Highlights
-
•
Overall prognosis of uterine leiomyosarcoma (ULMS) is poor with a low 5-year survival rate.
-
•
Microsatellite instability (MSI)-high ULMS is not well documented in current literature.
-
•
Immune checkpoint inhibitors such as pembrolizumab have been shown to have good efficacy in treating MSI-high solid tumors.
-
•
Targeting MSI-high ULMS with pembrolizumab can potentially maintain a patient’s quality of life and extend overall survival.
Keywords: Uterine leiomyosarcoma, Pembrolizumab, Microsatellite instability, Immune checkpoint inhibitors
1. Introduction
Uterine leiomyosarcoma (ULMS) is a rare, aggressive soft tissue sarcoma derived from the smooth muscle of the uterus. Only 2–5% of uterine malignancies are leiomyosarcomas, but due to their aggressive nature and limited effective treatment options they comprise a significant amount of uterine cancer-related deaths. (Ricci et al., 2017) Reported 5-year survival by stage is 76% for stage I, 60% for stage II, 45% for stage III, and 29% for stage IV disease. (Ricci et al., 2017) Current first-line treatment for uterine leiomyosarcoma consists of total abdominal hysterectomy and bilateral salpingo-oophorectomy with surgical debulking if intra-abdominal spread is found. The standard first-line chemotherapy is gemcitabine and docetaxel, but the 3-year progression free survival with this regimen is still only 50%, and most patients have recurrence within 8–16 months following treatment.2 There are limited options for second line therapy once a patient recurs. New techniques to target molecular changes have been proposed and studied, especially c-MYC, Bcl-2, K-ras, Ki-67, p16, p53, and Rb1, common mutations found in ULMS. (Momtahen et al., 2016) However, this has not yet translated into any new effective treatment options for patients.
Since their introduction in 2011, use of immune checkpoint inhibitors has improved overall survival rates for patients with other aggressive cancers including unresectable or metastatic melanoma and squamous non-small cell lung cancer. Ipilimumab, pembrolizumab, and nivolumab specifically are now recommended as part of first-line therapy for unresectable or metastatic melanoma in conjunction with chemotherapy and other treatment options. (La-Beck et al., 2015) In 2017 the FDA granted approval for the use of pembrolizumab, an immune checkpoint inhibitor, in the treatment of microsatellite instability (MSI)-high solid tumors, agnostic of tumor site or type. (Zhao et al., 2019) MSI is a reliable marker for mismatch repair (MMR) deficiency. Pembrolizumab targets a key receptor in the MMR pathway, PD-1. (Zhao et al., 2019) Other checkpoint inhibitors that target PD-1 and PD-L1 have been shown to have efficacy against renal cell carcinoma, bladder and urothelial cancers, squamous cell carcinoma of the head and neck, and Hodgkin lymphoma, and are being studied in the treatment of other cancers. (La-Beck et al., 2015)
We present the case of a woman with recurrent leiomyosarcoma who was found to be MSI-high who had a prolonged response to treatment with pembrolizumab.
2. Case
A 59-year-old G0 Caucasian female was diagnosed with Stage 2B leiomyosarcoma of the pelvic peritoneum after initial referral for right sided abdominal pain. Computed tomography (CT) imaging revealed a 30 × 23 × 23 cm pelvic mass with multiple noncalcified pulmonary nodules in the lung bases, and CA 125 of 63. She underwent exploratory laparotomy, total abdominal hysterectomy with bilateral salpingo-oophorectomy and suboptimal tumor debulking. Intraoperatively it was noted that the tumor arose from the pelvic peritoneum and was not attached to the uterus, which appeared normal. The decision was made not to proceed with further debulking, as complete resection could not be achieved due to residual disease requiring bladder resection and ileocecal resection. At the conclusion of the procedure, tumor remained in the pelvis, along the ileocecal valve, and a small volume along small and large bowel mesentery. Initial pathology described an undifferentiated pleomorphic sarcoma. The specimen was sent for additional testing to elucidate tumor subtype. Immunohistochemistry and molecular typing with both microarray analysis and NextGen Sequencing (NGS) confirmed leiomyosarcoma with massive genomic instability. The tumor was found to be MSI-high with loss of expression of MSH2, a homozygous deletion of Rb1, and Tp53 mutation (Fig. 1a, Fig. 1b, Fig. 1c, Fig. 1d, Fig. 2, Fig. 3). Further genetic testing was negative for hereditary non-polyposis colorectal cancer.
