Abstract
Background
Older surgical patients have a higher risk of postoperative mortality and morbidity compared to younger patients. Timely identification of high-risk patients facilitates comprehensive preoperative evaluation, optimization, and resource allocation to help reduce this risk. This review aims to identify a preoperative screening tool for older patients undergoing elective surgery predictive of poor short-term postoperative outcomes.
Methods
A scoping review was conducted. An Ovid MEDLINE search was used to identify systematic reviews or meta-analyses comprising older elective patients in at least two different surgical settings. International guidelines were reviewed for recommendations regarding preoperative tools in this population.
Results
Over 50 screening tools were identified. The majority showed a positive association with short-term postoperative mortality and morbidity in older patients. The most commonly described tools were the American Society of Anesthesiologists Physical Status (ASA-PS), frailty tools and domain-specific tools administered as part of comprehensive geriatric assessment (CGA). Due to heterogeneity in outcome measures and statistical methodology the predictive capacity between tools could not be compared. International guidelines described a comprehensive preoperative approach incorporating domain-specific tools rather than recommending a screening tool.
Conclusion
Multiple tools were associated with poor short-term postoperative outcomes in older elective surgical patients. No single superior tool could be identified. Frailty, cognitive and/or functional tools were most frequently utilized.
Electronic supplementary material
The online version of this article (10.1007/s11739-020-02415-y) contains supplementary material, which is available to authorized users.
Keywords: Geriatric assessment, Aged, Screening tool, Preoperative care, Surgery, Frailty
Background
Older people constitute the most rapidly growing group throughout the developed world [1]. This evolving demographic shift has led to an increased demand for surgery in older patients [2]. Older patients are more likely to suffer from multi-morbidity, frailty, cognitive and functional impairment [3]. As a result, they have poorer postoperative outcomes including higher mortality and complication rates, a prolonged length of stay and increased likelihood of discharge to supported accommodation compared to younger patients [4, 5]. Equally important measures of recovery, such as health-related quality of life, are infrequently reported and poorly defined [6].
Efficient and effective screening of older patients who may be at increased risk of these poor postoperative outcomes is a current challenge facing clinicians and service providers. Identification of high-risk older patients aims to improve postoperative outcomes through targeted comprehensive geriatric assessment (CGA) and medical optimization, shared decision-making, engagement of the perioperative multidisciplinary team and allocation of critical care resources [7–9]. Conversely, not all older patients will benefit from these interventions.
There is lack of consensus on which screening tools should be applied to older patients in an elective surgical setting [10]. Although there is an abundance of tools in existence, many are narrowly targeted towards specific surgical subtypes or require specialist training to administer. Thus, a preoperative assessment tool that can be easily and broadly applied to older elective surgical patients with a high ability to predict poor postoperative outcomes is sought.
This scoping review aims to examine the ability of preoperative assessment tools to predict poor short-term postoperative outcomes in older patients undergoing elective surgery and to determine if a single best screening tool can be recommended in this cohort. We also aim to summarize recommendations for the use of these preoperative assessment tools in relevant international guidelines on the perioperative care of the older patient.
Methods
Given the broad research question with anticipated heterogenous results, a scoping review based on Arksey and O’Malley’s framework was conducted [11].
Search strategy
We searched Ovid MEDLINE for systematic reviews and meta-analyses of preoperative tools applied to older patients undergoing elective surgery published between January 2000 and 8 February 2019. The literature search was conducted with assistance from a health sciences librarian. Keywords were combined with MeSH search terms ‘surgical procedures, operative’, ‘elective surgical procedures’, ‘risk assessment or risk factors’, ‘outcome assessment (health care)’, ‘decision support techniques’, ‘postoperative complications’, ‘mortality’, ‘morbidity’, ‘length of stay’ and ‘treatment outcome’. The detailed search string is listed in electronic supplementary material. The inclusion of international guidelines was deemed necessary after the literature search of systematic reviews and meta-analyses lacked a clear consensus on which screening tools were best to use in the population of interest.
Review procedure
Two investigators (RA, NSH) screened the titles and abstracts and selected articles for full-text review. Full-text articles were then examined for eligibility. A third researcher (ABM) resolved any differences that could not be decided by consensus. A manual search of the references of eligible articles was also performed. In addition, relevant international guidelines evaluating older patients undergoing elective surgery were screened for recommendations regarding evidence-based preoperative tools.
Inclusion criteria
Eligible articles consisted of systematic reviews or meta-analyses in which the majority of study participants were older patients undergoing elective surgery. Older patients were defined as a population mean or median age of 60 years or older. If the age range was not stated in the review article, original articles were examined. Screening tools needed to be tested in at least two different elective surgical populations. This ensured the tools were not limited to a specific surgical group and were therefore more broadly applicable. Tools needed to be able to be completed preoperatively. Outcomes of interest were short-term mortality (inpatient mortality, 30-day or 90-day mortality), length of stay and measures of short-term postoperative morbidity such as postoperative complications, postoperative delirium, quality of life and discharge to a care facility.
