, progressing rapidly to acute respiratory failure, acute respiratory distress syndrome (ARDS), metabolic acidosis, coagulopathy, and septic shock. Therefore, early detection and accurate evaluation of the severity and stage of infection of patients with SARS‐CoV‐2 may facilitate timely and appropriate clinical decision‐making.
Hematological parameters, such as white blood cell (WBCs) and their subpopulations, red cell distribution width (RDW), mean platelet volume (MPV), and plateletcrit (PCT), and derived biomarkers such as neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and monocyte‐to‐lymphocyte ratio (MLR), are easily measured at low cost using modern hematological analyzers. These parameters are widely used for risk stratification, diagnosis, and determination of prognosis. Recently, Liu et al identified the NLR as an independent risk factor for severe illness in patients infected with SARS‐CoV‐2. 4 However, no studies to date have evaluated the diagnostic value of other hematological parameters in such patients. Therefore, we aimed to investigate changes in hematological parameters in patients with SARS‐CoV‐2 infection and to evaluate their utility as diagnostic biomarkers of disease severity. To the best of our knowledge, this is the first time that these markers have been investigated simultaneously in a single study.
2. MATERIALS AND METHODS
2.1. Study population
We retrospectively recruited all patients aged at least 18 years with confirmed SARS‐CoV‐2 infection and treated at The First Affiliated Hospital of Zhejiang University in Zhejiang Province between January 19, 2020, and February 10, 2020. Diagnosis of SARS‐CoV‐2 was made according to National Health Committee guidelines and was confirmed by detection of SARS‐CoV‐2 viral RNA by the Chinese Center for Disease Prevention and Control. Exclusion criteria were hematological diseases, other infectious complications, and concurrent radiotherapy, chemotherapy, or immunosuppressive therapy. Disease severity was classified according to the following criteria: (i) mild disease: fever, respiratory symptoms, and imaging findings suggestive of pneumonia; (ii) moderate disease: respiratory distress with a respiratory rate of ≥ 30 breaths/min, mean oxygen saturation of ≤ 93% in a resting state, and/or arterial blood oxygen partial pressure of ≤ 300 mmHg; and (iii) severe disease: respiratory failure requiring mechanical ventilation, shock, and/or combined organ failure requiring admission to the intensive care unit.
This study was performed according to the principles laid out in the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
2.2. Data extraction
We extracted patient demographic information (including age, sex, and comorbidities) and hematological parameters recorded on the first day of admission to our hospital. Hematological parameters including WBCs and their subpopulations, lymphocyte count, neutrophil count, monocyte count, RDW, MPV, platelet count (PLT), hemoglobin levels, and PCT were analyzed using an automated analyzer (Sysmex XN‐9000, Kobe, Japan). The NLR was obtained by dividing the neutrophil count by the lymphocyte count, and LMR was calculated by dividing the number of lymphocytes by the number of monocytes. The PLR was calculated by dividing the platelet count by the number of lymphocytes. Pneumonia severity was assessed using the Pneumonia Severity Index (PSI). 5
2.3. Statistical Analysis
All continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]). Categorical data were expressed as percentages. Differences between patients with mild and severe SARS‐CoV‐2 infection were assessed using the independent sample t test, the Mann‐Whitney U test, or the chi‐squared test, as appropriate. Correlations between variables were examined using Spearman's rank correlation analysis. Univariate and multivariate logistic regression analyses were performed to identify independent correlates of severe illness in SARS‐CoV‐2‐infected patients. After reciprocal transformation of the monocyte count (1/monocyte count), lymphocyte count (1/lymphocyte count), and LMR (1/LMR), a receiver operating characteristic (ROC) curve was generated, and the area under the curve (AUC) was calculated to assess diagnostic value. All statistical analyses were performed using SPSS version 24 (IBM Corp., Armonk, NY, USA) or MedCalc version 15.2.1 (Ostend, Belgium). Values of P < .05 were considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
Of the 73 patients considered for enrollment in the study, five were excluded: Two had undergone radiotherapy or chemotherapy, two had hematological diseases, and one was undergoing immunomodulatory therapy. Finally, 68 patients (40 males and 28 females) with a mean age of 53.6 ± 11.4 years were enrolled. Of the total study population, 33 patients (48.5%) had underlying comorbidities. Twenty‐two patients were diagnosed with mild SARS‐CoV‐2 infection (mild group), and 46 were diagnosed with moderate or severe infection (severe group) at the time of admission. Demographic and hematological parameters in both groups are shown in Table 1. Patients with mild disease were significantly younger than those with severe disease. Patients with severe disease had lower mean lymphocyte and monocyte counts but higher mean WBCs, neutrophil counts, NLR, PLR, and PSI scores than those with mild disease. There were no significant differences in PLT, RDW, MPV, LMR, PCT, hemoglobin level, or gender between the groups. The PSI score was positively correlated with the NLR and PLR, but inversely correlated with lymphocyte and monocyte counts. There was no correlation between PSI score and WBC or neutrophil counts (Figure 1).