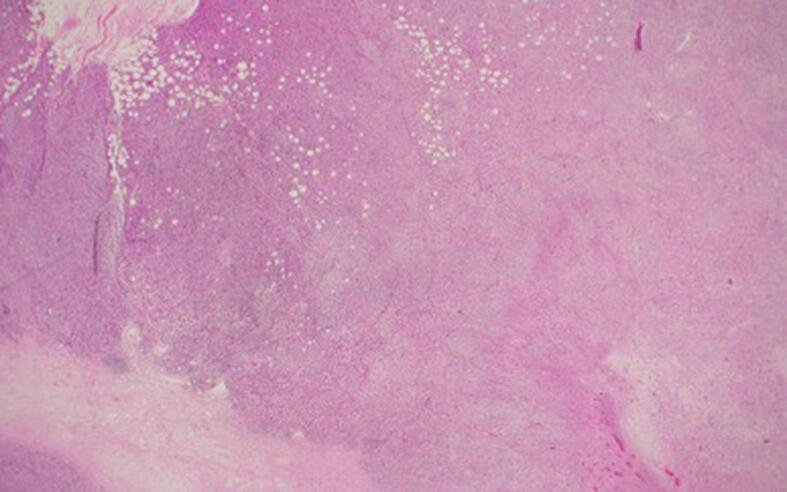
Fig. 1a.
20x magnification. Scanning power view of tumor shows areas of necrosis, infiltrating growth into fat, and spindle cell morphology in broad fascicles.
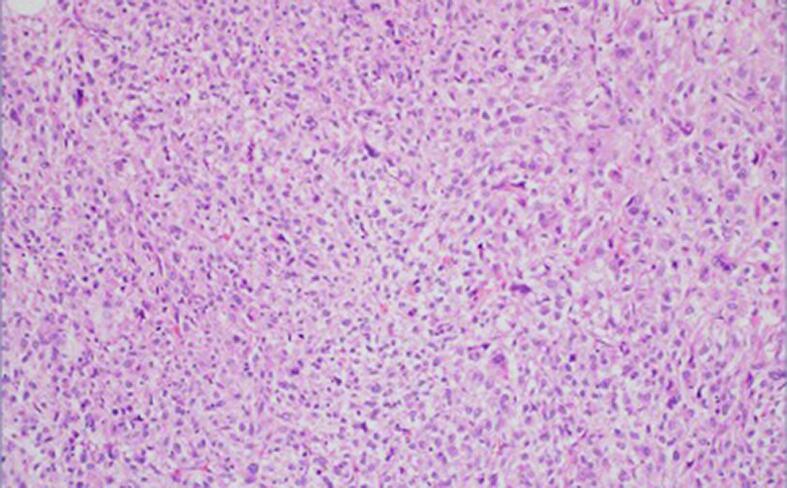
Fig. 1b.
200× magnification. Higher power view shows pleomorphism and nuclear atypia.
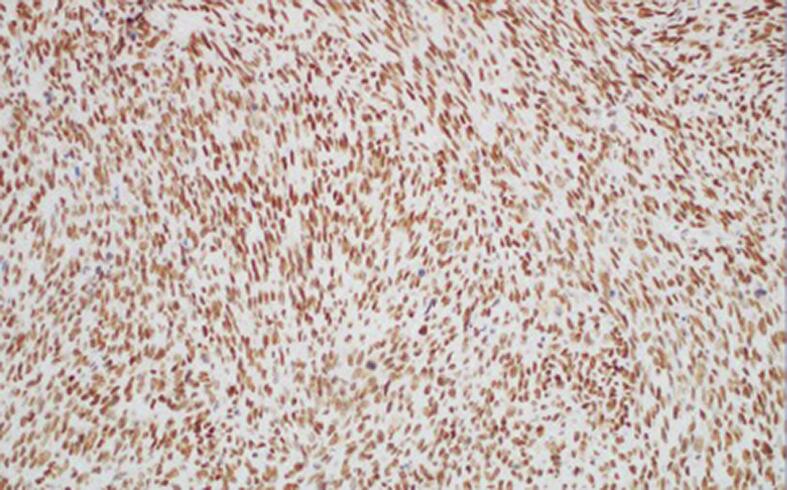
Fig. 1c.
200× magnification. PMS2 stain shown, with similar results with MLH1 and MSH6, with intact strong nuclear staining.
