Abstract
Staphylococcus aureus is a troublesome pathogen, responsible for a broad range of clinical manifestations, ranging from benign skin infections to life-threatening conditions such as endocarditis and osteomyelitis. The kidney can be affected through a rapidly progressive glomerulonephritis mediated by an inflammatory reaction against a superantigen deposited in the glomerulus during the infection’s course. This glomerulopathy has a poor prognosis, often leading to chronically impaired kidney function, eventually progressing to end-stage renal disease. Treatment rests on antibiotherapy. Despite the inflammatory role in this disease’s pathophysiology, most authors discourage a simultaneous immunosuppressive approach given the concomitant infection. However, there are some reports of success after administration of systemic corticosteroids in these patients. We present a 66-year-old man with a staphylococcus-induced glomerulonephritis brought on by a vascular graft infection, with rapidly deteriorating kidney function despite extraction of the infected graft and 3 weeks of antibiotherapy with achievement of infection control. Kidney function improved after the introduction of corticosteroids. This case highlights the potential role of corticosteroids in selected cases of staphylococcus-induced glomerulonephritis, particularly those in which the infection is under control.
Keywords: acute renal failure, contraindications and precautions, infectious diseases
Background
Staphylococcus aureus is a commensal bacterium and an important pathogen responsible for a variety of infections, either benign and easily treated (skin and soft tissue infections) or serious and with a protracted course (infective endocarditis and osteomyelitis). It is also responsible for an increasing number of healthcare-associated infections, particularly those related to prosthetic material (eg, endovascular and articular).
During these infections, a superantigen produced by the bacterium circulates in the bloodstream and can deposit in various tissues. In the case of staphylococcus-associated glomerulonephritis, these deposits end up in the kidney, stimulating an inflammatory reaction mediated by immune complexes that culminates in a diffuse endocapillary proliferative and exudative glomerulonephritis with a crescentic and necrotising pattern.1 2 Immunofluorescence most often shows C3 dominant, or codominant with IgG or IgA, glomerular staining.3
Treatment consists on antibiotherapy against the staphylococcal infection; successful eradication of the infection should result in resolution of the glomerulonephritis. Most authors discourage a simultaneous immunosuppressive approach due to the concomitant infection.3–6 Nonetheless, there are some reports of success after introduction of systemic corticosteroids in patients whose glomerulonephritis did not resolve even after control of the infection.7–10 Staphylococcus-induced glomerulonephritis usually carries a relatively poor prognosis, with only 25% of patients recovering renal function.5
Case presentation
We present the case of a 66-year-old man with a history of abdominal aorta pseudoaneurysm submitted to corrective surgery with endovascular aortobifemoral prosthesis placement in 2006. Thirteen years later, he was admitted with vascular graft infection at the right femoral segment of the prosthesis and acute kidney injury, with a serum creatinine (sCr) of 1.6 mg/dL. Haemocultures grew a methicillin-sensitive Staphylococcus aureus. The endovascular device was replaced by an iliofemoral cadaver allograft, and the patient completed a 15-day antibiotic treatment course with vancomycin and gentamicin. He was discharged with oral trimetropim–sulfamethoxazol (TMP/SMX) 960 mg 2id (two times a day). His sCr on discharge was 1.5 mg/dL, and his C reactive protein was 3.5 mg/dL. One week later, follow-up analytical workup showed declining renal function to an sCr of 5.21 mg/dL (C reactive protein of 1.09 mg/dL), so the patient was immediately admitted to the hospital for medical treatment and aetiological investigation. He had no complains and reported a preserved urine output. He did not show oedema, although his blood pressure was slightly elevated (154/73 mm Hg). Urine analysis showed microscopic haematuria (>100 erythrocytes per high-power field (HPF)). Urinary protein/creatinine ratio was 600 mg/g. Further diagnostic workup of acute renal injury was unremarkable, except for a dubious positive titre for ANCA-PR3 antibody (table 1). Cultures were negative, although this may have been influenced by simultaneous antibiotic treatment. Renal ultrasound showed normal-sized kidneys with an adequate thickness and differentiation of the parenchymal rim. Renal biopsy showed endocapillary and mesangial proliferative glomerulonephritis with an identified crescent (figures 1 and 2). Immunofluorescence reported C3 and IgA mesangial and glomerular wall deposits (figures 3 and 4). Electron microscopy was not performed.