Results
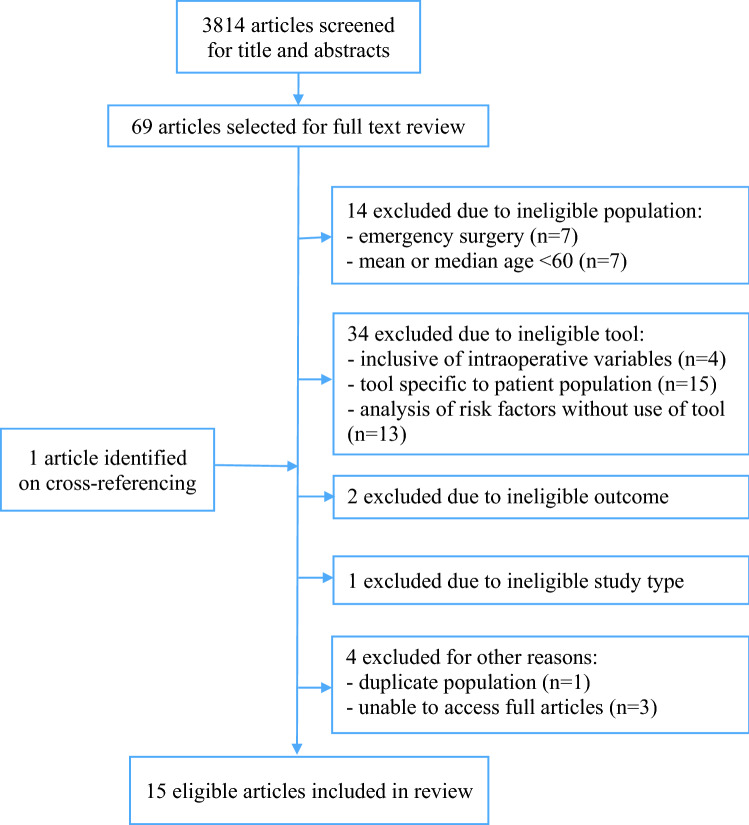
The literature search yielded 3814 articles. Screening of titles and abstracts resulted in 69 articles selected for full-text review. Following the exclusion of articles based on study type, patient population, tools and outcomes, 15 articles were selected for inclusion [12–26] as detailed in Fig. 1.
Fig. 1.
Flowchart of selection of articles for inclusion in review
More than 50 different preoperative tools were identified. The American Society of Anesthesiologists Physical Status (ASA-PS) tool, several frailty tools and domain-specific tools included as part of CGA were most frequently reported. Characteristics of the included studies are listed in Table 1. Tools and association with postoperative mortality and morbidity are detailed in Table 2.
Table 1.
Characteristics of systematic reviews and meta-analyses
| Author, year | Study design | Surgical Population | Urgency | Age, years | Articles, N |
Patients, N | Tool | Outcome |
|---|---|---|---|---|---|---|---|---|
| Abdullahi 2017 [12] | SR | Cardiac | Majority elective | Mean > 65 | 6 | 4819 | Gait speed, Katz IADL, Mini-Cog, CCI, Anemia, Geriatric syndrome of falls, CAF, FORECAST, TUGT, gait speed, Nagi scale | Mortality (inpatient, 30-day and 1 year), postoperative complications, length of stay |
| Buignes 2015 [13] | SR | Mixed major | Majority elective | Majority mean ≥ 60 | 32 | NA | mFI, Fried criteria, gait speed, CHS, MSSA4, CAF, Katz IADL, EFS, CGA |
Postoperative mortality, postoperative complications, Length of stay |
| Fagard 2016 [14] | SR | Colorectal cancer | Majority elective | Mean > 65 | 5 | 486 | Fried, GFI, CGA (Barthel index, NEADL, CIRS, polypharmacy, MNA, MMSE, GDS), Katz, TUGT, CCI, Anemia, Mini-Cog, Albumin, falls | Mortality (30-day, 1 year, 5 year), postoperative complications, length of stay, 30-day readmission |
| Hewitt 2018 [15] | MA & SR | Gastro-intestinal | Majorityelective | Mean age ≥ 60 | 9 | 2281 | Physical Frailty Phenotype, DAI, GFI, 7-point clinical frailty score | 30-day mortality, postoperative complications, length of stay |
| Huisman 2017 [16] | SR | Oncology | Elective | Mean ≥ 60 | 9 | NA | CGA domains: function (ADL impairment, ADL, IADL, Barthel, functional limitations, NEADL, falls, TUGT), nutrition (MNA, weight loss, MMC), cognition (MMSE, mini-cog), social support (MOS-SSS), mood (GDS, HADS, MHI), comorbidity (CIRS, SIC, CCI), polypharmacy (> 5), frailty (CGA-based, GFI, frailty phenotype) | Mortality (short-term, long-term, disease-free survival), postoperative complications |
| Lin 2016 [17] | SR | Mixed major | Majority elective | Mean > 75 | 23 | 17,117 | Fried, modified CHS, MSSA4, gait speed, Katz, SHERPA-risk, MMSE, MNA, TUGT, BADL, IADL, Comprehensive assessment of frailty, CAF, FORECAST, Balducci, frailty criteria, John Hopkins, CFS, Vulnerable elderly survey, MFS, Addenbrooke’s vascular frailty score, EFS, FI, mFI, KCCQ-OS QoL time | Mortality (in-hospital, 30 day and long term), postoperative complications, length of stay, discharge to institutional care, functional decline, QoL |