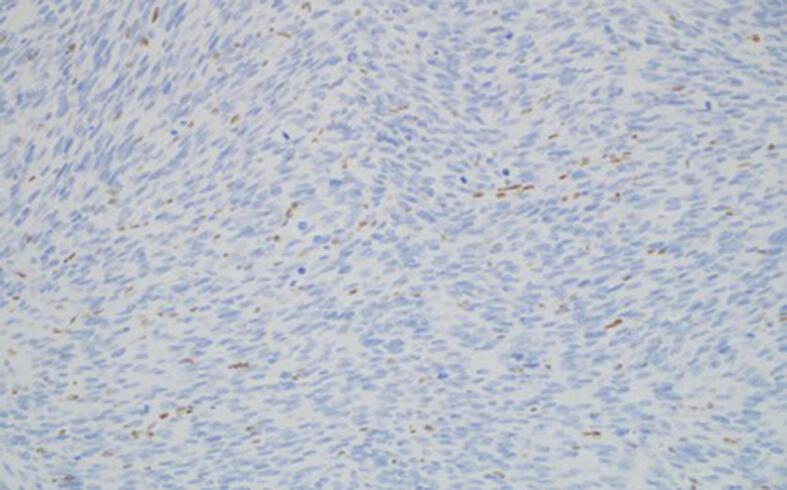
Fig. 1d.
200× magnification. MSH2 stain negative in tumor nuclei with background staining of nuclei of stromal and vascular endothelial cells.
Fig. 2.
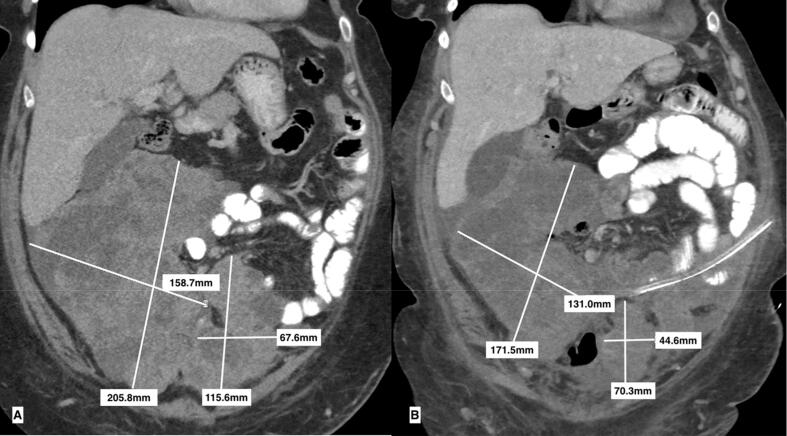
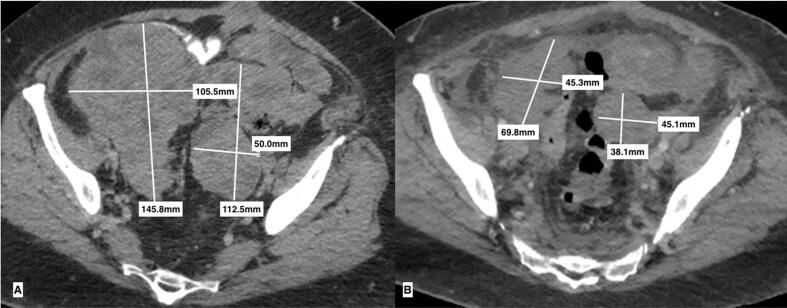
Coronal images of uterine leiomyosarcoma abdominopelvic tumors prior to starting pembrolizumab (a) and decreased size of abdominopelvic tumors after completing 3 cycles (b).
Fig. 3.
Axial images of uterine leiomyosarcoma abdominopelvic tumors prior to starting pembrolizumab (a) and decreased size of abdominopelvic tumors after completing 3 cycles (b).
The patient was then started on adjuvant treatment with gemcitabine and docetaxel. She completed 11 cycles with good response and stabilization of disease on imaging. Due to difficulties with neutropenia, she was transitioned to temozolomide 5 days monthly, which she tolerated well. The patient completed 3 cycles before CT showed progression of disease with development of large abdominopelvic tumors measuring 18.2 × 9.8 × 23.1 cm and 13.0 × 9.0 × 14.0 cm (Fig. 2, Fig. 3).
Ultimately the patient was admitted to the hospital for abdominal pain, large volume bloody ascites, anemia requiring transfusion, and vomiting. Her Eastern Cooperative Oncology Group (ECOG) status had declined from 1 to 3. Due to her poor performance status and known MSI-high status, the decision was made to start the patient on pembrolizumab 2 mg/kg every 3 weeks. During treatment she reported better energy, decreased nausea and vomiting, increased appetite, and decreased abdominal pain and swelling. She had resolution of ascites and within 1.5 months of starting treatment with pembrolizumab, her performance status improved to ECOG 1.
