Abstract
BRCA1/2 pathogenic variant prevalence in Japanese breast cancer is unclear. Here, we analyzed BRCA1/2 pathogenic variant prevalence with a particular focus on age factors, using the Japanese HBOC consortium database. All registered subjects were Japanese individuals who underwent BRCA1/2 genetic testing from January 1996 to July 2017 according to the Japanese HBOC consortium database. Cases were extracted and analyzed for each evaluation item. Overall BRCA1 and BRCA2 pathogenic variant prevalence was 11.2% and 9.0% in the cohort of 2366 proband patients, respectively. The age at onset of breast cancer for patients with BRCA1/2 pathogenic variants was significantly lower than that for patients without a BRCA1/2 pathogenic variant. In both BRCA1/2 patients, ages at onset were not statistically significantly different between two subtype groups (ER-positive vs. TNBC). We analyzed the BRCA1/2 pathogenic variant prevalence among age groups in patients with no family history of breast or ovarian cancer. In the TNBC group, the rate of genetic variants was more frequent among younger patients. Our results demonstrated that early breast cancer onset is associated with a BRCA1/2 pathogenic variant in the Japanese population. Younger TNBC patients were more likely to have a BRCA1/2 pathogenic variant irrespective of a family history of breast or ovarian cancer.
Subject terms: Breast cancer, Cancer epidemiology
Introduction
Breast cancer is the most commonly diagnosed cancer in both developed and less-developed countries. Various risk factors for breast cancer such as age, reproductive history, and lifestyle factors have been known [1], however, the causes of individual breast cancers are mostly unknown. Furthermore, ~10% of breast cancer cases are thought to be hereditary, and about 25% of these are caused by inherited variants in the tumor suppressor genes BRCA1 and BRCA2 (BRCA1/2) [2, 3]. Hereditary breast and ovarian cancer syndrome (HBOC) is an autosomal dominant inherited cancer susceptibility disorder caused by deleterious germline variants in BRCA1 or BRCA2. Nowadays, about 1 in 8 women in the U.S. (about 12.8%) and 1 in 10 Japanese women (about 10.2%) will develop infiltrated breast cancer over the course of their lifetime [4, 5]. In contrast, retrospective studies suggest an estimated cumulative risk of breast cancer to 70 years of age of 40–87% for BRCA1 variant carriers and 27–84% for BRCA2 variant carriers [3, 6–14]. There have been various reports from around the world on the statistics for each race, but in many cases the detailed statistics have not been disclosed for the Japanese population.
In October 2012, the Japanese HBOC Consortium (JHC) was established to raise awareness about HBOC and to provide integrate healthcare for patients with HBOC and their families in Japan. We established a registration committee for JHC in October 2013 and pushed it forward as a nationwide registration project. The first report from JHC was published by Arai et al., and they reported genetic and clinical characteristics in Japanese patients with HBOC [15]. This database is becoming increasingly crucial for HBOC research in Japan as the number of cases increases each day.
One of the clinical characteristics of hereditary tumors is its early age of onset. As it is also one of the Lynch syndrome criteria [16, 17], young-onset disease has been revealed as a very important clinical feature in hereditary breast cancer [18, 19]. It has been reported that women with germline mutations are more prone to develop breast cancer at a younger age with more aggressive subtypes than those without germline variants [20–22], but almost all reports are from foreign countries; only a few studies have used the Japanese cohort.
Here, we analyzed the cases of BRCA-associated breast cancer with BRCA1/2 germline pathogenic variants registered in the JHC database. We especially focused on age factors.
Patients and methods
Study registration
The subjects enrolled were all Japanese patients who underwent BRCA1/2 genetic testing from January 1996 to July 2017 in the participating facilities of the JHC. Ethical approval for this study was obtained from the Ethical Review Boards of the JHC and each institution. Written informed consent was obtained from all subjects. From all the registered cases, we extracted and analyzed the data from patients who have information such as their age at breast cancer onset, family history, and pathological features, that were required for each analysis.