Table 1.
Complementary diagnostic exams at admission
| Complementary diagnostic exams | Results | Normal range |
| Leucocytes | 6.7×109/L | 4.0–10.0×109/L |
| Haemoglobin | 9.6 g/dL | 13.0–17.5 g/dL |
| Platelets | 390×109/L | 150–400×109/L |
| BUN | 83 mg/dL | 7.9–20.9 mg/dL |
| Creatinine | 5.26 mg/dL | 0.72–1.18 mg/dL |
| Albumin | 3.7 g/dL | 3.5–5.2 g/dL |
| C reactive protein | 1.09 mg/dL | <0.5 mg/dL |
| ANAs | Negative | |
| ENAs | Negative | |
| Anti-dsDNA | 0.5 IU/L | Negative if <10 IU/L |
| ANCAs (ELISA) | Dubious positivity for anti-PR3 (2.4 IU/mL) Negative anti-MPO (<0.2 IU/mL) |
Negative if <2.0 IU/mL |
| ANCAs (immunofluorescence) | Negative c-ANCA Negative p-ANCA |
|
| Anti-GBM antibody | Negative | |
| C3 | 1.18 g/L | 0.82–1.85 g/L |
| C4 | 0.33 g/L | 0.15–0.53 g/L |
| Crioglobulins | Negative | |
| Serum immunofixation | No monoclonal proteins identified | |
| Urinary sediment | Erythrocyturia (>100 erythrocytes per high power field); proteinuria (70 mg/dL); no leucocyturia; rare hyaline casts | |
| Protein-to-creatinine ratio | 608 mg/g | <200 mg/g |
| HBV, HCV and HIV | All negative |
ANAs, Antinuclear antibodies; ANCAs, Antineutrophil cytoplasmic antibodies; Anti-dsDNA, Anti-double stranded DNA antibodies; Anti-GBM, Anti-Glomerular basement membrane; BUN, Blood urea nitrogen; C3, Complement component C3; C4, Complement component C4; ELISA, Enzyme-Linked Immunosorbent Assay; ENAs, Extractable nuclear antigen antibodies; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus.
Figure 1.
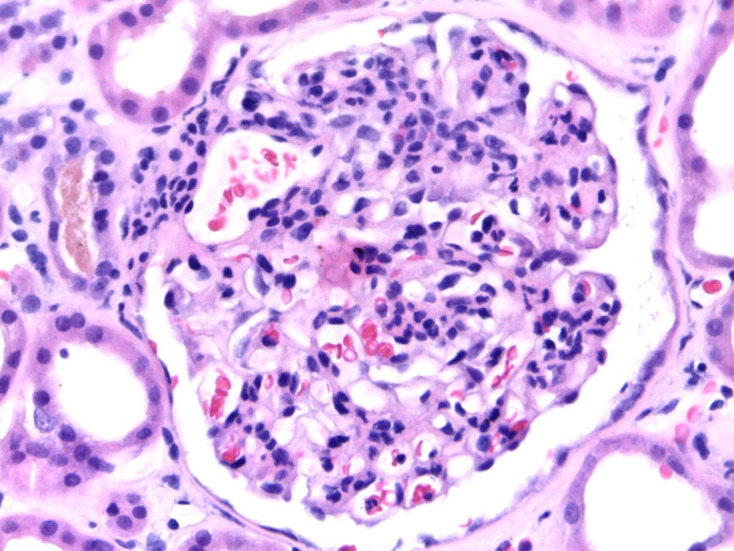
Light microscopy from kidney biopsy showing endocapillary and mesangial proliferation with intense congestion of the glomerular tuft, H&E 400×.
Figure 2.