After completion of cycle 3, CT imaging showed decreased size of the abdominopelvic masses and identified a new solitary right upper lobe pulmonary metastasis measuring 1.4 cm (Fig. 2, Fig. 3). Given drastic increase in performance status, significant improvement in symptom control, and reduced abdominopelvic disease burden, our multidisciplinary tumor board recommended continuation of therapy with pembrolizumab despite the newly identified pulmonary disease. Ultimately CT imaging after completion of 7 cycles demonstrated an increase in pulmonary and pelvic metastases.
The patient received two 28-day cycles of doxorubicin with olaratumab and progressed. She then received 1 cycle of cisplatin, ifosfomide, and doxorubicin but decided to stop treatment and enroll in hospice. Her overall survival was 22 months.
3. Discussion
Uterine leiomyosarcoma is a rare tumor known for early hematogenous spread and metastasis, especially to the lungs. Current treatments for ULMS are limited to surgical intervention, conventional chemotherapy regimens, and targeted radiation, partly because the molecular changes leading to ULMS are not well understood. Many genes have been identified as being involved, including c-MYC, Bcl-2, K-ras, Ki-67, p16, p53, Rb1, ING2, D14S267, CDC7, CDC20, GTSE1, CCNA2, CCNB1, and CCNB2. (Barlin et al., 2015) However, few cases of MSI-high ULMS have been documented, with 11 cases by Risinger et al. (Risinger et al., 1995) in 1995, and 0 cases of high-frequency MSI with 4 cases of low-frequency MSI by Amant et al. (Amant et al., 2001) in 2001. There have been some documented cases of treating ULMS with PD-1 inhibitors pembrolizumab and nivolumab which have demonstrated varied results (Table 1).
Table 1.
Three reports of immunotherapy in the treatment of ULMS.
| Author | Demographic | Previous Treatments | Treatment | Prognosis | Side Effects |
|---|---|---|---|---|---|
| George et al. (2017) | 48 y/o F | Surgical resection of primary tumor | Pembrolizumab 10 mg/kg every 2 weeks, 9 cycles total with surgical resection of 1 unresponsive metastasis | Complete remission >2 years |
|
| Cousin et al. (2016) | 55 y/o F | Subtotal hysterectomy, doxorubicin | Pembrolizumab and oral cyclophosphamide per NCT02406781, 2 cycles total | Progression within 2 cycles | Pulmonary sarcoidosis |
| Ben-Ami et al. (2017) | 29–73 y/o F (12 participants) |
Surgical resection of primary tumor, 1–6 prior lines of chemotherapy | Nivolumab 3 mg/kg every 2 weeks | Progression in 9 participants, 5 of them within 2–4 cycles | 4 patients with grade 3 + toxicity related to the drug, including increased serum amylase or lipase |
George et al. (George et al., 2017) describes a case that showed complete remission of metastatic ULMS after 9 cycles of pembrolizumab as monotherapy in a treatment-naïve patient. However, two other cases showed not only progression of the patients’ ULMS within just a few cycles, but also significant side effects of pembrolizumab and nivolumab, including increased serum amylase and lipase, and pulmonary sarcoidosis. (Cousin et al., 2016, Ben-Ami et al., 2017) Even though these cases demonstrate a mixed response to pembrolizumab, there is still reason to investigate the use of pembrolizumab in patients with ULMS who have either MSI high tumors, PD-1 or PD-L1 positive tumors, or tumors with high mutational burden.
After first-line therapy with gemcitabine and docetaxel, there are limited treatment options for unresponsive, recurrent, or metastatic ULMS. The National Comprehensive Cancer Network (NCCN) recommends enrollment in a clinical trial after failing first-line therapy for ULMS. Current second-line treatments are mainly limited to standard chemotherapy with ifosfamide or doxorubicin, whose response rates are 17% and 25% respectively, with significant side effects. (O'Cearbhaill and Hensley, 2010) Few therapies have shown meaningful improvement in progression-free survival (PFS) or overall survival (OS). There is therefore a significant need for further investigation of immunotherapy and other targeted therapies in the treatment of ULMS.
Though we cannot demonstrate an improved OS in this case, the significance of this patient’s improved functional status and quality of life cannot be overstated. With ECOG 3 prior to pembrolizumab treatment, this patient would most likely have been ineligible for a clinical trial and would not have tolerated cytotoxic chemotherapy, leaving her with limited options. The use of immunotherapy offered a treatment that was well tolerated, led to resolution of severe disease-related symptoms, and gave her 5 months of improved quality of life, while ultimately attaining a functional status that again made chemotherapy an option.
Our patient notably presented with large volume tumor burden, both at initial diagnosis and at the time of abdominopelvic recurrence. The initial designation as undifferentiated sarcoma and massive genomic instability on NGS may indicate a high level of aggressiveness in this tumor. We may postulate that with smaller volume tumors or fewer mutational changes, patients may have a more robust or prolonged response to immunotherapy treatment.