BRCA1 and BRCA2 gene testing
All BRCA1 and BRCA2 genetic testing (sequence and large rearrangement analysis) were performed at Myriad Genetic Laboratories or FALCO Biosystems. The detected variants were interpreted according to the criteria of Myriad Genetic Laboratories.
Clinical subtypes of BRCA-related breast cancer
Based on the registered clinical data, we examined what subtypes of cancer were frequently observed in Japanese BRCA1/2 mutation carriers. Subtypes were determined based on estrogen receptor (ER), progesterone receptor, and human epidermal growth factor-2 (HER2) Immunohistochemistry (IHC) status. If the HER2 IHC result is 2+ and equivocal, the Fluorescence In Situ Hybridization assay for determination of HER2 status was performed. Since very few HER2-positive cases were found, this study compared the ER-positive (ER+) group and the triple-negative breast cancer (TNBC) group.
Statistical analysis
Statistical significance was analyzed by one-way analysis of variance and the Student’s t test using R software (http:///www.rproject.org/) and Bioconductor (http://bioconductor.org/). Results were considered statistically significant at p < 0.05.
Results
Patient demographics and variant prevalence in the JHC cohort
The total number of registrations is 11,711 Japanese individuals between January 1996 and August 2017, including their relatives who have not received genetic testing.
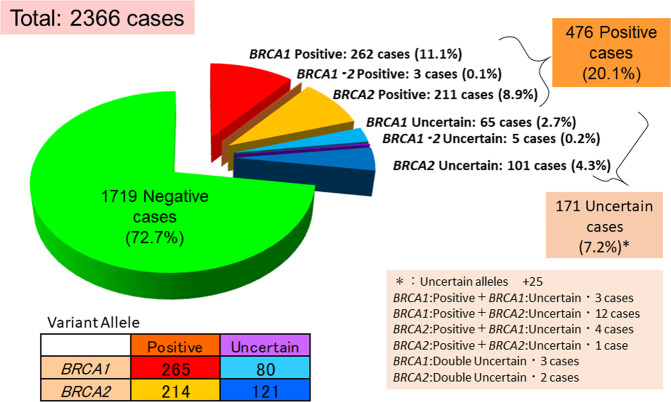
Out of all those registered, 2366 cases were the probands who underwent genetic testing, and the pathogenic variant detection rate was evaluated only for these cases. As a result, 265 (11.2%) cases had a BRCA1 pathogenic variant, 214 (9.0%) cases had a BRCA2 pathogenic variant, and 3 (0.1%) cases had both BRCA1 and BRCA2 pathogenic variants (Fig. 1). This database includes 2054 female patients with breast cancer, 89 female patients with ovarian cancer patients, 62 female patients with both breast and ovarian cancers, 14 male breast cancer patients, and 147 individuals who had neither breast nor ovarian cancer. We compared the rate of each cohort with previous reports [23–37] (Table 1).
Fig. 1.
The prevalence of BRCA1 and BRCA2 variant among the cases who underwent the screening test. The total number of cases was 2366
Table 1.
