Abstract
To report the clinical course and management of interface keratitis due to Enterococcus faecalis after Descemet membrane endothelial keratoplasty (DMEK). A 64-year-old man underwent DMEK, with unevenful immediate postoperative course, with a visual recovery of 20/30 at 2 weeks. At 3 months of clinical visit, interface keratitis was noted. DMEK graft removal with stromal bed scrapings was performed. A diagnosis of E. faecalis interface keratitis was made. The patient responded favourably to antibiotic susceptibility-guided intensive treatment with vancomycin 5% with complete resolution of infection. After 2 months of graft removal, Descemet stripping endothelial keratoplasty (DSEK) was performed. The corneal clarity was restored and the best corrected visual acuity was 20/40 at last follow-up of 1 year. E. faecalis should be kept as a differential in delayed onset interface keratitis after DMEK. After microbiological cure with antibiotic therapy, visual rehabilitation with DSEK restores corneal clarity and results in favourable visual outcome.
Keywords: ophthalmology, infections
Background
Descemet membrane endothelial keratoplasty (DMEK) involves selective replacement of diseased endothelium with healthy endothelium-Descemet membrane. In Descemet stripping endothelial keratoplasty (DSEK), the diseased endothelium and Descemet membrane of host is replaced with posterior corneal stroma, Descemet membrane and endothelium of donor. In DMEK absence of posterior stromal lamella in scroll, reduces posterior astigmatism, hyperopic shift and altered higher order optical aberrations caused by lenticule in DSEK.1 Similar to DSEK, DMEK also creates a graft host interface that can harbour microorganisms in the sequestered space leading to peculiar complication of interface keratitis.
Interface keratitis after any type of lamellar keratoplasty (LK) is challenging to diagnose and manage due to an impaired access to the site of infiltration for microbiological analysis and antibiotic penetration.2 3 Often the treatment is governed by an empirical approach and indirect evidence such as cultures from the donor rim. Several organisms have been implicated in interface keratitis after DMEK,2–8 of which fungus is the most common aetiological agent reported.
Herein, we report the clinical course and management of a case of delayed interface keratitis after DMEK with Enterococcus faecalis
Case presentation
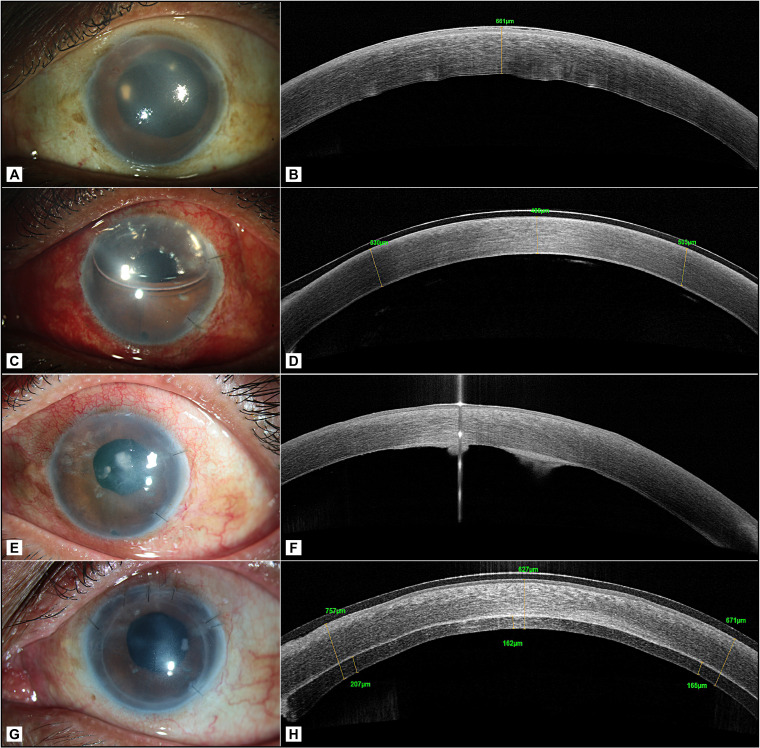
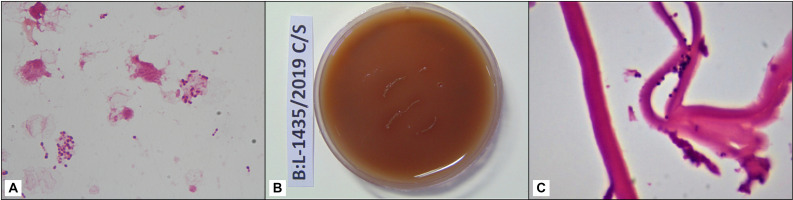
A 64-year-old man underwent DMEK for pseudophakic corneal oedema in the left eye (figure 1A, B). A subconjunctival injection of 0.3% moxifloxacin was administered at the conclusion of the surgery. The DMEK graft was prepared immediately prior to the surgery in the operating room from a 72-year-old phakic, male donor, collected from a hospital source, with an endothelial cell density of 2600 cells/mm2. The death to preservation time was 4 hours and preservation to utilisation time in MaCarey Kaufman medium (MK medium) containing