Abstract
Introduction
The overall survival in patients with gliomas has not significantly increased in the modern era, despite advances such as immunotherapy. This is in part due to their notorious ability to suppress local and systemic immune responses, severely restricting treatment efficacy.
Methods
We have reviewed the preclinical and clinical evidence for immunosuppression seen throughout the disease process in gliomas. This review aims to discuss the various ways that brain tumors, and gliomas in particular, co-opt the body’s immune system to evade detection and ensure tumor survival and proliferation.
Results
A multitude of mechanisms are discussed by which neoplastic cells evade detection and destruction by the immune system. These include tumor-induced T-cell and NK cell dysfunction, regulatory T-cell and myeloid-derived suppressor cell expansion, M2 phenotypic transformation in glioma-associated macrophages/microglia, upregulation of immunosuppressive glioma cell surface factors and cytokines, tumor microenvironment hypoxia, and iatrogenic sequelae of immunosuppressive treatments.
Conclusions
Gliomas create a profoundly immunosuppressive environment, both locally within the tumor and systemically. Future research should aim to address these immunosuppressive mechanisms in the effort to generate treatment options with meaningful survival benefits for this patient population.
Keywords: Glioblastoma, Gbm, Glioma, Immune suppression, Immunosuppression, Immunotherapy
Introduction
The overall survival in patients with gliomas has not improved significantly over the past decades, despite aggressive treatments [1]. Recent research within the field has shown an increased emphasis on understanding the complex relationship between the immune system and these deadly central nervous system (CNS) tumors. The present findings have significant implications not only from a research standpoint, but also in the daily management and treatment of glioma patients. This review aims to discuss the various ways that brain tumors, and gliomas in specific, co-opt the body’s immune system to evade detection and ensure their proliferation and survival.
Immune cell dysfunction
Lymphocyte dysfunction
T-cells
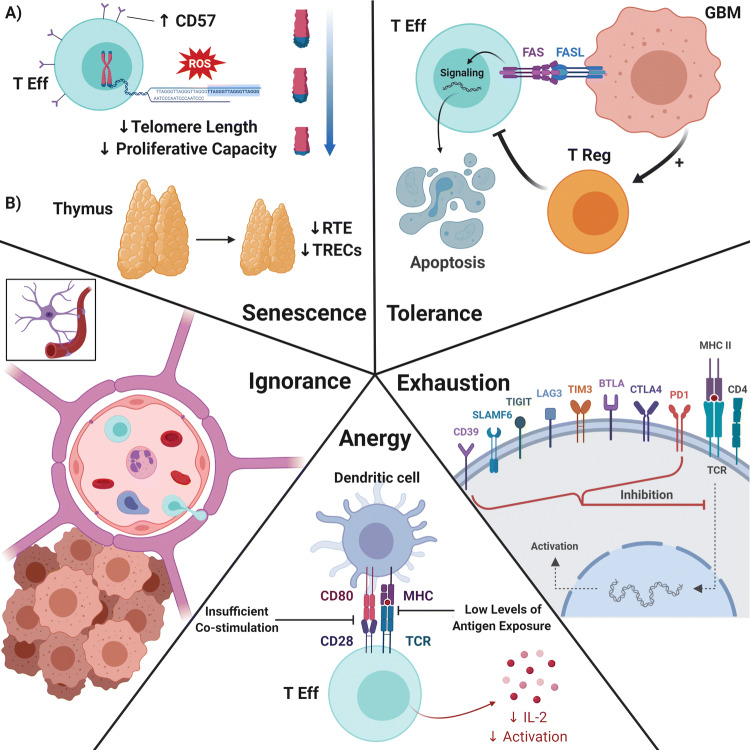
High grade gliomas (HGG) are one of the most immunosuppressive solid tumors despite rare metastasis outside the CNS [2]. The ability to cause severe, systemic T-cell deficits is one of the most prominent and earliest reported immune-related effects of HGGs (1). T-cell dysfunction in HGG (and glioblastoma [GBM] in specific) can be molecularly categorized into 5 domains: senescence, tolerance, anergy, exhaustion, and ignorance (Fig. 1) [3].