Table 1.
Demographic features and hematological parameters of patients with SARS‐CoV‐2 infection at the time of admission due who later developed mild or severe illness
| Characteristics | All patients (n = 68) | Mild disease (n = 22) | Severe disease (n = 46) | P value |
|---|---|---|---|---|
| Demographic features | ||||
| Age (years) | 52.4 ± 13.9 | 44.0 ± 11.3 | 56.4 ± 13.4 | <.001 |
| Gender (M/F) | 40/28 | 11/11 | 29/17 | .448 |
| Comorbidity | ||||
| Hypertension, n (%) | 18 (26.5%) | 3 (15.8%) | 15 (32.6%) | .172 |
| Diabetes, n (%) | 4 (5.9%) | 1 (4.5%) | 3 (6.5%) | .821 |
| Chronic hepatitis, n (%) | 2 (3.0%) | 0 | 2 (4.3%) | – |
| Chronic obstructive, n (%) | 1 (1.5%) | 0 | 1 (2.2%) | – |
| Bronchitis, n (%) | 1 (1.5%) | 0 | 1 (2.2%) | – |
| Renal diseases, n (%) | 4 (5.9%) | 0 | 4 (8.7%) | – |
| Cardiovascular disease, n (%) | 3 (4.4%) | 0 | 3 (6.5%) | – |
| Hematological parameters | ||||
| WBC count (× 10⁹/L) | 5.7 (4.2‐8.9) | 5.1 (3.7‐5.5) | 7.9(4.8‐10.3) | .001 |
| Neutrophil count (× 10⁹/L) | 4.4(2.8‐7.7) | 2.9 (2.5‐3.9) | 6.5 (3.6‐8.9) | <.001 |
| Lymphocyte count (× 10⁹/L) | 0.9 (0.5‐1.2) | 1.3 (0.9‐1.5) | 0.7 (0.5‐0.9) | <.001 |
| Monocyte count (× 10⁹/L) | 0.3 (0.2‐0.5) | 0.5(0.3‐0.6) | 0.3 (0.2‐0.5) | .006 |
| Platelet count (× 10⁹/L) | 201.0 ± 82.3 | 206.6 ± 74.8 | 198.4 ± 86.3 | .074 |
| Hemoglobin (g/L) | 132.6 ± 16.6 | 133.6 ± 12.5 | 131.4 ± 18.1 | .602 |
| RDW (%) | 12.4 (11.9‐13.0) | 12.4 (11.9‐12.8) | 12.4 (12.0‐13.1) | .355 |
| PCT (%) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | .838 |
| NLR | 6.1 (3.3‐12.1) | 2.4 (1.4‐4.0) | 9.0 (6.0‐14.8) | <.001 |
| LMR | 2.7 (2.2‐2.9) | 2.7 (2.0‐3.7) | 2.5 (1.6‐3.3) | .409 |
| PLR | 228.6 (155.9‐337.0) | 152.2 (115.8‐233.9) | 284.0 (195.0‐441.7) | <.001 |
| MPV(fl) | 10.5 (10.1‐11.2) | 10.5 (9.7‐11.4) | 11.6 (10.1‐11.1) | .555 |
| PSI score | 55.5 (42.3‐65.8) | 5.5 (36.0‐53.8) | 61.0 (50.0‐68.0) | <.001 |
Data are expressed as number (%), mean ± SD, or median (IQR).