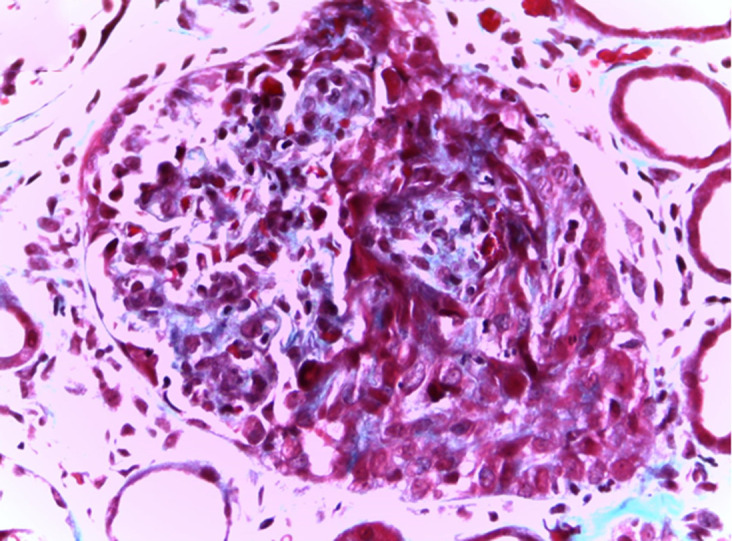
Light microscopy from kidney biopsy showing fibrocellular crescent, trichrome Masson 400×.
Figure 3.
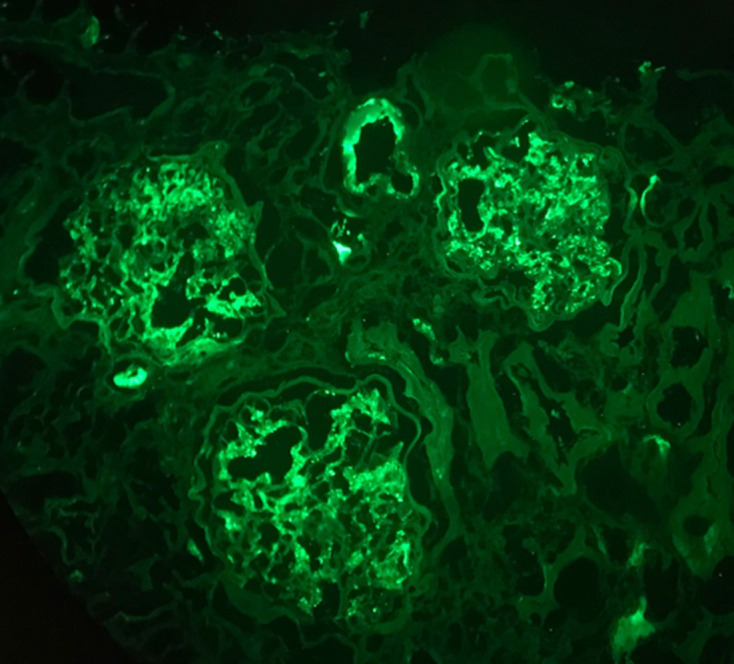
Immunofluorescence microscopy from kidney biopsy showing C3 glomerular deposits (2+), 200×.
Figure 4.
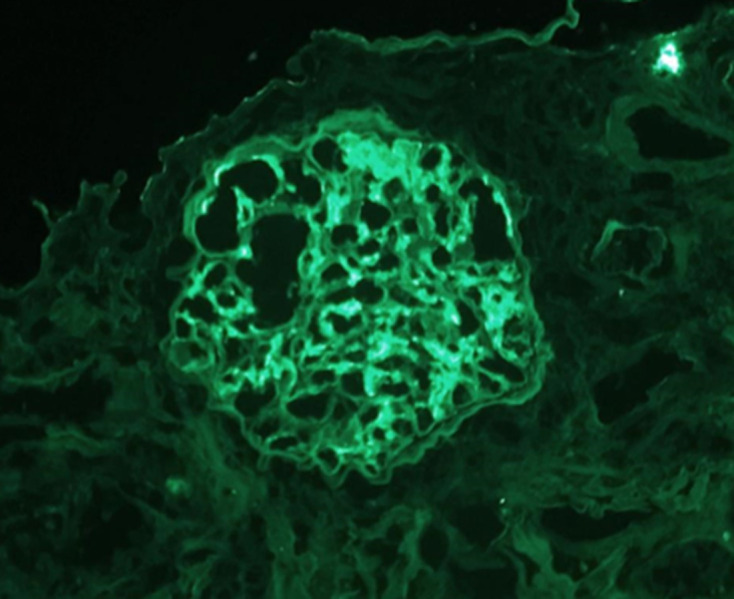
Immunofluorescence microscopy from kidney biopsy showing IgA glomerular deposits (1+), 200×.