Fig. 1.
Five domains of T-cell dysfunction. Clockwise from top left—Senescence: a Repetitive T-cell proliferation/activation and DNA damage events cause telomere shortening, decreasing the proliferative capacity of effector T-cells. b Thymic involution develops prematurely in patients with GBM, reducing T-cell output from the thymus. Tolerance: Gliomas induce T-cell apotosis via the FasL-Fas pathway, as well as generate proliferation of Tregs, which have suppressive effects on effector T-cells. Exhaustion: After repeated exposure under suboptimal conditions, T-cells end up expressing inhibitory immune checkpoints, with the major ones shown here. The degree of exhaustion is correlated with expression of specific checkpoints. Anergy: T-cell anergy can be caused by two broad mechanisms: insufficient co-stimulation leading to clonal anergy and impairment of T-cell activation, and continuous low level antigen exposure, leading to adaptive tolerance and reduced T-cell proliferation. Ignorance: T-cell ignorance is the result of fully functional T-cells that are prevented from antigen exposure by anatomical barriers or insufficient antigen expression levels, such as is the case with the blood brain barrier and T-cell sequestration. T Eff effector T-cell, ROS reactive oxygen species, RTE recent thymic emigrants TRECs T-cell receptor excision circles, T reg regulatory T-cell, MHC major histocompatibility complex, TCR T-cell receptor. Created with BioRender.com
T-cell senescence is thought to be caused by telomere shortening from repetitive T-cell proliferation/activation and DNA damage events, such as exposure to reactive oxygen species (ROS) [4]. Proposed signature markers of T-cell senescence are upregulation of CD57, an indicator for T-cell terminal differentiation, as well as loss of CD27 and CD28, which are costimulatory markers [5, 6]. These phenotypes correlate well with telomere shortening and telomerase activity loss. In GBM, T-cell senescence phenotype suggests poor prognosis, as GBM patients with higher level of CD4+CD28−CD57+ T-cells have shorter overall survival [7]. Additionally, thymic involution develops prematurely in patients with GBM. This phenomenon results in a reduced output of naïve T-cells (known as recent thymic emigrants [RTE]) from the thymus [8]. Lower RTE, as measured by lower T-cell receptor excision circles (TREC, indicating thymic senescence), was also shown to correlate with poor clinical outcomes in GBM patients [9].
In the normal physiologic state, the body prevents autoimmunity through T-cell tolerance [10]. Central tolerance, mediated by negative selection in the thymus, is imperfect, with the chance for self-antigen reactivity. Therefore, peripheral tolerance outside the thymus serves as an additional safety net against autoimmunity. Peripheral T-cell tolerance is normally comprised of peripheral deletion and suppression by regulatory T-cells (Tregs). However this mechanism is hijacked by tumors, preventing an effective antitumor immune response [11]. T-cell apoptosis, mediated by the FasL-Fas pathway, has been described as a mechanism to delete T-cells in several types of cancer, including GBM [12]. The role that Tregs play in this peripheral T-cell tolerance in HGG will be discussed in a subsequent section.
T-cell anergy was originally used to describe the lack of type IV hypersensitivity response found in GBM patients who failed to react to recall antigen [13]. However, the term anergy now covers two separate entities: clonal/in vitro anergy and adaptive tolerance/in vivo anergy [13]. Clonal anergy is caused by insufficient co-stimulation, leading to defective RAS/MAPK activation and AP-1 transcription, which impairs T-cell activation [14]. Alternatively, adaptive tolerance arises from continuous low levels of antigen exposure, which leads to NF-κB impairment, low IL-2 production, and reduced T-cell proliferation [14]. While each entity represents different T-cell molecular states, both are present in GBM patients and contribute to global T-cell dysfunction.