| Oldroyd 2017 [18] | MA & SR | Vascular | Majority Elective | > 65 | 16 | 3617 | Risk factors including ASA > 2 | Postoperative delirium |
| Panayi 2018 [19] | MA & SR | Mixed major | Majority elective | ≥ 60 | 16 | 683,487 | mFI | 30-day mortality, postoperative complications, readmission, discharged to facility |
| Partridge 2014 [20] | SR | Mixed major | Elective | Majority > 60 | 5 | 1364 | CGA tools: MMSE, Barthel, ADL, IADL, TUGT, MNA, clock, GDS, social support scale | Postoperative complications, length of stay, change in QoL |
| Sandini 2017 [21] | MA & SR | Mixed major | Elective | Mean age ≥ 65 | 35 | 1,153,684 | Frailty tools: tools not published in paper. Domains listed: activity, sarcopenia, comorbidities, nutrition, cognition, depression, walking incl | Mortality (90-day, 1 year), 30 day major morbidity |
| Scholz 2016 [22] | MA & SR | Gastro-intestinal | Elective, mixed | > 65 | 11 | 1427 | Risk factors including ASA > / = 3, CCI | Postoperative delirium |
| Sepehri 2014 [23] | SR | Cardiac | Majority elective | > 60 | 6 | 4756 | Fried, Katz IADL, CAF, mFI, CHS, MSSA4, gait speed, MMGA, MGBE (MMSE, MNA, TUGT, BADL) frailty tools | Mortality (in-hospital, all-cause, 1 year), MACCE, discharge to institution, functional decline |
| Visser 2015 [24] | SR | Mixed major | Majority elective |
Majority mean ≥ 60 |
30 | UNK | Risk factors including ASA grade | Postoperative mortality, postoperative complications |
| Warnell 2015 [25] | SR | Oesophagectomy | Elective | Majority mean > 60 | 20 | 13,887 | ASA, POSSUM, P-POSSUM, O-POSSUM, CCI, Karnofsky index | Mortality (in-hospital or 30 day) |
| Zhu 2017 [26] | MA | Head and neck cancer | Elective | Majority mean > 60 | 8 | 1940 (incl controls) | Risk factors including ASA ≥ 3 | Postoperative delirium |
IADL instrumental activities of daily living, CCI Charlson comorbidity index, CAF Comprehensive Assessment of Frailty, FORECAST Frailty predicts death One year after Elective Cardiac Surgery Test, TUGT timed up and go test, mFI modified frailty index, CHS cardiovascular health study frailty tool, MSSA4 4 item frailty scale (gait speed, handgrip strength, inactivity, cognitive impairment), EFS Edmonton frailty scale, GFI Groningen frailty indicator, NEADL Nottingham extended ADL scale, CIRS cumulative illness rating scale, SIC Seattle index of comorbidity, MNA mini-nutritional assessment, MMSE mini-mental status examination, DAI deficit accumulation index, MMC mid arm muscle circumference, MOS-SSS medical outcomes study social support survey, GDS geriatric depression scale, HADS hospital anxiety and depression scale, MHI mental health inventory, MFS Morse fall scale, MMGA modified multidimensional geriatric assessment, MGBE modified geriatric baseline examination
Table 2.