Investigations
Complementary diagnostic exams (table 1) done at admission exhibited acute kidney failure with an active urinary sediment. Autoimmunity workup was negative.
[Atraia a atenção do seu leitor colocando uma boa citação no documento ou utilise este espaço para enfatizar um ponto chave. Para colocar esta caixa de texto noutro local da página, arraste-a.]
Figures 1–4 show kidney biopsy results. The first figure reveals glomerular capillary congestion with marked neutrophil infiltration as well as some mesangial proliferation. In figure 2, we can see a glomerulus containing a fibrocellular crescent (mostly cellular). Immunofluorescence (figures 3 and 4) shows C3 (++) and IgA (+) deposits in the mesangium as well as in the capillary wall.
Differential diagnosis
It is essential to differentiate staphylococcus-induced glomerulonephritis from other possible entities such as IgA nephropathy, C3 glomerulonephritis, poststreptococcal glomerulonephritis and other causes of crescentic glomerulonephritis.
The distinction from IgA nephropathy is difficult. It consists of a mesangial proliferative glomerulonephritis accompanied by mesangial dominant IgA deposits. However, it can present with endocapillary proliferation and, rarely, with crescent formation. Glomerular C3 deposition is also frequent; however, IgA deposits should be dominant. Clinical presentation, such as with macroscopic haematuria and/or kidney injury is usually synpharyngitic (simultaneous with a respiratory infection). Other infections may sometimes be identified as triggers. In our case, the kidney injury developed 3 weeks after the infection was detected; this is unusual in IgA nephropathy and favours our diagnosis. Besides, immunofluorescence from the kidney biopsy showed C3 dominance, as opposed to what usually happens in IgA nephropathy (IgA dominance). Hence, timing of kidney injury and histological presentation were the most striking hints that allowed us to make the diagnosis.
In C3 glomerulonephritis, C3 deposits are not typically accompanied by immunoglobulin deposits, as we see in this patient’s kidney biopsy. Besides, there is usually complement consumption in this disorder, contrary to what happened in our case.
Poststreptococcal glomerulonephritis would present in a similar way. However, it is normally associated with a respiratory tract infection and occurs mainly in children. In any case, the bacteria identified was a staphylococcus species.
Other crescentic glomerulonephritis, such as ANCA vasculitis (ANCA-PR3 titre was dubious) could be responsible for this clinical picture. However, the glomerulonephritis presented is not pauci-immune (C3 and IgA deposits), and ANCA immunofluorescence was negative.
Treatment
A sole methylprednisolone pulse (500 mg) was administrated followed by prednisolone 1 mg/kg/day. Corticosteroids were rapidly tapered (10 mg/day by the second month) and eventually stopped after 3 months of therapy. Linezolid was substituted for TMP/SMX on admission, given the possibility of an interstitial nephritis attributed to the later antibiotic; it was kept throughout the entire corticosteroid treatment.
Outcome and follow-up
Patient’s kidney function improved after introduction of corticosteroid treatment, and he was discharged 2 weeks later with an sCr of 3.56 mg/dL. He was kept on prednisolone (with a rapid tapering scheme) and linezolid 600 mg 2id. Two months later, his kidney function improved further to an sCr of 1.75 mg/dL, under prednisolone 10 mg/day. The patient completed 3 months of corticosteroid therapy. On his last follow-up visit (12 months after the admission), his sCr had stabilised on 1.9 mg/dL, his proteinuria had decreased to 125 mg/g (protein-to-creatinine ratio) and microscopic haematuria had disappeared. He did not have any further infectious complications.