Comparison with previous reports
| Study | Country | Year | Race | High-risk or sporadic | Total | BRCA1+ | BRCA2+ | BRCA1/2+ | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | (%) | Cases | (%) | Cases | (%) | |||||||
| Sugano et al. | Japan | 2008 | Japanese | h | 122 | 16 | (13.1) | 18 | (14.8) | 34 | (27.9) | [23] |
| Hall et al. | USA | 2009 | Mixed | h | 46,276 | 3351 | (7.2) | 2444 | (5.3) | 5795 | (12.5) | [26] |
| Weitzel et al. | USA | 2012 | Mixed | h | 746 | 124 | (16.6) | 65 | (8.7) | 189 | (25.3) | [27] |
| Couch et al. | USA | 2015 | Mixed | h | 1824 | 155 | (8.5) | 49 | (2.7) | 204 | (11.2) | [25] |
| Kang et al. | South Korea | 2015 | Korean | h | 2403 | 157 | (6.5) | 224 | (9.3) | 378 | (15.7) | [31] |
| Kast et al. | Germany | 2016 | Germany | h | 59,304 | 10,195 | (17.2) | 5542 | (9.3) | 15,737 | (26.5) | [32] |
| Kemp et al. | UK | 2019 | NA | h | 1184 | 57 | (4.8) | 60 | (5.1) | 117 | (9.9) | [37] |
| Okano et al. | Japan | 2020 | Japanese | h | 2,366 | 262 | (11.1) | 211 | (8.9) | 476 | (20.1) | |
| Tung et al. | USA | 2015 | Mixed | mixed | 1,781 | 78 | (4.4) | 87 | (4.9) | 165 | (9.3) | [30] |
| Buys et al. | USA | 2017 | Mixed | mixed | 35,409 | 814 | (2.3) | 828 | (2.3) | 1642 | (4.6) | [34] |
| Palomba et al. | Italy | 2014 | Italian | s | 726 | 7 | (1.0) | 14 | (1.9) | 21 | (2.9) | [28] |
| Ghadirian et al. | Canada | 2014 | French-Canadian | s | 1093 | 13 | (1.2) | 43 | (3.9) | 56 | (5.1) | [29] |
| Susswein et al. | USA | 2016 | Mixed | s | 5209 | 63 | (1.2) | 73 | (1.4) | 136 | (2.6) | [33] |
| Wen et al. | Malaysia | 2017 | Asian | s | 2575 | 55 | (2.1) | 66 | (2.6) | 121 | (4.7) | [35] |
| Momozawa et al. | Japan | 2018 | Japanese | s | 7051 | 102 | (1.45) | 191 | (2.71) | 293 | (4.16) | [36] |
Age of breast cancer onset is lower in patients with BRCA1/2 pathogenic variants
Since the age of cancer onset is one of the most important characteristics of hereditary breast cancer, we investigated whether it was associated with the presence or absence of the BRCA1/2 variant.
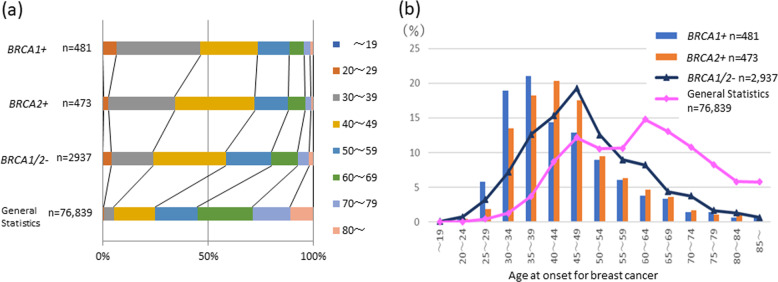
In our database, there were 3891 cases who underwent genetic testing and whose data clearly reported age of onset. Figure 2 and Supplementary Table 1 show the distribution of age at onset for breast cancer among different age groups. The general statistical data for comparison were data from the 2017 Japanese cancer statistics [38]. The mean age of onset was 43.6, 45.2, 48.8 years in the BRCA1 variant group, BRCA2 variant group, and BRCA1/2 pathogenic variant free groups, respectively (Table 2). A statistically significant earlier age of onset was observed in the group of patients with the BRCA1/2 pathogenic variant compared to the group of patients without the BRCA1/2 pathogenic variant (Table 2).
Fig. 2.
a Comparison of frequency distribution by age at onset of breast cancer in cases with or without BRCA1/2 variants and the general statistics. b Age distribution of the cases with or without BRCA1/2 variants
Table 2.