Predictive capacity of preoperative assessment tools
| Tool | Mortality | Morbidity and length of stay |
|---|---|---|
| ASA |
AUROC 0.64 [25] OR 1.54–11.6 [24] |
Postop complications: OR 1.77–7.1 [24] ASA > 2: OR 3.44 (2.02–5.87) [18] ASA-3: Clavien-Dindo 4 OR 6.8 [13] ASA ≥ 3: pooled OR 2.71 (1.64–4.48) [22] Delirium: ASA ≥ 3: OR 5.65 (1.57–20.36) [26] Cardiac arrest: ASA-3: OR 1.2, ASA-4: OR 3.5, ASA-5: OR 7.5 [13] Perioperative MI: ASA-3: OR 3, ASA-4: 6.9, ASA-5: 14.9 [13] |
| CAF | ≤ 11 30d mortality OR 1.1 (1.06–1.2) [12] | |
| CCI |
AUROC 0.57 [25] All-cause mortality HR 1.03 (0.9–1.17) [16] |
Postop complications: OR 0.93 (− 1.68–3.54) [22] |
| CGA assessment of frailty |
2 frailty markers: 6 mo mortality HR 3.86 (0.41–36.02)–8.88 (1.09–72.29) [16] ≥ 3 markers: 6 mo mortality HR 4.51 (0.49–41.25)–8.5 (1.1–65.87) [16] |
Postop complications: OR 3.13 (1.65–5.92)–6 [13, 16] RR 1.59 (1.25–2.01)–1.75 (1.28–2.41) [16] Length of stay LOS > 2 days OR 4.2 [13] |
| Fried | 30d mortality OR 2.67 (p = 0.029) [17] |
Postop complications: OR 2.54 (1.12–5.77) [13] Major Cx OR 3.13 (1.65–5.92)—4.1 [13] ≥ Clavien 2 Cx OR 4.08 (p = 0.006) [17] Mortality or procedural Cx OR 2.2 (p = 0.04) [17] QoL Mortality or poor QoL at 6 mo OR 2.21 (p = 0.03) [17] Length of stay LOS intermediately frail OR 1.49 (1.24–1.8) [13] |
| GFI | GFI ≥ 5 30d mortality ES 0.08 (0.02–0.21) [15] |
Postop complications: GFI ≥ 5 Postop Cx ES 0.15 (0.06–0.31) [15] GFI ≥ 3 ≥ Clavien 3a OR 3.62 [13] Length of stay GFI ≥ 5 ES 7.17 (6.02–8.54) [15] GFI ≥ 3 ES 15.8 (12.79–19.51) [15] |
| Katz IADL | Dependence in ≥ 1 ADL inpatient mortality OR 1.8 (1.1–3) [23] | |
| mFI |
OR 11–11.7 [13] RR 4.19 (2.96–5.92) [19] |
Postop complications: OR 11[13] Clavien 4 and 5 postop Cx OR 14.4 [13] mFI > 0.27: Clavien 4 Cx OR 4.8 [13] mFI > 0.12: postop Cx OR 2.71 [13] mFI > 0: postop Cx pooled RR 1.48 (1.35–1.61), major postop Cx pooled RR 1.48 (1.35–1.61) [19] Discharge to care facility: RR 2.15 (1.92–2.4) [19] |
| Slow gait speed 5 m ≥ 6 s | OR 2.63 [13] | Mortality or major morbidity OR 2.63 (1.17–5.9)–3.17 (1.7–2.59) [12] |
American Society of Anesthesiologists Physical Status (ASA-PS)
The ASA tool is a simple ranking of physical health status from 1 to 5 (independent—moribund), which can be completed quickly by a wide range of clinicians [27]. It is broadly applied to all ages and to both emergency and elective populations. An association of high ASA grade with postoperative delirium [18, 22, 26] and postoperative mortality as well as complications [24] was reported in older patients undergoing a range of elective surgery. Conversely, a poor AUROC of 0.64 for the ability of the ASA to predict postoperative mortality following oesophagectomy was described [25].
Frailty
Of the multitude of frailty tools applied to older surgical patients across nine reviews [12–17, 19, 21, 23], including cardiothoracic surgical patients [12, 13, 17], the modified frailty index (mFI) and Fried criteria were the most frequently reported, followed by the Comprehensive Assessment of Frailty (CAF), Groningen Frailty Index (GFI) and Balducci frailty criteria. A strong association between the mFI and postoperative mortality and Clavien–Dindo grade 4 or 5 postoperative complications were reported in frail patients undergoing mixed major surgery [13]. In a meta-analysis and systematic review, frail patients (defined as any mFI score > 0) had a higher 30-day mortality (RR 4.19, CI 2.96–5.92), higher major postoperative complications (RR 2.03, CI 1.26–3.29) and an higher likelihood of discharge to skilled care accommodation (RR 2.15, CI 1.92–2.4) compared to non-frail patients (mFI score of 0) [19]. Similarly, frail patients meeting at least 3 of 5 phenotypic Fried scale criteria were more likely to die (30-day mortality OR 2.67, p = 0.029) [17], develop major postoperative complications [13] and have a longer length of stay (median LOS 9 vs 6 days, p = 0.004) [19]. Sandini et al. reported a strong association between frailty and 90-day postoperative mortality [OR 5.77, (CI 4.41–7.55)] and major morbidity [OR 2.56 (CI 2.08–3.16)] in older patients undergoing mixed major surgery, although did not specify a suggested frailty tool [21]. Overall, the majority of frailty tools summarized in this review reported a positive association with morbidity and mortality in older patients undergoing elective surgery.
Function
Tools to assess function were applied as part of frailty screening and CGA. Gait speed and the timed up and go test (TUGT) were described as bedside preoperative functional tests. Slow gait speed defined as 5 m ≥ 6 s was associated with higher postoperative mortality [13], and composite endpoint of postoperative mortality or major morbidity (OR ranging 2.63 (CI 1.17–5.9) to 3.17 (CI 1.7–2.59) [12, 23]. TUGT over 20 s was associated with postoperative complications [OR ranging from 3.1 (CI 1.1–8.6) to 4.1 (CI 1.6–10.5)] [16] in older patients undergoing oncologic surgery. Clinician or patient-measured functional scales including the Katz, Barthel, Instrumental Activities of Daily Living (IADL) and Nottingham extended ADL scale (NEADL) tools demonstrated an association between functional impairment and increased postoperative mortality [12, 16] and 30-day postoperative complication rate [16].