Classically described in chronic viral infection, T-cell exhaustion occurs after repeated antigen exposure under suboptimal conditions. This results in activation of a specific transcriptional program that generates a hyporesponsive T-cell state [15]. Recently, gliomas have been shown to induce similar phenotypes of T-cell exhaustion [16]. Transcription factors involved in programmed T-cell exhaustion include T-bet, Eomesodermin (Eomes), and NFAT. Exhausted T-cells express high levels of Eomes and low levels of T-bet [17]. While in the exhausted state, failure of NFAT to form a complex with AP-1 results in expression of inhibitory immune checkpoints, such as PD-1 and CTLA-4 [18]. In addition to these conventional ones, other recently characterized checkpoints involved in T-cell exhaustion include TIM-3, LAG-3, BTLA, 2B4, SLAMF6, CD160, TIGIT, and CD39 [3]. A recent study looking at a variety of these exhaustion markers demonstrated that T-cell exhaustion is particularly severe in GBM compared to other types of cancer [16]. The authors showed that co-expression of PD-1, TIM-3, and LAG-3 rendered human GBM tumor-infiltrating lymphocytes (TILs) in a severely hypofunctional state.
The last domain of T-cell dysfunction is T-cell ignorance, which occurs when fully functional T-cells are prevented from antigen exposure by anatomical barriers or insufficient antigen expression levels [19]. Theoretically, ignorance can be overcome by a sufficient quantity of T-cells undergoing antigen exposure. However, GBM patients frequently exhibit clinically significant lymphopenia [20]. A recent study again demonstrated this fact, and was able to show this is at least partially produced by T-cell sequestration in the bone marrow due to the loss of S1P1 receptors from the T-cell surface [20]. Lymphopenia combined with the blood brain barrier (BBB) limiting access into the immunologically-distinct brain prevents the antigen exposure necessary to produce robust, T-cell mediated immune responses in the tumor microenvironment (TME).
Regulatory T-cells (Tregs)
Tregs are characterized by their ability to suppress effector T-cell activation through a variety of mechanisms (Fig. 2), most notably secretion of immunosuppressive cytokines and downmodulation of co-stimulatory molecules on antigen presenting cells (APCs) [21]. The glioma TME favors recruitment and survival of Tregs by maintaining high concentrations of cytokines that support Treg persistence, such as transforming growth factor-β (TGF-β) and indoleamine 2,3-dioxygenase (IDO) [22, 23]. While Tregs normally represent 5–10% of circulating CD4+T-cells, they are found in increased numbers and frequencies in a multitude of cancers, with higher numbers of Tregs associated with a worse prognosis [24, 25]. Glioma patients have higher proportions of circulating Tregs compared to healthy controls (even though absolute Treg numbers were decreased), and these patients have increased Treg numbers infiltrating the tumors themselves [26, 27]. These findings were recapitulated in murine glioma models, with subsequent studies demonstrating that Treg depletion prolonged survival in glioma-bearing mice [26]. Consequently, novel therapeutic approaches to either inhibit or reduce Treg numbers are an active area of research [27–29].
Fig. 2.