Abbreviations: F, female; LMR, lymphocyte‐to‐monocyte ratio; M, male; MPV, mean platelet volume; NLR, neutrophil‐to‐lymphocyte ratio; PCT, plateletcrit; PLR, platelet‐to‐lymphocyte ratio; PSI, Pneumonia Severity Index; RDW, red cell distribution width; WBC, white blood cell.
Figure 1.
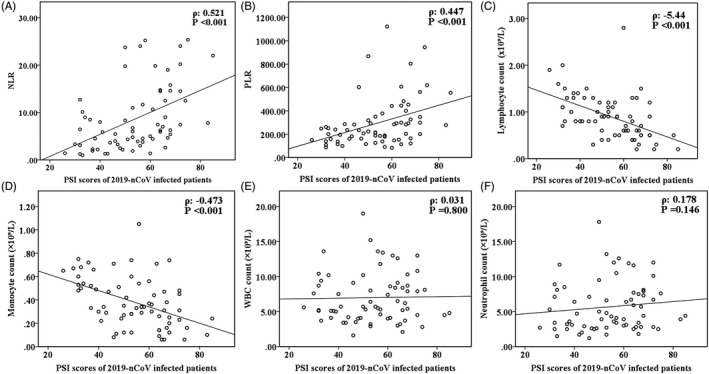
Scatterplots illustrating the correlations between Pneumonia Severity Index score and (A) NLR, (B) PLR, (C) lymphocyte count, (D) monocyte count, (E) white blood cell count, and (F) neutrophil count in patients SARS‐CoV‐2 infection. NLR, neutrophil‐to‐lymphocyte ratio; PSI, Pneumonia Severity Index; PLR, platelet‐to‐lymphocyte ratio; WBC, white blood cell
3.2. Factors associated with severe illness
Potential risk factors for the development of severe disease identified using univariate and multivariate analyses are shown in Table 2. Univariate analysis revealed older age, high WBC count, high neutrophil count, high NLR, high PLR, low monocyte count, and low lymphocyte count as independent risk factors for severe illness. Multivariate analysis identified only high NLR as an independent risk factor for severe illness (Table 2). We used ROC analyses to examine the ability of hematological markers to discriminate between patients with severe and mild illness. Monocyte count, lymphocyte count, and LMR were inversely transformed into 1/monocyte count, 1/lymphocyte count, and 1/LMR. The AUC was higher for NLR than for age, WBC, neutrophil count, lymphocyte count, monocyte count, PLR, PSI score, and LMR. The optimal cutoff value for NLR was calculated as 3.63, with a sensitivity of 93.5% and a specificity of 72.7% (Figure 2).
Table 2.
Logistic regression analysis to identify risk factors associated with disease severity in patients with SARS‐CoV‐2 infection
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Age (years) | 1.076 | 1.029‐1.126 | .001 | |||
| WBC count (×10⁹/L) | 1.422 | 1.124‐1.800 | .003 | |||
| Neutrophil count (×10⁹/L) | 1.818 | 1.282‐2.587 | .001 | |||
| Lymphocyte count (×10⁹/L) | 0.031 | 0.005‐0.196 | <.001 | |||
| Monocyte count (×10⁹/L) | 0.052 | 0.004‐0.676 | .023 | |||
| NLR | 2.648 | 1.566‐4.479 | <.001 | 2.648 | 1.566‐4.479 | <.001 |
| PLR | 1.007 | 1.001‐1.013 | .010 | |||
| LMR | 0.961 | 0.695‐1.329 | .811 | |||
| PSI score | 1.082 | 1.034‐1.133 | .001 | |||
Abbreviations: CI, confidence interval; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PSI, Pneumonia Severity Index; WBC, white blood cell.
Figure 2.