Discussion
Staphylococcus-associated glomerulonephritis is an uncommon but increasingly reported renal disease.11 Even though this glomerulonephritis occurs with an infectious process, it may take up to 10 weeks (median time is 1 week) to develop, which is in agreement with the notion that it is driven by immunocomplex deposition, since these require some time to develop.3 7 Kidney injury was detected in our patient 3 weeks after the infection diagnosis. However, one can easily presume it started sooner as the patient’s creatinine was already 5.26 mg/dL at the time kidney injury was detected. These patients usually present with acute kidney injury (mean sCr of 5.1 mg/dL), microscopic haematuria and variable proteinuria, as was found in our patient.12 Hypocomplementaemia may occur but is not universal.5 Our patient had a marginally positive ANCA titre that can occur in staphylococcus-associated glomerulonephritis, particularly in those cases with associated endocarditis.13 Light microscopy findings include endocapillary and exudative glomerulonephritis, sometimes accompanied by mesangial proliferation and, rarely, crescent formation.4 Immunofluorescence usually reveals a coarsely granular mesangial and glomerular wall staining for IgA and C3 (‘starry sky pattern’). However, other distributions are sometimes found, such as the ‘garland pattern’ and the ‘mesangial pattern’, with predominant glomerular wall and mesangial staining, respectively.12 14 Electron microscopy is helpful in order to verify the presence of subepithelial humps, which is a very common finding.5 Our patient’s kidney biopsy, along with his clinical data, was consistent with the diagnosis of staphylococcus-associated glomerulonephritis. Unfortunately, we were unable to perform electron microscopy.
Intravenous methylprednisolone is usually used to suppress renal inflammatory activity, given its fast and strong immunosuppressive effect. However, this is also the reason why its use is disapproved in infection associated glomerulonephritis (shunt nephritis and staphylococcal glomerulonephritis).3 12 15 Sometimes kidney function does not improve in spite of the adequate antibiotherapy.7–10 This is understandable, owing to the fact that an immunological process is contributing to this glomerulonephritis and, although it is a response to the background infectious process, it can sometimes be disproportionally intense and require an immunosuppressive approach.
There are some case reports of staphylococcus-associated glomerulonephritis successfully treated with steroid therapy together with antibiotics.7–10 Steroids were usually prescribed when there was refractoriness of the renal manifestations in spite of systemic antibiotics or when crescent formation was verified.7 9 The authors recommend continuing antibiotherapy during the administration of corticosteroids.7 8
Facing a scenario in which the infectious process is under control and the patient has not recovered kidney function, physicians have to consider potential benefits and risks of starting corticosteroid therapy. In this case, the risk was considered to be low, since the patient had already been on 21 days of antibiotherapy, had been without fever for at least 2 weeks and had a C reactive protein of 1.09 mg/dL at admission. The benefits were theoretically very significant, considering we were facing a rapidly progressive glomerulonephritis with at least one crescent formation (others might have been missed since this is a focal segmental lesion) and steroids could make the difference between initiation of renal replacement therapy or partial recovery of kidney function. In our case, this option was very rewarding, as the patient was discharged with an sCr of 3.56 mg/dL and a C reactive protein of 0.36 mg/dL. Antibiotics were provided during the entire corticosteroid therapy course. On admission, it was switched from TMP/SMX to linezolid due to the acute kidney injury. We must add that the biopsy also showed some degree of interstitial nephritis that may have been related to the former antibiotic. This lesion pattern might partly justify the results achieved with corticosteroid administration, although a favourable impact on the glomerular lesions was certainly made as witnessed by the resolution of haematuria and the marked reduction of proteinuria. Presently, 12 months have passed and the patient maintains partial recovery of kidney function (sCr of 1.9 mg/dL) and a benign urine urinalysis without further infectious complications.
Hence, this patient was treated with antibiotics and corticosteroids for a staphylococcal glomerulonephritis with a good clinical and analytical response. Based on ours and other’s published experiences, corticosteroids may be a reasonable option for staphylococcal glomerulonephritis patients who fail to respond to isolated antibiotic therapy despite adequate control of the infectious process. Despite the need for further studies to more precisely define the role of steroid therapy in these patients, the potential benefits may be substantial as the need for long-time renal replacement therapy may be avoided with significant long-term impact on prognosis and quality of life. It is important to note, however, that there is no evidence for routine use of immunosuppression in this glomerulopathy.
Learning points.
Staphylococcus-induced glomerulonephritis is an immune complex-mediated disease with a poor prognosis.
Appropriate antibiotics are the most important aspect of treatment.
Immunosuppressive therapy is contraindicated when there is active infection.
Although there is no evidence regarding the use of corticosteroids use in staphylococcus-associated glomerulonephritis, it may be beneficial in cases refractory to adequate antibiotic control of the infection, particularly when kidney biopsy shows aggressive glomerular inflammatory activity (eg, endocapillary proliferation and crescent formation).