Summary of glioma-immune interactions. Gliomas secrete or express a variety of factors that attract or induce immunosuppressive cell types, or have direct inhibitory effects on immune effector cells. T Eff effector T cell, ROS reactive oxygen species, NO nitric oxide, GAM glioma-associated microglia/macrophage, MDSC myeloid-derived suppressor cell, T reg regulatory T cell, MHC major histocompatibility complex, APC antigen presenting cell. Created with BioRender.com
Natural killer (NK) cells
NK cells are innate lymphoid cells capable of directly lysing infected or malignant cells. NK cells can target other cells missing MHC Class I, an adaptive process that is used by many viruses and tumors to evade detection by T-cells [30, 31]. By expressing a combination of inhibitory and stimulatory receptors, NK cells can tailor their response to specific insults [32]. For example, killer cell immunoglobulin-like receptors (KIR) can recognize MHC Class I present on healthy cells, preventing NK cell activation. In contrast, stressed or infected cells upregulate ligands that bind NKG2D, an activating receptor that triggers NK cell-mediated killing of the target cell. The importance of NK cells in cancer is demonstrated by the fact that mice and humans with NK cell deficiencies are at a higher risk to develop certain malignancies [33, 34]. In GBM, some populations of patients have decreased levels of NKG2D on the surface of their NK cells, leading to decreased NK cell activation [35]. Additionally, HLA-G, an inhibitory ligand found on gliomas, is able to bind to NK receptors in the KIR family (such as KIR2DL4 and ILT2) and inhibit NK cytotoxicity, IFN-γ secretion, NKG2D activation, and chemotaxis (Fig. 2) [36]. Despite NK cells making up a relatively small proportion of tumor-infiltrating cells, studies have shown that these NKs residing in the GBM TME display characteristics that allow them to be considerably cytotoxic to tumor cells in other cancers [37]. Therefore, potential therapeutic opportunities are actively being pursued that focus on either modulating NK cell numbers/activation status, or utilizing chimeric antigen receptor (CAR) technology to generate NK cells expressing receptors that specifically target tumor antigens.
Myeloid dysfunction
G/M-MDSCs
Myeloid-derived suppressor cells (MDSCs), identified as CD11b+CD33+HLA-DR−/low cells, are a heterogeneous population of immature myeloid cells that also play an important role in tumor-induced immunosuppression [38]. MDSCs, whose phenotype comprises 20–30% of the bone marrow, make up only 0.5% of peripheral blood mononuclear cells (PBMCs) as they quickly differentiate into mature subtypes in a normal, non-pathologic state. However, in disease states such as cancer, this population increases significantly due to alterations in myelopoiesis [39]. To date, elevated levels of MDSCs have been found in melanoma, glioma, renal, gastric, bladder, esophageal, and pancreatic cancers [40]. GBM, however, has one of the highest levels of circulating MDSCs of these cancers, with ~ 12 × greater than normal levels [41–43].
MDSCs, whose two major subsets include granulocytic (G-MDSC, identified as CD15+ in addition to the previously mentioned markers) and monocytic (M-MDSC, additionally CD14+), exert their immunosuppressive effects through inhibition of innate antitumor immunity by several mechanisms (Fig. 2) [44, 45]. These mechanisms include: expression of arginase, which decreases the level of L-arginine in the blood/tumor (an amino acid needed for normal T-cell function, specifically translation of the T-cell CD3 zeta chain); secretion of nitric oxide and production of ROS, which themselves are capable of inducing T-cell suppression; and expression of PD-L1 to participate in checkpoint blockade [46, 47]. Raychudhuri et al. demonstrated that T-cells obtained from GBM patients have suppressed IFN-γ production, and that removal of MDSCs from the patients’ PBMC population restored T-cell function [41]. In addition, several other studies have shown secretion of immunosuppressive cytokines, Treg stimulation, and the positive relationship between immunosuppression and tumor angiogenesis, which is mediated by MDSCs and dependent on STAT3 activation [39, 48, 49].
In light of their widespread immunosuppressive effects, elevated levels of MDSCs have been shown to be correlated with clinical cancer stage, histologic tumor grade, metastatic tumor burden, radiographic progression, and/or prognosis in a variety of cancers [46, 50, 51]. While the volume of literature linking MDSCs to these clinical variables in glioma is not as robust as in other types of cancer, recent publications have focused on this topic. Alban et al. found that GBM patients with a better prognosis had decreasing numbers in their peripheral circulation over time, as well as reduced MDSCs in their tumors [52]. Another study found that a subtype of G-MDSCs accumulated in the peripheral blood of GBM patients, and correlated with reduced numbers of effector immune cells, early recurrence, and disease progression [53]. In light of these results, a trial was performed in GBM patients to reduce MDSCs in peripheral circulation and increase cytotoxic immune infiltration into the TME [54]. Future studies are needed to further assess the association of MDSCs to clinical disease course.