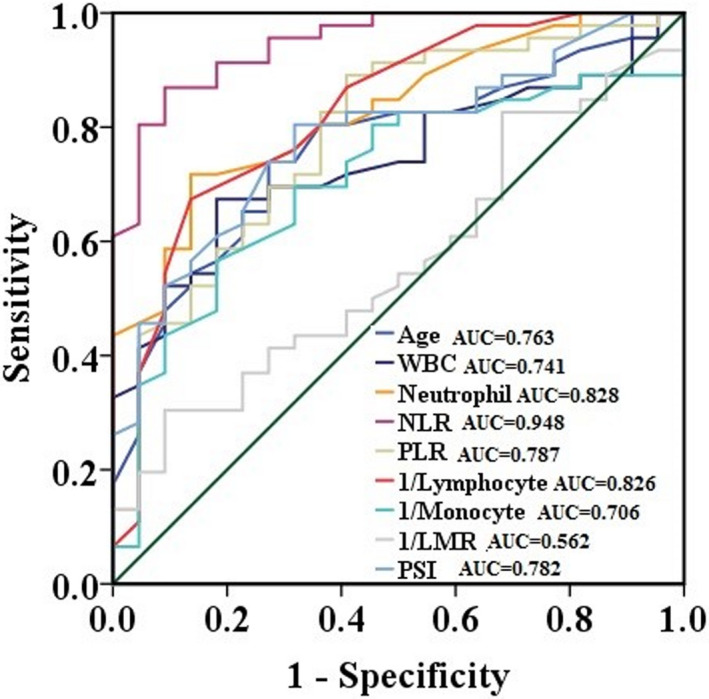
ROC curves showing the relative diagnostic performance of age and hematological parameters for prediction of disease severity in patients with SARS‐CoV‐2 infection. WBC, white blood cell; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; LMR, lymphocyte‐to‐monocyte ratio; PSI, Pneumonia Severity Index
4. DISCUSSION
This study is, to the best of our knowledge, the first to investigate associations between a large panel of hematological parameters and disease severity in patients with SARS‐CoV‐2 infection. We retrospectively analyzed data from 68 patients with SARS‐CoV‐2 infection and found that severe illness was associated with a lower lymphocyte count and monocyte count but higher WBC count, neutrophil count, NLR, PLR, and PSI score compared with mild illness. Univariate analysis showed that older age, high WBC count, high neutrophil count, high NLR, high PLR, low monocyte count and low lymphocyte count were independent risk factors for severe illness. Multivariate analysis identified only high NLR as an independent risk factor for severe illness. ROC analysis revealed NLR to have the highest AUC among hematological parameters.
A previous study demonstrated that both lymphocytes and neutrophils play key roles in the pathogenesis of various diseases and immune defense. 6 Levels of neutrophils have been suggested to reflect the inflammatory state during disease progression, while lymphocyte levels represent the outcome of controlled immune responses. 7 In the present study, we found that the PSI score was inversely correlated with lymphocyte count and that severe illness following SARS‐CoV‐2 infection was associated with a higher neutrophil count and lower lymphocyte count than mild infection. These findings support those of Wang et al, who found that fatal cases of SARS‐CoV‐2 infection had increased neutrophil counts and decreased lymphocyte counts. 8 More recently, Liu et al also observed decreased lymphocyte counts in patients with ARDS compared with those without ARDS. Thus, we propose that SARS‐CoV‐2 infection may affect T lymphocytes, causing a reduction in their levels and consequent disease progression.