gentamicin was 70 hours. Postoperative day 1, the graft was attached well (figure 1C, D). At 2-week review visit after surgery, the cornea was clear with a best corrected visual acuity (BCVA) of 20/30. After 3 months, he presented to the clinic with reports of poor vision, redness and discomfort in the operated eye. Further, on enquiry, he revealed that the decrease in vision began at 1 month after the surgery and was gradually progressive. However, he was unable to visit the clinic then. The visual acuity at this visit had reduced to 20/600. Slit-lamp examination showed mild congestion, yellowish- white infiltrate in the interface (figure 1E). High-resolution optical coherence tomography corroborated with the clinical findings and revealed that the infiltrate was localised at the interface level (figure 1F). DMEK graft removal was done by scoring with reverse sinskey hook and interface scraping was performed. The recipient bed was scraped with a blunt cannula and aspirated using a 3 mL syringe attached to it. The aspirate was plated and inoculated on the culture media. Intracameral amphotericin B 25 mg/0.1 mL was administered at the time of the surgery. The Descemet membrane removed was bisected at the site of the infiltrate; one half was sent in 10% formalin for histological evaluation and the second half was plated on chocolate agar for microbiological evaluation. The gram stained smears showed the presence of gram-positive cocci in groups and culture media grew E. faecalis (figure 2A, B) that was identified by ViTEK 2 and found to be sensitive only to vancomycin and resistant to cefazoline, ciprofloxacin, ofloxacin, gatifloxacin, moxifloxacin, clindamycin, tetracycline, chloramphenicol and gentamycin. The histology sample showed the Descemet membrane with colonies of gram-positive cocci on gram stain (figure 2C). As a part of our eye bank’s protocol of investigation of an adverse event after keratoplasty, the details of donor history and the mate pair of the donor cornea used for DMEK were reviewed. The mate pair of this donor was pseudophakic with unrecordable endothelial cell density and a pterygium scar and hence was not used for transplantation. The practice at our eye bank is to preserve the corneoscleral rim after keratoplasty for a week after surgery, beyond which these are discarded. Hence, the corneoscleral rim was unavailable for microbiological culture at 3 months when the patient was seen in the clinic. The patient was treated with vancomycin 5% eye drops every 1 hour for 1 week, every 2 hours thereafter for the next 2 weeks and atropine sulphate eye drops two times per day for 2 weeks. After 1 week of intensive antibiotic treatment, prednisolone acetate 1% and sodium chloride 5% were prescribed every 4 hours. The patient responded favourably to the treatment and there was resolution of infiltrate in 5 weeks duration. The B scan ultrasonography performed at the time of diagnosis of interface keratitis, a week, and 5 weeks after DMEK graft removal was echo free.
Figure 1.