Comprehensive geriatric assessment (CGA)
Several objective tools as part of CGA were evaluated and categorized into functional, nutritional, cognitive, mood, comorbidity, polypharmacy and frailty domains [16]. Patients at risk of malnourishment using the Mini Nutritional Assessment (MNA) had a higher risk of short-term postoperative mortality (HR 2.39, CI 1.24–4.61) [16]. Those with a mini-mental status examination (MMSE) score < 24 points had an increased risk of mortality (HR 1.13, CI 1.04–1.22) and postoperative complications (OR 4.55, CI 1.15–18.05) within 6 months following surgery [16]. Older surgical patients with a geriatric depression scale ≥ 5 points were also less likely to survive 6 months (HR 3.62, CI 1.77–7.4) and were more likely to experience postoperative complications [OR range 3.68 (CI 0.96–14.08) to 4.58 (CI 125–16.84)] [16]. Partridge et al. reviewed overall CGA application encompassing the use of objective tools and demonstrated lower postoperative complications and length of stay (4.9 vs 8.9 days, p < 0.001) [20].
Current guidelines on perioperative management of older patients
Recommendations summarized in international guidelines on the perioperative care of older patients are given in Table 3 [28–33]. Most are based on expert consensus opinion. Where validated screening tools have been used to assess individual domains, these are highlighted.
Table 3.
Current guidelines on perioperative management of older patients
| Society | Guideline title | Year | Domain assessed | Evidence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | Function | Frailty | Mood | Nutrition | Medication | Comorbidity | Other/comments | ||||
| ACS NSQIP/ American Geriatrics Society [28] | Optimal Perioperative Management of the Geriatric Patient | 2016 | – | – | – | – | – |
+ NSQIP 2012 |
– | References NSQIP 2012 for assessment of individual domains, covers advance directives, preoperative fasting, antibiotic use and venous thromboembolism prevention | Expert opinion |
| ACS NSQIP/American Geriatrics society [29] | Optimal Preoperative Assessment of the Geriatric Surgical Patient | 2012 |
+ Mini-COG |
+ TUGT |
+ |
+ PHQ-2 |
+ |
+ AGS Beers Criteria |
+ RCRI |
Recommends preoperative diagnostic tests including Hb, renal function, serum albumin ± WCC, platelet count, coagulation profile, serum glucose, CXR, ECG, RFT, noninvasive stress testing, BMI and unintentional weight loss |
Level 1 + Expert opinion |
| Association of Anaesthetists Great Britain and Ireland [30] | Perioperative care of the elderly | 2014 |
+ NSQIP 2012 |
+ | + | – | – |
+ NSQIP 2012 |
– |
Recommends risk scores such as NSQIP preoperative assessment and Nottingham Hip Fracture Score Recommends multidisciplinary care |
Level 1 + Expert opinion |
| British Geriatric Society [31] | Perioperative Care for Older Patients Undergoing Surgery | 2013 | + | + | + | – | – | – | + | Social domain assessed | Expert opinion |
| New South Wales Government Health [32] | The Perioperative toolkit | 2018 | + | + | + | – | – | + | + | Social domain assessed, involves families in decision-making, multidisciplinary team, patient care pathways, shared decision-making | Expert opinion |
| Society for Perioperative Assessment and Quality Improvement (SPAQI) [33] | Recommendations for Preoperative Management of Frailty from the SPAQI | 2018 |
+ Mini-COG |
+ |
+ Frailty/Edmonton score |
+ | – | – | + | Recommends multidisciplinary care and shared decision-making, prehabilitation principles (eg nutritional intervention), requires further studies prior to inclusion in standard recommendations |
Level 1 + Expert opinion |
ACS NSQIP American College of Surgeons National Surgical Quality Improvement Program, TUGT timed up and go test, PHQ-2 Patient Health Questionnaire 2, RCRI revised cardiac risk index
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) 2012 guideline [29] is one of the earliest publications released in this field. It is relatively prescriptive and recommends specific preoperative testing, such as full blood examination and baseline ECG. Validated domain-specific assessment tools are recommended according to expert consensus. The NSQIP 2016 guideline [28] includes sections relating to the immediate perioperative period. It does not discuss screening tools, however, refer to the NSQIP 2012 guideline where screening tools are discussed in further detail, for example, in the medication management domain [28, 29].
The guidelines of the Association of Anaesthetists of Great Britain and Ireland [30] similarly refer to NSQIP 2012 for assessment of domains including cognition and medication management. These guidelines also recommend preoperative risk score calculation tailored to specific surgical situations, for example, use of the Nottingham Hip Fracture Score in the prediction of 30-day mortality after hip fracture surgery [30]. The British Geriatric Society guideline [31] and an Australian guideline, the New South Wales Government Health Perioperative toolkit [32], recommend assessing several domains to risk stratify patients, but do not specify which tools to use. Both these guideline emphasize the importance of assessing social domains which are not included in NSQIP guidelines [31, 32]. The Society for Perioperative Assessment and Quality Improvement (SPAQI) [33] covers several domains including cognition, functional status, frailty, mood disorder and medical comorbidity. Specific screening tools are suggested for some of the domains, such as mini-COG to assess cognition. The more recently published guidelines, including SPAQI and the New South Wales Government Health Perioperative Toolkit, tend to state broader expert consensus recommendations such as multidisciplinary care and shared decision-making [32, 33].