The mean of age onset of each group
| Gene variant | n (%) | Age at onset (year) | Pairwise comparison p value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | BRCA1+ | BRCA2+ | BRCA1/2− | ||
| BRCA1+ | 481 | 43.6 ± 12.5 | 21–99 | – | ||
| BRCA2+ | 473 | 45.2 ± 11.1 | 19–84 | 0.0175 | – | |
| BRCA1/2− | 2937 | 48.8 ± 12.6 | 17–95 | <0.001 | <0.001 | – |
SD standard deviation
In both BRCA1/2 positive patients, age at onset did not statistically differ between ER-positive group and TNBC group
Furthermore, we examined the relationship between the breast cancer clinical subtype and each BRCA1/2 pathogenic variant using a group of patients with subtype information. In our cohort, 971 ER+ patients and 515 TNBC patients had IHC information that could be analyzed. The mean age at breast cancer onset of patients with the BRCA1 pathogenic variant, with the BRCA2 mutation, and without the BRCA1/2 pathogenic variant in the ER+ group were 41.6, 41.8, and 46.4 years, respectively. There was a statistically significant difference in the age of onset between the group with BRCA1/2 variant and the group without BRCA1/2 variant in both ER+ and TN subgroup (p < 0.01). In TNBC group, the average ages were 40.8, 41.7, and 45.6 years, respectively. There was no statistically significant difference between the two subtype groups in each group (Table 3).
Table 3.
Subtype details and onset age in each BRCA condition
| Gene variant | Subtype | n | Age at onset | p value | |
|---|---|---|---|---|---|
| Mean ± SD | Range | ||||
| BRCA1+ | Luminal | 30 | 41.6 ± 10.7 | 25–71 | NS |
| TNBC | 147 | 40.8 ± 9.8 | 23–75 | ||
| BRCA2+ | Luminal | 97 | 41.8 ± 9.5 | 23–73 | NS |
| TNBC | 25 | 41.7 ± 12.3 | 19–71 | ||
| BRCA1/2− | Luminal | 844 | 46.4 ± 10.5 | 22–83 | NS |
| TNBC | 343 | 45.6 ± 11.6 | 22–85 | ||
SD standard deviation
The prevalence of pathogenic variants is more frequent among younger patients in the TNBC group even with no family history
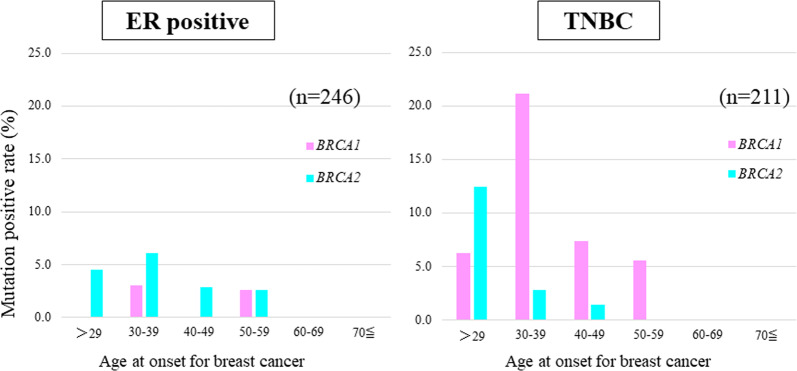
The relationship between subtype and age with no family history is also interesting. We analyzed the prevalence of pathogenic variants among age groups in cases with no family history of breast and ovarian cancer. There were 246 ER+ cases and 211 TNBC cases in the cohort where we extracted patients that had no family history and had the onset age and subtype information.
Figure 3 and Supplementary Tables 2 and 3 show that the rate of BRCA1/2 pathogenic variant showed no trend by age in the ER-positive group. On the other hand, the prevalence of pathogenic variants was higher among younger patients in the TNBC group. Also, no variant in both genes was observed in this analysis past the age of 60.
Fig. 3.
The prevalence of BRCA1 and BRCA2 variants stratified by subtype and age groups in the cases with no family history of breast or ovarian cancer. Red bars indicate BRCA1 variants and blue bars indicate BRCA2 variants
Discussion
The purpose of this study was to characterize breast cancer patients with BRCA1/2 variants in a Japanese-only cohort, with a particular focus on age factors. To date, only a few studies have examined the details of the prevalence of BRCA1/2 variants in a Japanese-only cohort.