(A and B) Preoperative slit lamp and OCT image of the left eye; (C and D) slit lamp photograph and OCT image on postoperative day 1 after DMEK; (E and F) slit lamp photograph and OCT at 3 months visit showing 2 foci of interface infection; (G and H) slit lamp photograph and OCT image at 1 month post DSEK. DMEK, Descemet membrane endothelial keratoplasty; DSEK, Descemet stripping endothelial keratoplasty; OCT, Optical coherence tomography.
Figure 2.
(A) Direct smear examination of interface scrapings showing polymorphonuclear cells 0–4/oil immersion field (OIF), and purple to deep pink cocci in pairs and short chains 0–10/OIF (×1000); (B) growth of confluent tiny, smooth cream bacterial colonies on the streaked regions on chocolate agar (incubation 37°C, 3 days); (C) photomicrograph of Descemet membrane showing gram-positive cocci in small clusters (Gram stain, ×1000).
The cornea was oedematous and developed paracentral haze. At 2 months after graft removal, DSEK was performed in view of relatively poorer visualisation of the anterior segment compared with the earlier when he underwent DMEK.
Outcome and follow-up
The postoperative course was uneventful after DSEK and visual acuity was improved to 20/80 at 1 month postoperative time (figure 1G, H). The cornea clarity recovered over the next 3 months when BCVA was 20/40. At the last follow-up of 1 year, the patient’s cornea had cleared, with a BCVA of 20/30.
Discussion
Interface keratitis is a rare complication after LK. With the growing popularity of DMEK, there has been an increased reporting of interface keratitis after DMEK. Although majority of reports of the interface keratitis after DMEK were due to Candida sp, there are isolated case reports of interface keratitis caused by other organisms such as Mycobacterium chelonae and Nocardia asteroides. We report E. faecalis as another cause of interface keratitis after DMEK.
E. faecalis is a Gram-positive, commensal bacterium in the gastrointestinal tracts of humans. D’Oria et al9 reported a case of microbiologically proven E. faecalis interface keratitis post deep anterior lamellar keraoplasty in which therapeutic penetrating keratoplasty (PK) was performed with good outcome. Hannush et al10 reported E. faecalis deep stromal abscess post DSEK with venting incisions treated with medical management and PK post infection resolution with fair visual outcome. A case of infectious crystalline keratopathy (ICK) after DSEK caused by E. faecalis was reported by Porter et al and treated with PK and vitrectomy to resolve infection.11 Our case presented in the interface between posterior stroma and Descemet membrane, while the case of ICK reported by Porter et al was in the stroma–stroma interface.
Managing interface keratitis can be difficult unless the diagnosis can be made with certainty. There are several challenges faced in appropriate diagnosis. The deep-seated nature of the infiltrate makes microbiological assays inaccessible. In eyes with early onset infection, the rim cultures may aid in making a diagnosis. However, in those cases presenting with delayed onset of infection, the corneoscleral rim may not be available (as was the case here) and hence other methods of microbiological diagnosis are required. The rim cultures cannot be relied on for the diagnosis of postoperative problems and they are a poor substitute for specimens from the site of infection. Our patient had symptoms of gradual decrease in vision from 1 month after DMEK. There was no evidence of infection at 2 weeks of clinical visit postsurgery, when the interface was clear and vision was 20/30. He presented to the clinic only after 3 months when his vision had dropped significantly. Considering the delayed onset and indolent course of the interface infection, although Candida interface infection was suspected and kept as a differential diagnosis, a microbiological confirmation was clearly needed. In view of this, our approach involved DMEK graft extraction along with stromal bed scrapings for microbiological and histological diagnoses.
The patient responded to intensive treatment with vancomycin 5%. In view of graft removal, there was significant corneal oedema and some degree of stromal haze ensued, that precluded repeated DMEK due to visualisation concerns; hence, DSEK was performed instead. There was gradual and satisfactory improvement in corneal clarity and visual acuity over a period of 1 year.