Overall, there is heterogeneity in the approach taken by each guideline committee towards the perioperative management of older patients. Assessment domains and tools differ between guidelines. Almost all guidelines recommend an assessment of cognition, functional status and frailty, although many do not specify which tool to use.
Discussion
This scoping review of systematic reviews and meta-analyses demonstrates the broad range of tools that are applied preoperatively to older patients undergoing elective surgery. The most commonly described tools include the ASA, frailty tools and tools utilized during CGA. The majority of tools show a positive association with short-term postoperative mortality and morbidity as measures of postoperative recovery in various older surgical patient populations, including cardiothoracic patients. Due to the differences in utilized cut-off points and outcome parameters, tools are unable to be compared in order to support one tool over another. Perioperative guidelines offer recommendations for pre-assessment approach in older surgical patients but lack consensus regarding the selection of preoperative tools. As a result, there is no evidence to support a distinct tool which should be applied universally to older surgical patients.
The ASA is simple to apply and routinely used by anaesthetists to broadly stratify patients in all perioperative settings. Whilst there is a consistent association between a higher ASA score and poor postoperative outcomes [34], it remains a subjective score with high inter-observer variability [35].
The inherent value of identifying frailty, defined as an age-related cumulative decline in multiple physiological systems [36], has been increasingly recognized as a measure of high-risk in older surgical patients [9, 37, 38]. However, standardized assessment is often lacking due to the absence of a universal or ‘gold standard’ frailty tool as demonstrated in this review.
CGA is a time-consuming patient-specific evaluation which might not be appropriate to administer to all older patients preoperatively [39]. Whilst there is supportive evidence for CGA in both emergency [40] and elective [41] older surgical patients, it requires specialist training to administer the domain-specific tools [20, 21]. Adaptations of the CGA into screening tools such as the G-8 questionnaire [42] and CGA-GOLD [43] require further research in broad surgical populations and were not published in a meta-analysis or systematic review format for inclusion. Additional commonly utilized screening tools did not meet the inclusion criteria for this review. For example, the P-POSSUM uses intraoperative variables [44] and the Revised Cardiac Risk Index was only included in one systematic review within our literature search [45].
International guidelines are fairly consistent in terms of recommending a complete preoperative medical assessment based on geriatric domains included in a CGA. Most recommendations are based on expert opinion. Although cognition, functional status and frailty are consistently prioritized, with corresponding tools given as an example in each guideline, there is no consensus regarding which tool to use. This suggests that completing any chosen assessment may be more important than which tools are specifically used. The ease of use of the guidelines and ability to apply the recommendations quickly and effectively in an outpatient setting, such as a preadmission clinic, has not been validated. Furthermore, a comprehensive approach might not necessary for all older patients.
The 2018 Royal College of Surgeons High-Risk General Surgical Guideline recommends all patients undergo risk assessment prior to surgery and classifies patients with a predicted postoperative mortality risk of ≥ 5% as high risk [9]. This can be estimated using a preoperative risk assessment tools and frailty assessment. Resources can consequently be targeted towards high-risk patients including planning postoperative critical care beds, senior anaesthetic and surgical intraoperative presence and engagement of the multidisciplinary perioperative team. Whilst no screening tool has been identified as the single best option in the older general surgical patient, it appears that making a screening assessment using any validated tool to guide the application of comprehensive geriatric assessment is warranted. Given the shared recommendation of guidelines to assess cognition, functional status and frailty, it is reasonable for clinicians to choose a tool within one or all of these domains.
There were limitations met throughout this scoping review which contributed to the inability to define a single appropriate screening tool. The high number of tools reported and marked heterogeneity in outcomes measured significantly limited the ability to compare tools in this review. Whilst narrowing the search to a more specific population may have been more achievable, we aimed to find a broadly applicable tool to reflect clinical need and simplify perioperative pathways. There were multiple selection biases including skewed subsurgical groups, the underrepresentation of oldest old patients and geography.
Geriatrician-led multidisciplinary perioperative care targeting older patients undergoing surgery is growing in clinical practice. The establishment of the ‘Perioperative Care of Older Patients Undergoing Surgery’ (POPS) service in the UK is an example of a successful collaborative perioperative model for older patients, which has led to improved mortality and morbidity in older surgical patients [41, 46]. In this model, preoperative screening is not limited to a specific tool but encourages identification of geriatric syndromes and clinical judgement [47]. Despite strong evidence and UK national endorsement of the POPS model of care, clinical uptake is not yet widely disseminated with an acknowledged ‘implementation gap’. A logic implementation model of the POPS service has successfully led to translation of core components to a smaller setting [48].
Conclusion
The use of screening tools to predict postoperative outcomes in older patients prior to elective surgery is important in identifying high-risk patients and developing safe, efficient and effective clinical pathways for the perioperative team. A number of screening tools have been identified as associated with poor postoperative outcomes and the selection of a frailty, functional and/or cognitive tool is proposed. International consensus guidelines recommend a complete and thorough medical and geriatric assessment of the older patient prior to surgery; screening tools can help guide which patients will benefit from this comprehensive approach.