Footnotes
Contributors: RFN (corresponding author) was responsible for writing the paper, was involved in the acquisition, analysis and interpretation of data as well as in the revision of the article for important intellectual content; was also involved in the final approval of the version published; and contributed to the patient's follow-up. NO was responsible for the patient's care as well as his follow-up and was involved in the revision of the article for important intellectual content and in the final approval of the version published. VS was responsible for analysing the kidney biopsy samples and provide the results, revised the work critically for important intellectual content and was involved in the final approval of the version published. RA was involved in conception and design of the paper as well as acquisition and interpretation of data and revised it critically for intellectual content and approved it for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nasr SH, Radhakrishnan J, D'Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int 2013;83:792–803. 10.1038/ki.2012.407 [DOI] [PubMed] [Google Scholar]
- 2.Fujigaki Y, Yousif Y, Morioka T, et al. . Glomerular injury induced by cationic 70-kD staphylococcal protein; specific immune response is not involved in early phase in rats. J Pathol 1998;184:436–45. [DOI] [PubMed] [Google Scholar]
- 3.Feehally J, Floege J, Tonelli M. Comprehensive clinical nephrology.
- 4.Glassock RJ, Alvarado A, Prosek J, et al. . Staphylococcus-related glomerulonephritis and poststreptococcal glomerulonephritis: why defining "post" is important in understanding and treating infection-related glomerulonephritis. Am J Kidney Dis 2015;65:826–32. 10.1053/j.ajkd.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 5.Nasr SH, Fidler ME, Valeri AM, et al. . Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol 2011;22:187–95. 10.1681/ASN.2010060611 [DOI] [PubMed] [Google Scholar]
- 6.Nadasdy T, Hebert LA. Infection-Related glomerulonephritis: understanding mechanisms. Semin Nephrol 2011;31:369–75. 10.1016/j.semnephrol.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 7.Kapadia AS, Panda M, Fogo AB. Postinfectious glomerulonephritis: is there a role for steroids? Indian J Nephrol 2011;21:116. 10.4103/0971-4065.82141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeledon JI, McKelvey RL, Servilla KS, et al. . Glomerulonephritis causing acute renal failure during the course of bacterial infections. Histological varieties, potential pathogenetic pathways and treatment. Int Urol Nephrol 2008;40:461–70. 10.1007/s11255-007-9323-6 [DOI] [PubMed] [Google Scholar]
- 9.Okuyama S, Wakui H, Maki N, et al. . Successful treatment of post-MRSA infection glomerulonephritis with steroid therapy. Clin Nephrol 2008;70:344–7. 10.5414/CNP70344 [DOI] [PubMed] [Google Scholar]
- 10.Carbayo J, Rodriguez-Benitez P, Diaz-Crespo F, et al. . IgA dominant postinfectious glomerulonephritis secondary to cutaneous infection by methicillin-resistant Staphylococcus aureus. Nefrologia 2019;39:447–9. 10.1016/j.nefroe.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 11.Pestana N, Silva F, Vieira P, et al. . IgA dominant glomerulonephritis associated to Staphylococcus infection: a peculiar case report. Pjnh 2019;33 10.32932/pjnh.2019.07.025 [DOI] [Google Scholar]
- 12.Nasr SH, Markowitz GS, Stokes MB, et al. . Acute postinfectious glomerulonephritis in the modern era. Medicine 2008;87:21–32. 10.1097/md.0b013e318161b0fc [DOI] [PubMed] [Google Scholar]
- 13.Satoskar AA, Suleiman S, Ayoub I, et al. . Staphylococcus Infection-Associated GN - Spectrum of IgA Staining and Prevalence of ANCA in a Single-Center Cohort. Clin J Am Soc Nephrol 2017;12:39–49. 10.2215/CJN.05070516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorger K, Gessler M, Hübner FK, et al. . Follow-up studies of three subtypes of acute postinfectious glomerulonephritis ascertained by renal biopsy. Clin Nephrol 1987;27:111–24. [PubMed] [Google Scholar]
- 15.Montseny JJ, Meyrier A, Kleinknecht D, et al. . The current spectrum of infectious glomerulonephritis. experience with 76 patients and review of the literature. Medicine 1995;74:63–73. 10.1097/00005792-199503000-00001 [DOI] [PubMed] [Google Scholar]