In Japan, Sugeno et al. were the first to report the prevalence of BRCA1/2 variants. In the study, the prevalence of deleterious variants in a cohort of Japanese women was considered to be about 1.9 times higher than that in non-Ashkenazi American women, provided that background factors such as medical history and family history are similar [23, 24].
In our result, the prevalence rate in a Japanese high-risk cohort has decreased (27.9–20.1%) with the increase in the size of the cohort, which is closer to the actual clinical situation. Nonetheless, one in five in the Japanese high-risk group still had BRCA1/2 variants. This seems to be a slightly higher rate than reports from other countries analyzing high-risk cohorts (Table 1). It is highly likely that our cohort included the cases with higher risk because BRCA1/2 genetic testing was not covered by the national health insurance in Japan, until recently. Now that the tests are covered by national health insurance, further studies are required.
Momozawa et al. investigated the prevalence of BRCA1/2 variants in sporadic breast cancer cohort and control cohort of healthy women in 2018 [36]. According to this report, the prevalence of BRCA1/2 variants in 7051 Japanese patients with breast cancer was about 4.16%, which was clearly lower than our data. Although it is important to investigate the prevalence of gene mutations in sporadic breast cancer, in clinical practice, our data, which includes many high-risk cases, are more likely to be useful when clinicians consider whether genetic testing should be recommended for individual cases with suspected familial breast cancer based on these factors: clinical presentation, family history, age, and subtype.
The age factor is one of the testing criteria of the NCCN guidelines for familial breast and ovarian cancer [39]. Although it is clear that there is a strong association between familial breast cancer and a younger onset of the disease, it has also been reported there were differences among races and ethnicities [26, 40] in this aspect. There are reviews from various races, but many have no clear family history, or the cohort conditions are different [41]. It is necessary to confirm the details in a large cohort of the Japanese population. Our data showed that breast cancers with BRCA1/2 mutations were also diagnosed at a younger age in a Japanese cohort, and particularly those with BRCA1 mutations had a younger age at onset (43.6 years). Compared to the previous reports, the age of onset in BRCA patients of our cohort was slightly higher than those of previous studies (around 42 years) [42, 43]. Study populations of these reports consisted of dominantly Caucasian/white patients. The racial differences about the effect of BRCA variants on the age of onset or other clinical characteristics have been largely unknown. Thus, further studies, especially in non-Caucasian race populations, are warranted for the better understanding of clinical features of BRCA variants.
There have been many reports of the association between BRCA-related breast cancer and its subtypes. Several studies have demonstrated that BRCA1-associated tumors are usually TNBC and BRCA2 mutation carriers commonly develop ER-positive breast cancer [43–45]. A similar pattern was reported in metastatic and recurrent breast cancer [46]. However, only a few reports have included age factors. Data from Singapore showed that TNBC patients with BRCA1 variants were diagnosed at a relatively younger age than non-TNBC patients with the same gene variants (38 vs. 46 years, p = 0.028) [47]. On the other hand, there was no difference in the age of onset between luminal type BC and TNBC in our present study (both around 41 years) (Table 3). Though both cohorts are Asian and high-risk groups with a family history and/or early onset, this discrepancy may have occurred due to differences in race and other environmental factors.