Author contributions and authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. RA: 40% contribution (study design, literature search, results Tables 1, 2, Fig. 1, manuscript drafting and editing, reference collation). NSH: 40% contribution (study design, literature search, review of guidelines, results Table 3, manuscript drafting and editing). ABM: 20% contribution (study design, manuscript editing, research supervisor).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Mr Patrick Condron, Senior Liaison Librarian, Melbourne Medical School, Melbourne Dental School, Melbourne School of Psychological Sciences, The University of Melbourne.
Funding
This research is based on an unrestricted grant provided by the University of Melbourne received by Prof. Andrea B. Maier.
Compliance with ethical standards
Conflict of interests
There are no conflicts of interest to disclose.
Statement of human and animal rights
This review was conducted following accepted princlples of ethical and professional conduct. The research did not involve human participants or animals.
Informed consent
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rachel Aitken and Nur-Shirin Harun have contributed equally.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division (2015) World Population Ageing 2015 (ST/ESA/SER.A/390)
- 2.Etzioni DA, Liu JH, Maggard MA, et al. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon AL, Evans BJ, Dhesi J. The physician’s role in perioperative management of older patients undergoing surgery. Clin Med (London) 2017;17(4):357–359. doi: 10.7861/clinmedicine.17-4-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing non cardiac surgery. Ann Intern Med. 2001;134:637–643. doi: 10.7326/0003-4819-134-8-200104170-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hamel MB, Henderson WG, Khuri SF, et al. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatric Soc. 2005;53(5):424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 6.Bowyer AJ, Royse CF. Postoperative recovery and outcomes—what are we measuring and for whom? Anaesthesia. 2016;71(S1):72–77. doi: 10.1111/anae.13312. [DOI] [PubMed] [Google Scholar]
- 7.Shipway DJH, Harari D, Dhesi J. Peri-operative management of older people undergoing surgery. Rev Clin Gerontol. 2014;24(1):78–92. doi: 10.1017/S095925981300018X. [DOI] [Google Scholar]
- 8.Dhesi J. Improving outcomes in older patients undergoing elective surgery. Aging Health (London) 2012;8(4):329–332. doi: 10.2217/ahe.12.38. [DOI] [Google Scholar]
- 9.The Royal College of Surgeons of England and Department of Health (2018) The high-risk general surgical patient: raising the standard. Updated recommendations on the perioperative care of the high-risk general surgical patient. https://www.rcseng.ac.uk/-/media/files/rcs/news-and-events/media-centre/2018-press-releases-documents/rcs-report-the-highrisk-general-surgical-patient--raising-the-standard--december-2018.pdf. Accessed 25 June 2020
- 10.Eamer G, Al-Amoodi MJH, Holroyd-Leduc J, et al. Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am J Surg. 2018;216:585–594. doi: 10.1016/j.amjsurg.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 12.Abdullahi YS, Athanasopoulos LV, Casula RP, et al. Systematic review on the predictive ability of frailty assessment measures in cardiac surgery. Interact Cardiovasc Thorac Surg. 2017;24:619–624. doi: 10.1093/icvts/ivw374. [DOI] [PubMed] [Google Scholar]
- 13.Buignes C, Juarros-Folgado P, Fernandez-Garrido J, et al. Frailty syndrome and pre-operative risk evaluation: a systematic review. Arch Gerontol Geriatr. 2015;61:309–321. doi: 10.1016/j.archger.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Fagard K, Leonard S, Deschodt M, et al. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: a systematic review. J Geriatr Oncol. 2016;7:479–491. doi: 10.1016/j.jgo.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt J, Long S, Carter B, et al. The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis. Age Ageing. 2018;47:793–800. doi: 10.1093/ageing/afy110. [DOI] [PubMed] [Google Scholar]
- 16.Huisman MG, Kok M, de Bock GH, et al. Delivering tailored surgery to older cancer patients: preoperative geriatric assessment domains and screening tools—a systematic review of systematic reviews. EJSO. 2017;43:1–14. doi: 10.1016/j.ejso.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Lin H, Watts JN, Peel NM, et al. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157. doi: 10.1186/s12877-016-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldroyd C, Scholz AFM, Hinchliffe RJ, et al. A systematic review and meta-analysis of factors for delirium in vascular surgical patients. J Vasc Surg. 2017;66:1269–1279. doi: 10.1016/j.jvs.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 19.Panayi AC, Orkaby AR, Sakthivel D, et al (2018) Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. ISSN 0002-9610. 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed]
- 20.Partridge JSL, Harari D, Martin FC, et al. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2014;69(Suppl. 1):8–16. doi: 10.1111/anae.12494. [DOI] [PubMed] [Google Scholar]