Tumor-associated macrophages/microglia
Tumor-associated macrophages (TAMs) and their resident CNS correlate, microglia, are able to infiltrate gliomas and comprise a substantial proportion of cells in the TME, up to 15–30% depending on glioma grade [55]. While microglia are yolk sac–derived with the capacity for limited self-renewal, TAMs are monocyte-derived from the bone marrow and peripheral circulation, extravasating into the tumor as a result of the breakdown of the BBB near the tumor [56]. While glioma-infiltrating TAMs and microglia (termed glioma-associated microglia/macrophages [GAMs] as a group) have been identified in the past by the markers CD163, CD200, CD204, CD68, and Iba-1, the most common identification strategy in the literature considers microglia to be CD11bhighCD45low, while TAMs are CD11bhighCD45high [51]. Multiple studies have shown the correlation between the number and morphology of GAMs with glioma grade (higher numbers and amoeboid morphology), as well as increases in GAM numbers correlating with increased aggressiveness within specific tumor grades [57–61].
GAMs are noted to have a significant degree of plasticity in regards to their effector functions. The M1 phenotype is considered pro-inflammatory and anti-tumor, typically acquired after stimulation with GM-CSF, toll-like receptor 4 (TLR4) ligands, and/or IFN-γ [51, 62]. Conversely, the M2 phenotype is considered cytoprotective, immunosuppressive, and protumorigenic, occurring after M-CSF (expressed by glioma cells, as well as normal human astrocytes), IL-4, IL-10 and/or IL-13 exposure. The M2 polarized GAMs produce high levels of IL-10, transforming growth factor (TGF)-β, epithelial growth factor (EGF), matrix metalloproteinase (MMP)-2 and MMP-9, and low levels of IL-12, which overall promotes tumor cell immune evasion, invasion, proliferation and angiogenesis (Fig. 2) [51, 62]. However, it should be noted that these phenotypes were generated in vitro under ideal conditions, and thus GAMs in vivo likely have a variety of functions along the M1/M2 spectrum (moreover, additional subpopulations have also been defined, such as M2a, M2b, M2c, etc.) [55]. Recent work now aims to go beyond cell surface markers to gather in depth gene expression profiling data, to gain greater understanding of the functions of GAMs and discern potential therapeutic targeting strategies [63, 64].
Tumor-related immunosuppressive factors
Glioma cell surface factors and cytokine secretion/dysregulation
Gliomas employ several mechanisms to evade the immune system. Among others, these include modulation of cell surface molecules, and secretion of cytokines. Gliomas can express PD-L1, and when bound to PD-1 on T-cells, can suppress T-cell activation. In addition, gliomas downregulate HLA-class I and can upregulate certain HLA-class II molecules, resulting in a deficient cytotoxic T-cell response and skewing toward a CD4+T-cell response. Gliomas also have the capacity to interfere with antigen processing or presentation on HLA [65, 66].
Cytokines play an important role in glioma progression, as they can affect proliferation, angiogenesis and aggressiveness of the tumor. Classic immunosuppressive cytokines associated with glioma are TGF-β and IL-10. TGF-β levels are associated with glioma grade, triggering proliferation in HGGs. It is also a regulator of VEGF (vascular endothelial growth factor), implicated in angiogenesis [67]. TGF-β suppresses lymphocytes and NK cells and can cause inhibition of antigen presentation [68]. In addition to TGF-β, IL-10 is largely responsible for shifting the TME toward an immunosuppressive phenotype. IL-10 can be produced from the glioma directly or gliomas can stimulate the production of IL-10 by macrophages and microglia [67, 68]. IL-1β, a classical pro-inflammatory cytokine, is also overexpressed in gliomas as compared to healthy controls, and has been shown to regulate both the survival and invasiveness of GBM. IL-6, TNF-α, and IL-8 have all also been shown to be upregulated in gliomas as compared to healthy individuals and play a role in tumor growth and invasion [69].