Furthermore, our data showed that TNBC onset in a patient’s fourth decade of life can have BRCA1 variants in more than 20% of those without family history, even in a Japanese cohort. On the other hand, no cases had BRCA1/2 variants in patients over 60 years with no family history of breast and ovarian cancer in our cohort. The data from Tung et al. and Momozawa et al. showed that the prevalence of pathogenic variants in breast cancer-related genes in patients aged 60 and older is 6.4% and 4.1%, respectively, but these data may include cases with a family history [36, 42]. A study from Germany showed that there was a 6.9% prevalence of BRCA1/2 variants in TNBC patients aged 60–69 years [48] where the authors concluded that TNBC patients diagnosed before the age of 50 years with no familial history of breast and ovarian cancer should be tested for BRCA variants. A report by Cough et al. demonstrated that the prevalence of BRCA1/2 variants in patients without a family history that are 60 years older in Western populations was only 2%, even in TNBC. Although the number of TNBC patients without family history of breast and ovarian cancers were limited in our cohort and the results should be confirmed in larger studies, this information could potentially reduce the number of patients undergoing tests that bring no potential benefit. In addition, although it is now covered by national health insurance, a BRCA1/2 gene test costs around 2000 USD in Japan. Considering that about 86% of Japan’s national health expenditure is covered by public medical insurance and medical expenses are increasing annually, it is better to avoid unnecessary genetic testing. The availability of olaparib, which has proven to be effective for the treatment of HER2-negative metastatic breast cancer with a pathogenic variant in BRCA1/2 [49], has steadily increased the number of germline gene variant tests. BRCA genetic testing is recommended in any age for the use of olaparib in HER2-negative metastatic breast cancer. It is therefore necessary for health care professionals to provide correct information and appropriate genetic testing for their patients, which would have both clinical and economic benefits.
This study has some limitations. A major limitation of our study is the relatively small cohort. It is expected that more cases will be registered since BRCA1/2 pathogenic variant testing is now covered by national health insurance in Japan. Second, as mentioned above, there is a possibility of the bias that there are more patients at higher risk. Due to the high cost of genetic testing, patients who are not high-risk may have refrained from genetic testing in Japan. Breast cancers with BRCA2 variants have a higher age of onset than breast cancers with BRCA1 variants [50] and have weaker hereditary cancer characteristics, which may reduce its proportion in high-risk cohorts. These data can be seen in Table 1.
Conclusion
Our data indicate that breast cancer with a BRCA1/2 mutation has an early onset even in the Japanese population. Furthermore, more than 20% of young TNBC patients had a BRCA1/2 mutation even if they have no family history of breast and ovarian cancer.
Supplementary information
Acknowledgements
Registration Committee, Japanese HBOC Consortium [Committee]: Seigo Nakamura (Showa University), Takayuki Enomoto (Niigata University), Tadashi Nomizu (Hoshi General Hospital), Akihiro Sakurai (Sapporo Medical University), Masayuki Sekine (Niigata University), Hiroyuki Nomura (Fujita Medical University), Megumi Ohkawa (St. Luke’s International Hospital), Junko Yotsumoto (International University of Health and Welfare), [Data center] Chie Watanabe (Sophia University), [Office] Shiro Yokoyama (Showa University). Participating Facilities of Nationwide registration projects: Showa Medical University Hospital, St. Lukes Hospital, Cancer Institute Hospital, Hoshi General Hospital, Shikoku Cancer Center, Hokkaido Cancer Center, Sapporo Medical Center, Niigata University Faculty of Medicine, Keio University, Kitano Hospital, Kochi University Faculty of Medicine, Tawara IVF Clinic, Kochi Medical Center, Nagoya University Hospital, St. Marianna University School of Medicine, Asahikawa Medical University, Aichi Cancer Center Hospital, Saku Medical Center, Tsukuba Medical University Hospital, Yokohama City University Hospital, Fujita Medical University Hospital, Ehime University Hospital, Gunma Prefectural Cancer Center, Nagasaki University Hospital, Shinshu University Hospital, Juntendo University Hospital, Kitazato University Hospital, Aichi Cancer Center Aichi Hospital, Okayama University Hospital, Saitama Medical University International Medical Center, Tokyo Medical Center, Tokyo Metropolitan Komagome Hospital, Japanese Red Cross Ishinomaki Hospital, Nagoya City University Hospital, Kawasaki Medical School Hospital, Yamanashi Prefectural Central Hospital, Nara Medical University Hospital, Kanagawa Cancer Center, Shiga Medical University, Shizuoka Cancer Center, Gifu University Hospital, Hiroshima Prefectural Hospital, Niigata Cancer Center Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Tottori University Hospital, Kansai Rosai Hospital, Kokura Medical Center, Nippon Medical School Musashi Kosugi Hospital, Kansai Medical University Hospital, Sagara Hospital Breast Center, Chiba University Hospital, Ibaraki Prefectural Hospital, Fukui University Hospital, Hokkaido University Hospital, Saitama Medical Center, Osaka University, Japanese Red Cross Yamaguchi Hospital, Kyoto University Hospital, Konan Kosei Hospital, Japanese Red Cross Medical Center, Shonan Memorial Hospital, Shizuoka General Hospital, Hiroshima University Hospital, National Hospital Organization Kyoto Medical Center, Shiroyama Hospital, Saitama Cancer Center, National Hospital Organization Osaka National Hospital, Kitakyushu Municipal Medical Center, Iwate Medical University, and Tokuyama Central Hospital.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s10038-020-00849-y) contains supplementary material, which is available to authorized users.