- 21.Sandini M, Pinotti E, Persico I, et al. Systematic review and meta-analysis of frailty as a predictor of morbidity and mortality after major abdominal surgery. BJS Open. 2017;1:128–137. doi: 10.1002/bjs5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz AFM, Oldroyd C, McCarthy K, et al. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. BJS. 2016;103:e21–28. doi: 10.1002/bjs.10062. [DOI] [PubMed] [Google Scholar]
- 23.Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–3117. doi: 10.1016/j.jtcvs.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 24.Visser A, Geboers B, Gouma DJ, et al. Predictors of surgical complications: a systematic review. Surgery. 2015;158:58–65. doi: 10.1016/j.surg.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Warnell I, Chincholkar M, Eccles M. Predicting perioperative mortality after oesophagectomy: a systematic review of performance and methods of multivariate models. Br J Anaesth. 2015;114(1):32–43. doi: 10.1093/bja/aeu294. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Wang G, Liu S, et al. Risk factors for postoperative delirium in patients undergoing major head and neck cancer surgery: a meta-analysis. Jpn J Clin Oncol. 2017;47(6):505–511. doi: 10.1093/jjco/hyx029. [DOI] [PubMed] [Google Scholar]
- 27.American Society of Anesthesiologists. ASA Physical Status Classification System. Last amended Oct 15 2014. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Accessed 12 Jan 2019
- 28.Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222(5):930–947. doi: 10.1016/j.jamcollsurg.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215(4):453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths R, Beech F, Brown A, et al. Peri-operative care of the elderly 2014: Association of Great Britain and Ireland. Anaesthesia. 2014;69(Suppl 1):81–98. doi: 10.1111/anae.12524. [DOI] [PubMed] [Google Scholar]
- 31.Dhesi, J. Peri-operative care for Older Patients Undergoing Surgery. British Geriatrics Society. Last amended Feb 4 2018. https://www.bgs.org.uk/resources/peri-operative-care-for-older-patients-undergoing-surgery. Accessed 27 Feb 2019
- 32.Anaesthesia Perioperative Care Network Surgical Services Taskforce. The perioperative toolkit. Last amended Dec 13 2016. https://www.aci.health.nsw.gov.au/data/assets/pdf_file/0010/342685/The_Perioperative_Toolkit.pdf. Accessed 27 Feb 2019
- 33.Alvarez-Nebreda ML, Bentov N, Urman RD, et al. Recommendations for preoperative management of frailty from the Society for Perioperative Assessment and Quality Improvement (SPAQI) J Clin Anesth. 2018;47:33–42. doi: 10.1016/j.jclinane.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Wolters U, Wolf T, Stutzer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. BJA. 1996;77(2):217–222. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 35.Mak PH, Campbell RC, Irwin MG, et al. The ASA physical status classification: inter-observer consistency American Society of Anesthesiologists. Anaesth Intensive Care. 2002;30(5):633–640. doi: 10.1177/0310057X0203000516. [DOI] [PubMed] [Google Scholar]
- 36.Xue Q-L. The Frailty Syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147. doi: 10.1093/ageing/afr182. [DOI] [PubMed] [Google Scholar]
- 38.Cesari M. Why and how do we measure frailty? Intern Emerg Med. 2019;14(1):5–6. doi: 10.1007/s11739-018-1986-8. [DOI] [PubMed] [Google Scholar]
- 39.Partridge JSL, Harari D, Martin FC, et al. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2014;69(Suppl 1):8–16. doi: 10.1111/anae.12494. [DOI] [PubMed] [Google Scholar]
- 40.Eamer G, Taheri A, Chen SS, et al. Comprehensive geriatric assessment for older people admitted to a surgical service. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD012485.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Partridge JSL, Harari D, Martin FC, et al. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2013;69(Suppl. 1):8–16. doi: 10.1111/anae.12494. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M, Takahashi M, Komine K, et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: a retrospective, single institutional study. PLoS ONE. 2017;12(6):e0179694. doi: 10.1371/journal.pone.0179694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittle AK, Kalsi T, Babic-Illman G, et al. A comprehensive geriatric assessment screening questionnaire (CGFiA-GOLD) for older people undergoing treatment for cancer. Eur J Cancer Care (Engl) 2017 doi: 10.1111/ecc.12509. [DOI] [PubMed] [Google Scholar]
- 44.Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. BJS. 1998;85(9):1217–1220. doi: 10.1046/j.1365-2168.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 45.Ford MK, Beattie SB, Wljeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 46.Gragnano F, Cattano D, Calabro P. Perioperative care of cardiac patient’s candidate for non-cardiac surgery: a critical appraisal of emergent evidence and international guidelines. Intern Emerg Med. 2018;13(8):1185–1190. doi: 10.1007/s11739-018-1927-6. [DOI] [PubMed] [Google Scholar]
- 47.Partridge JSL, Sbai M, Dhesi J. Proactive care of older people undergoing surgery. Aging Clin Exp Res. 2018;30(3):253–257. doi: 10.1007/s40520-017-0879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasper EV, Dhesi JK, Partridge JSL, et al. Scaling up perioperative medicine for older people undergoing surgery (POPS) services; use of a logic model approach. Clin Med (Lond) 2019;19(6):478–484. doi: 10.7861/clinmed.2019-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.