TME hypoxia
Tumor cell viability and response to therapeutic agents is highly influenced by several factors, including tissue hypoxia. Hypoxia, defined as an oxygen saturation of less than 2% (compared to 2–9% in healthy tissue), is a hallmark of the GBM TME [70]. Low oxygen tension (i.e. hypoxia) is caused by the tumor cells rapidly outgrowing their blood and nutrient supply, resulting in increased cellular necrosis and acidosis [71, 72]. Gliomas adapt to the hypoxic TME via oxygen-sensitive transcription factors called hypoxia-inducible factors (HIFs), the most notable of them being HIF-1α and HIF-2α [72]. These HIFs play an important role in tumor growth and survival through regulation of several key components of tumor biology, including glycolytic metabolism, pH homeostasis, angiogenesis, mitochondrial autophagy and resistance to apoptosis [72, 73].
HIF activation is also important for tumor immunogenicity, as certain immune cells that promote tumorigenesis can infiltrate and preferentially target these areas of hypoxia [72, 74]. TAMs have been shown to infiltrate hypoxic regions within solid tumors, with VEGF increasing TAM recruitment in a HIF-dependent manner [72, 74]. Likewise, tumor-associated fibroblast expression of the chemoattractant CXCL12 is upregulated under hypoxic conditions and also plays an important role in TAM recruitment [72]. While TAM polarization in the M1 or M2 phenotype is mainly induced by interferon-regulatory factor/signal transducer and activator of transcription (IRF/STAT) signaling pathways, hypoxia also can regulate this phenomena and activate HIFs differently to induce an M1 or M2-like phenotype [75]. Specifically, HIF2α activation is involved in the M2 polarization axis, with these TAMs being associated with immunosuppression, tumor cell proliferation, angiogenesis, and local invasion, resulting in poor patient outcomes [76, 77]. Similarly, elevated expression of HIF-2α is associated with poor prognosis and higher tumor grade in numerous cancer types [78]. Due to these reasons, HIFs may be a promising treatment target, with studies in several murine models showing that HIF inhibition (e.g. acriflavine) improves destruction of cancer cells and increases survival [73].
Systemic/treatment-related immune suppression
Steroid therapy
The use of high-dose glucocorticoids, such as dexamethasone, is standard of care to reduce the life-threatening vasogenic edema seen in patients with CNS tumors. Although the exact mechanism is not well understood, several studies have proposed that glucocorticoids reduce cerebral edema by stabilizing the capillary membrane and blocking expression of VEGF [79, 80]. However, the potent anti-inflammatory and immunomodulatory effects of dexamethasone are well described in the literature, producing clinically significant lymphopenia via signaling through the lymphotoxic glucocorticoid receptors on both B and T lymphocytes, and attenuating the CD28 co-stimulatory pathway [81, 82]. Studies have shown that dexamethasone doses as little as 0.25 mg/kg/day result in reduced numbers of TILs and other important immune cells in the TME [83]. Therefore, the positive benefits of edema reduction are countered by the negative sequelae of immune suppression. While steroid administration is an absolute necessity in many circumstances, their immunosuppressive side effects should prompt dose reduction or cessation by clinicians whenever possible, especially in patients that are on immunotherapies.
Chemotherapy
Glioma patients may be repeatedly pancytopenic for periods of time due to chemotherapy-induced myelosuppression and myeloablation, exposing them to the risk of infection and limiting mechanisms of innate anti-tumor immunity (Table 1). The most commonly used chemotherapeutic in glioma treatment is temozolomide, a DNA methylator that is known to cause long-lasting lymphopenia [84, 85]. Additionally, the use of temozolomide is associated with an upregulation of T-cell exhaustion markers such as LAG-3 and TIM-3, which has unique implications for its concomitant use with immunotherapy [86]. As studies have shown that treatment-related immunosuppression from temozolomide/radiation therapy is long-lasting and associated with early death from tumor progression in HGG patients, new approaches need to be devised to overcome these detrimental effects [85]. Recent work by Karachi et al. demonstrated that metronomic dosing of temozolomide in combination with anti-PD-1 therapy decreased TIL exhaustion markers and rescued the survival benefit seen with immunotherapy in two syngeneic murine GBM models. As temozolomide is part of the current standard of care treatment of GBM, further evaluation of this study and others is needed [86].