References
- 1.Breast Cancer Risk and Prevention. https://www.cancer.org/cancer/breast-cancer/risk-and-prevention.html. 2020.
- 2.Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. 2017;152:589–94. doi: 10.1001/jamasurg.2017.0552. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury AR, Olopade OI. Genetic susceptibility to breast cancer. Rev Endocr Metab Disord. 2007;8:255–67.. doi: 10.1007/s11154-007-9038-0. [DOI] [PubMed] [Google Scholar]
- 4.SEER Stat Fact Sheets. Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html. 2015–2017.
- 5.National Cancer Center Japan. https://ganjoho.jp/reg_stat/statistics/stat/summary.html. (2017).
- 6.Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457–66.. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30.. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begg CB, Haile RW, Borg A, Malone KE, Concannon P, Thomas DC, et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299:194–201.. doi: 10.1001/jama.2007.55-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brohet RM, Velthuizen ME, Hogervorst FB, Meijers-Heijboer HE, Seynaeve C, Collée MJ, et al. Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet. 2014;51:98–107. doi: 10.1136/jmedgenet-2013-101974. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–71.. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DG, Shenton A, Woodward E, Lalloo F, Howell A, Maher ER. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89.. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA. 2014;111:14205–10.. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 15.Arai M, Yokoyama S, Watanabe C, Yoshida R, Kita M, Okawa M, et al. Genetic and clinical characteristics in Japanese hereditary breast and ovarian cancer: first report after establishment of HBOC registration system in Japan. J Hum Genet. 2018;63:447–57.. doi: 10.1038/s10038-017-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch HT, Lynch PM. The cancer-family syndrome: a pragmatic basis for syndrome identification. Dis Colon Rectum. 1979;22:106–10.. doi: 10.1007/BF02586773. [DOI] [PubMed] [Google Scholar]
- 17.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 18.Nomizu T, Abe R, Tsuchiya A, Utsunomiya J, Watanabe F, Yamaki Y, editors. A clinical study of familial cancer in Japan. Berlin, Heidelberg: Springer Berlin Heidelberg; 1992. [Google Scholar]
- 19.Nomizu T, Tsuchiya A, Kanno M, Katagata N, Watanabe F, Yamaki Y, et al. Clinicopathological features of hereditary breast cancer. Breast Cancer (Tokyo, Jpn) 1997;4:239–42.. doi: 10.1007/BF02966513. [DOI] [PubMed] [Google Scholar]
- 20.Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64:830–5. doi: 10.1158/0008-5472.CAN-03-2970. [DOI] [PubMed] [Google Scholar]
- 21.Nicoletto MO, Donach M, De Nicolo A, Artioli G, Banna G, Monfardini S. BRCA-1 and BRCA-2 mutations as prognostic factors in clinical practice and genetic counselling. Cancer Treat Rev. 2001;27:295–304. doi: 10.1053/ctrv.2001.0233. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S, et al. Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. 2018;9:909. doi: 10.3389/fphar.2018.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugano K, Nakamura S, Ando J, Takayama S, Kamata H, Sekiguchi I, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci. 2008;99:1967–76.. doi: 10.1111/j.1349-7006.2008.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–90.. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 25.Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–11.. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–33.. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitzel JN, Clague J, Martir-Negron A, Ogaz R, Herzog J, Ricker C, et al. Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol. 2013;31:210–6. doi: 10.1200/JCO.2011.41.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palomba G, Budroni M, Olmeo N, Atzori F, Ionta MT, Pisano M, et al. Triple-negative breast cancer frequency and type of BRCA mutation: clues from Sardinia. Oncol Lett. 2014;7:948–52.. doi: 10.3892/ol.2014.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghadirian P, Robidoux A, Nassif E, Martin G, Potvin C, Patocskai E, et al. Screening for BRCA1 and BRCA2 mutations among French-Canadian breast cancer cases attending an outpatient clinic in Montreal. Clin Genet. 2014;85:31–5. doi: 10.1111/cge.12174. [DOI] [PubMed] [Google Scholar]