Based on our case and cases in current literature, we think it would be beneficial to test ULMS tumors for MSI status, PD-L1/PD-1 expression. This would allow us to identify and treat patients that may benefit from immune checkpoint inhibitor therapy in an effort to extend overall survival in a disease with poor prognosis and limited treatment options.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Amant F., Dorfling C.M., Dreyer L., Vergote I., Lindeque B.G., Van Rensburg E.J. Microsatellite instability in uterine sarcomas. Int. J. Gynecol. Cancer. 2001;11(3):218–223. doi: 10.1046/j.1525-1438.2001.01013.x. PMID: 11437928. [DOI] [PubMed] [Google Scholar]
- Barlin J.N., Zhou Q.C., Leitao M.M., Bisogna M., Olvera N., Shih K.K., Jacobsen A., Schultz N., Tap W.D., Hensley M.L., Schwartz G.K., Boyd J., Qin L.X., Levine D.A. Molecular subtypes of uterine leiomyosarcoma and correlation with clinical outcome. Neoplasia. 2015;17(2):183–189. doi: 10.1016/j.neo.2014.12.007. PMID: 25748237; PMCID: PMC4351299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami, E., Barysauskas, C.M., Solomon, S., Tahlil, K., Malley, R., Hohos, M., Polson, K., Loucks, M., Severgnini, M., Patel, T., Cunningham, A., Rodig, S.J., Hodi, F.S., Morgan, J.A., Merriam, P., Wagner, A.J., Shapiro, G.I., George, S., 2017. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer., 123, 17, 3285–3290. http://doi.org/10.1002/cncr.30738. Epub 2017 Apr 25. PMID: 28440953; PMCID: PMC5762200. [DOI] [PMC free article] [PubMed]
- Cousin S., Toulmonde M., Kind M., Cazeau A.L., Bechade D., Coindre J.M., Italiano A. Pulmonary sarcoidosis induced by the anti-PD1 monoclonal antibody pembrolizumab. Ann. Oncol. 2016;27(6):1178–1179. doi: 10.1093/annonc/mdw125. Epub 2016 Apr 18 PMID: 27091806. [DOI] [PubMed] [Google Scholar]
- George S., Miao D., Demetri G.D., Adeegbe D., Rodig S.J., Shukla S., Lipschitz M., Amin-Mansour A., Raut C.P., Carter S.L., Hammerman P., Freeman G.J., Wu C.J., Ott P.A., Wong K.K., Van Allen E.M. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity. 2017;46(2):197–204. doi: 10.1016/j.immuni.2017.02.001. PMID: 28228279; PMCID: PMC5408320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La-Beck N.M., Jean G.W., Huynh C., Alzghari S.K., Lowe D.B. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy. 2015;35(10):963–976. doi: 10.1002/phar.1643. Erratum. In: Pharmacotherapy. 2015 Dec; 35(12):1205 PMID: 26497482. [DOI] [PubMed] [Google Scholar]
- Momtahen, S., Curtin, J., Mittal, K., 2016. Current Chemotherapy and Potential New Targets in Uterine Leiomyosarcoma. J. Clin. Med. Res., 8, 3, 181–189. http://doi.org.10.14740/jocmr2419w. Epub 2016 Jan 26. PMID: 26858789; PMCID: PMC4737027. [DOI] [PMC free article] [PubMed]
- O'Cearbhaill R., Hensley M.L. Optimal management of uterine leiomyosarcoma. Expert Rev. Anticancer Ther. 2010;10(2):153–169. doi: 10.1586/era.09.187. PMID: 20131992. [DOI] [PubMed] [Google Scholar]
- Ricci S., Stone R.L., Fader A.N. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol. Oncol. 2017;145(1):208–216. doi: 10.1016/j.ygyno.2017.02.019. Epub 2017 Feb 13 PMID: 28209496. [DOI] [PubMed] [Google Scholar]
- Risinger J.I., Umar A., Boyer J.C., Evans A.C., Berchuck A., Kunkel T.A., Barrett J.C. Microsatellite instability in gynecological sarcomas and in hMSH2 mutant uterine sarcoma cell lines defective in mismatch repair activity. Cancer Res. 1995;55(23):5664–5669. PMID: 7585651. [PubMed] [Google Scholar]
- Zhao P., Li L., Jiang X., Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019;12(1):54. doi: 10.1186/s13045-019-0738-1. PMID: 31151482; PMCID: PMC6544911. [DOI] [PMC free article] [PubMed] [Google Scholar]