Table 1.
Chemotherapeutic drugs commonly used alone or in combination for the treatment of malignant tumors of the CNS
| Chemotherapeutica | Mechanism of actionb | Myelosuppression scorec,d |
|---|---|---|
| Carmustine/Lomustine | DNA cross-linking/alkylating agent | 4 |
| Carboplatin | DNA cross-linking/alkylating agent | 3 |
| Cisplatin | DNA cross-linking/alkylating agent | 1 |
| Cyclophosphamide | DNA cross-linking/alkylating agent | 3–4 (based on dose) |
| Etoposide | DNA Topoisomerase II inhibitor | 4 |
| Irinotecan | DNA Topoisomerase I inhibitor | 4 |
| Methotrexate | Anti-metabolite (dihydrofolate reductase inhibitor) | 2 |
| Procarbazine | DNA cross-linking/alkylating agent | Unavailable |
| Temozolomide | DNA cross-linking/alkylating agent | 2 |
| Vinblastine | Cell cycle specific microtubule/tubulin inhibition | 2 |
| Vincristine | Cell cycle specific microtubule/tubulin inhibition | 0 |
aNational Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. V.2.2019. Accessed at www.nccn.org/professionals/physician_gls/pdf/cns.pdf on October 5, 2019
bLexicomp Online, Hudson, Ohio: Wolters Kluwer Clinical Drug Information, Inc.; 2013; January 28, 2020
cLalami Y, Paesmans M, Muanza F, et al. (2006) Can we predict the duration of chemotherapy-induced neutropenia in febrile neutropenic patients, focusing on regimen-specific risk factors? A retrospective analysis. Ann. Oncol, 17:507–514. 10.1093/annonc/mdj092
dBased upon single drug therapy. A weight (0–4) is assigned to each drug according to its expected frequency of severe neutropenia (0 unusual, 1 very rare, 2 rare, 3 frequent, 4 very frequent)
While these negative chemotherapy-induced side effects are well noted and should be minimized whenever possible, a recently-devised strategy uses the lymphotoxicity of temozolomide to the clinician’s advantage within a specific treatment paradigm. Suryadevara and colleagues were able to utilize a dose-intensified temozolomide regimen to deplete host lymphocytes prior to CAR administration, which was associated with dramatically improved CAR proliferation, complete tumor regression, and increased survival in a murine model of GBM [84]. Examples such as this one highlight the ability of clinicians and researchers to develop innovative and/or unconventional uses of traditional chemotherapeutics to enhance antitumor immunity.
Conclusions
Gliomas create a profoundly immunosuppressive environment both locally at the tumor and systemically in the body, creating a number of challenges that negatively impact patient well-being and efficacy of novel immunotherapeutic approaches. In attempting to understand the pathobiology of these complex tumors, a multitude of mechanisms have been uncovered by which neoplastic cells develop the ability to evade detection and destruction by the immune system. By targeting one or more of these mechanisms, researchers hope to discover the next major treatment breakthrough that provides a meaningful survival benefit to a patient population greatly in need of one.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by MG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was utilized in the preparation and submission of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As this study is a review article with no new data collection, no ethical approval was required.