The NLR has emerged as a potent inflammatory marker with diagnostic and prognostic value in various clinical syndromes. 10 , 11 , 12 , 13 In the present study, PSI score was positively correlated with the NLR. More importantly, we found that a high NLR was an independent risk factor for severe illness following infection with SARS‐CoV‐2. This corroborates the results of Liu et al, 4 although their study did not analyze other hematological parameters such as WBCs and their subpopulations, PLR, LMR, RDW, or MPV. Our study complements the results of Liu et al in assessing the diagnostic value of other hematological parameters. We identified differences in several hematological parameters associated with severity of illness, although no parameters other than NLR were found to be independent correlates of disease severity. There are two possible explanations for this result. First, the pathogenesis of SARS‐CoV‐2 infection is complex, 8 and it is likely that the early stages of the disease do not influence all hematological parameters. For example, we did not detect significant differences in hemoglobin levels, RDW, MPV, PCT, or PLT between patients with mild and severe illness at the time of admission, even though RDW and MPV are established inflammatory indicators. 13 Second, a limited number of patients were included in our study. Although neutrophil and lymphocyte counts were identified as risk factors for severe illness by univariate analyses, neither was found to be independent predictors of severe illness by multivariate analysis. This may be because the NLR, being a ratio, is more stable than its individual parameters, which may be influenced by factors such as hydration level. Therefore, we conclude that the NLR could be used to predict disease severity and may represent a more reliable biomarker than the neutrophil or lymphocyte count alone. Recently, pathological findings of autopsy examinations revealed inflammatory infiltration of lymphocytes in both lungs of a patient with SARS‐CoV‐2 pneumonia. 8 Furthermore, this report also indicated that a cytokine storm (previously known as the inflammatory storm) or immune hyperactivation could be an important cause of deterioration and death in patients with SARS‐CoV‐2 infection. 14 , 15 We propose that the association of NLR with disease severity may result, partly from the inflammatory response in patients with SARS‐CoV‐2 infection. Initial systemic inflammation following SARS‐CoV‐2 infection could lead to organ dysfunction and subsequent deregulation of the NLR. Our results indicate that the NLR has a higher diagnostic efficacy than the PSI score. Furthermore, unlike the PSI score, evaluation of the NLR requires measurement of two simple factors, both of which are objective. Important factors, such as inflammation, are not taken into consideration for calculation of the PSI score but can affect prognosis. Thus, we believe that the NLR could be beneficial for preliminary diagnosis and prediction of disease severity in patients with SARS‐CoV‐2 pneumonia. While our findings should be confirmed in large multicenter prospective studies, they provide useful preliminary information.
Our study had some limitations that warrant consideration. First, this was a single‐center study with a small sample size and no external validation cohort. Second, hematological parameters were not measured dynamically; thus, it remains unclear whether they exhibit stepwise changes when the patient's condition deteriorates. Finally, we did not evaluate several inflammatory markers, such as C‐reactive protein and interleukin‐6. Evaluation of these markers will help to elucidate the mechanism underlying the findings presented here.
5. CONCLUSIONS
We investigated the associations between hematological parameters and disease severity in patients with SARS‐CoV‐2 infection. We found that the NLR was superior to other hematological parameters for early identification of patients with SARS‐CoV‐2 infection at high risk of developing severe disease. This ratio may be a valuable measurement to complement other conventional assessments of disease severity in these patients. Early evaluation of the NLR may be useful for classification of patients to facilitate appropriate management and efficiently use scarce medical resources. Our findings warrant further large‐scale multicenter prospective studies.
ACKNOWLEDGMENT
We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Lin S, Mao W, Zou Q, Lu S, Zheng S. Associations between hematological parameters and disease severity in patients with SARS‐CoV‐2 infection. J Clin Lab Anal 2021;35:e23604 10.1002/jcla.23604
Contributor Information
Sha Lin, Email: 1508092@zju.edu.cn.
Shufa Zheng, Email: zsfzheng@zju.edu.cn.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200313‐sitrep‐53‐covid‐19.pdf?sfvrsn=adb3f72_2 (accessed March 13, 2020)
- 3. Zhu N, Zhang D, Wang W, et al. A Novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. J Transl Med. 2020;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243‐250. [DOI] [PubMed] [Google Scholar]
- 6. Wang W, Wang Y, Qu C, et al. The RNA genome of hepatitis E virus robustly triggers an antiviral interferon response. Hepatology. 2018;67(6):2096‐2112. [DOI] [PubMed] [Google Scholar]
- 7. Kwon JH, Jang JW, Kim YW, et al. The usefulness of C‐reactive protein and neutrophil‐to‐lymphocyte ratio for predicting the outcome in hospitalized patients with liver cirrhosis. BMC Gastroenterol. 2015;15:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet. Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lou B, Li TG, Zheng SF, et al. Serology characteristics of SARS‐CoV‐2 infection after exposure and post‐symptom onset. Eur Respir J. 2020;56(2):2000763 10.1183/13993003.00763-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 11. Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109(2):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18(6):1167‐1173. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Lou Y, Chen Y, Yang J. Prognostic value of the neutrophil‐to‐lymphocyte ratio in patients with acute‐on‐chronic liver failure. Int J Clin Pract. 2014;68(8):1034‐1040. [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]