Although the source of infection in this case could not be ascertained, we believe it is likely due to donor contamination. The donor cornea was harvested from hospital source and the Entercococcus spp isolated was resistant to several antibiotics. There is an increasing antimicrobial resistance in hospital setting and corneas harvested from donors with hospitalisation can have microbial contamination that can potentially cause donor-related infection, especially if the organism is found resistant to the antibiotic in the corneal preservation medium like gentamycin-enriched MK medium in our case.12
The case highlights the importance of diagnostic intervention in cases of delayed interface keratitis after DMEK. A definitive diagnosis is required to initiate a specific antibiotic therapy to achieve eradication of infection, following which visual rehabilitation should be considered.
Learning points.
Interface infectious keratitis (IIK) is a known complication after lamellar keratoplasty (LK) warranting surgical intervention for infected graft removal and for microbiology testing.
Culture sensitivity-guided medical management for LK results in infection resolution and secondary surgical intervention results in optimal outcome.
Fungus (Candida) is the most common reported aetiology for IIK, other causes like Enterococcus, Nocardia and Mycobacterium should be considered and appropriately evaluated.
Footnotes
Contributors: Concept and design of study: SC. Acquisition of data, analysis and interpretation of data: PS. Initial draft: PS. Final draft, editing, references: SC, JJ, DKM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tourtas T, Laaser K, Bachmann BO, et al. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 2012;153:1082–90. 10.1016/j.ajo.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, Moramarco A, Mandarà E, et al. Interface infectious keratitis after anterior and posterior lamellar keratoplasty. clinical features and treatment strategies. A review. Br J Ophthalmol 2019;103:307–14. 10.1136/bjophthalmol-2018-312938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y, Li C, Bu P, et al. Infectious interface keratitis (IIK) following lamellar keratoplasty: a literature review. Ocul Surf 2019;17:635–43. 10.1016/j.jtos.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Augustin VA, Weller JM, Kruse FE, et al. Fungal interface keratitis after Descemet membrane endothelial keratoplasty. Cornea 2018;37:1366–9. 10.1097/ICO.0000000000001727 [DOI] [PubMed] [Google Scholar]
- 5.Tu EY, Majmudar PA. Adjuvant stromal amphotericin B injection for late-onset DMEK infection. Cornea 2017;36:1556–8. 10.1097/ICO.0000000000001398 [DOI] [PubMed] [Google Scholar]
- 6.Doshi H, Pabon S, Price MO, et al. Overview of systemic Candida infections in hospital settings and report of Candida after DMEK successfully treated with antifungals and partial graft excision. Cornea 2018;37:1071–4. 10.1097/ICO.0000000000001608 [DOI] [PubMed] [Google Scholar]
- 7.Van Landeghem R, Foets B, Desmet S, et al. Donor-related nontuberculous mycobacterial interface infection after Descemet membrane endothelial keratoplasty. Cornea 2019;38:632–4. 10.1097/ICO.0000000000001895 [DOI] [PubMed] [Google Scholar]
- 8.Srirampur A, Mansoori T, Reddy AK, et al. Management of Nocardia interface keratitis after Descemet membrane endothelial keratoplasty. Cornea 2019;38:1599–601. 10.1097/ICO.0000000000002058 [DOI] [PubMed] [Google Scholar]
- 9.D'Oria F, Galeone A, Pastore V, et al. Multi-drug resistant Enterococcus faecium in late-onset keratitis after deep anterior lamellar keratoplasty: a case report and review of the literature. Medicine 2019;98:e17140. 10.1097/MD.0000000000017140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannush SB, Chew HF, Eagle RC. Late-onset deep infectious keratitis after Descemet stripping endothelial keratoplasty with vent incisions. Cornea 2011;30:229–32. 10.1097/ICO.0b013e3181eae8ff [DOI] [PubMed] [Google Scholar]
- 11.Porter AJ, Lee GA, Jun AS. Infectious crystalline keratopathy. Surv Ophthalmol 2018;63:480–99. 10.1016/j.survophthal.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 12.Das S, Ramappa M, Mohamed A, et al. Acute endophthalmitis after penetrating and endothelial keratoplasty at a tertiary eye care center over a 13-year period. Indian J Ophthalmol 2020;68:2445–50. 10.4103/ijo.IJO_71_20 [DOI] [PMC free article] [PubMed] [Google Scholar]