- 30.Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 31.Kang E, Seong MW, Park SK, Lee JW, Lee J, Kim LS, et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res Treat. 2015;151:157–68.. doi: 10.1007/s10549-015-3377-4. [DOI] [PubMed] [Google Scholar]
- 32.Kast K, Rhiem K, Wappenschmidt B, Hahnen E, Hauke J, Bluemcke B, et al. Prevalence of BRCA1/2 germline mutations in 21401 families with breast and ovarian cancer. J Med Genet. 2016;53:465–71.. doi: 10.1136/jmedgenet-2015-103672. [DOI] [PubMed] [Google Scholar]
- 33.Susswein LR, Marshall ML, Nusbaum R, Vogel Postula KJ, Weissman SM, Yackowski L, et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med. 2016;18:823–32.. doi: 10.1038/gim.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123:1721–30.. doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 35.Wen WX, Allen J, Lai KN, Mariapun S, Hasan SN, Ng PS, et al. Inherited mutations in BRCA1 and BRCA2 in an unselected multiethnic cohort of Asian patients with breast cancer and healthy controls from Malaysia. J Med Genet. 2018;55:97–103. doi: 10.1136/jmedgenet-2017-104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momozawa Y, Iwasaki Y, Parsons MT, Kamatani Y, Takahashi A, Tamura C, et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat Commun. 2018;9:4083. doi: 10.1038/s41467-018-06581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp Z, Turnbull A, Yost S, Seal S, Mahamdallie S, Poyastro-Pearson E, et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open. 2019;2:e194428. doi: 10.1001/jamanetworkopen.2019.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Center for Cancer Control and Information Services NCC. Cancer Statistics in Japan ‘17. 2017. https://ganjoho.jp/data/reg_stat/statistics/brochure/2017/cancer_statistics_2017.pdf.
- 39.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Genetic/familial high-risk assessment: breast and ovarian Version 1.2020. 2019.
- 40.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72–8. doi: 10.1097/GCO.0b013e328332dca3. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543–61.. doi: 10.2147/CLEP.S206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tung N, Lin NU, Kidd J, Allen BA, Singh N, Wenstrup RJ, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34:1460–8. doi: 10.1200/JCO.2015.65.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–8. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honrado E, Benitez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol. 2005;18:1305–20.. doi: 10.1038/modpathol.3800453. [DOI] [PubMed] [Google Scholar]
- 45.Musolino A, Bella MA, Bortesi B, Michiara M, Naldi N, Zanelli P, et al. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast. 2007;16:280–92.. doi: 10.1016/j.breast.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Barry WT, Seah DS, Tung NM, Garber JE, Lin NU. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer. 2020;126:271–80.. doi: 10.1002/cncr.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong ES, Shekar S, Chan CH, Hong LZ, Poon SY, Silla T, et al. Predictive Factors for BRCA1 and BRCA2 genetic testing in an Asian clinic-based population. PLoS ONE. 2015;10:e0134408. doi: 10.1371/journal.pone.0134408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engel C, Rhiem K, Hahnen E, Loibl S, Weber KE, Seiler S, et al. Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer. 2018;18:265. doi: 10.1186/s12885-018-4029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.