Informed consent
As this study is a review article with no new data collection or patient involvement, no informed consent was required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP, Fecci PE, Curry WT (2012) Cancer immunoediting in malignant glioma. Neurosurgery 71:201–22; discussion 222–3. 10.1227/NEU.0b013e31824f840d [DOI] [PubMed]
- 3.Woroniecka KI, Rhodin KE, Chongsathidkiet P, et al. T-Cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24:3792–3802. doi: 10.1158/1078-0432.CCR-18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Focosi D, Bestagno M, Burrone O, Petrini M. CD57 + T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 6.Strioga M, Pasukoniene V, Characiejus D. CD8+CD28- and CD8+CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornara O, Odeberg J, Solberg NW, et al. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. Oncoimmunology. 2015;4:1–14. doi: 10.1080/2162402X.2015.1036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler CJ, Black KL, Liu G, et al. Thymic CD8 + T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- 10.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716–724. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 12.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—A mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 14.Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ, Blattman JN, Murali-Krishna K, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/jvi.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24:4175–4186. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buggert M, Tauriainen J, Yamamoto T, et al. T-bet and eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014 doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez GJ, Pereira RM, Äijö T, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018 doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels V, Barrett JM, Bockman S, et al. Immunocytochemical study of transforming growth factor expression in benign and malignant gliomas. Am J Pathol. 1989;134:894–902. [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaefer C, Kim GG, Albers A, et al. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 26.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 27.El AA, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme1. Neuro Oncol. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 29.Poirier M-D, Haban H, El Andaloussi A. A combination of systemic and intracranial anti-CD25 immunotherapy elicits a long-time survival in murine model of glioma. J Oncol. 2009;2009:963037. doi: 10.1155/2009/963037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 31.Kriegsman BA, Vangala P, Chen BJ, et al. Frequent loss of IRF2 in cancers leads to immune evasion through decreased MHC Class I antigen presentation and increased PD-L1 expression. J Immunol. 2019;203:1999–2010. doi: 10.4049/jimmunol.1900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart CA, Laugier-Anfossi F, Vély F, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci USA. 2005;102:13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon WY, Powis SJ. Does natural killer cell deficiency (NKD) increase the risk of cancer? NKD may increase the risk of some virus induced cancer. Front Immunol. 2019;10:1703. doi: 10.3389/fimmu.2019.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane CA, Han SJ, Barry JJ, et al. TGF-β downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12:7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin A, Yan WH. Human leukocyte antigen-G (HLA-G) expression in cancers: roles in immune evasion, metastasis and target for therapy. Mol Med. 2015;21:782–791. doi: 10.2119/molmed.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kmiecik J, Poli A, Brons NHC, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Otvos B, Silver DJ, Mulkearns-Hubert EE, et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34:2026–2039. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Raychaudhuri B, Ireland PRJ, Ko J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. In: Shurin MR, Smolkin YS, editors. Immune-mediated diseases. Advances in experimental medicine and biology, vol 601. New York: Springer; 2007. [DOI] [PubMed] [Google Scholar]
- 43.Raychaudhuri B, Rayman P, Huang P, et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J Neurooncol. 2015;122:293–301. doi: 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- 44.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubinski D, Wölfer J, Hasselblatt M, et al. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol. 2016;18:807–818. doi: 10.1093/neuonc/nov280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gieryng A, Pszczolkowska D, Walentynowicz KA, et al. Immune microenvironment of gliomas. Lab Investig. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 52.Alban TJ, Alvarado AG, Sorensen MD, et al. Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI insight. 2018 doi: 10.1172/jci.insight.122264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chai E, Zhang L, Li C. LOX-1+ PMN-MDSC enhances immune suppression which promotes glioblastoma multiforme progression. Cancer Manag Res. 2019;11:7307–7315. doi: 10.2147/CMAR.S210545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peereboom DM, Alban TJ, Grabowski MM, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019 doi: 10.1172/jci.insight.130748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2015;19:20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science(80-) 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esiri MM, Morris CS. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2 Non-neoplastic diseases. J Neurol Sci. 1991;101:59–72. doi: 10.1016/0022-510X(91)90018-3. [DOI] [PubMed] [Google Scholar]
- 58.Wierzba-Bobrowicz T, Kuchna I, Matyja E. Reaction of microglial cells in human astrocytomas (preliminary report) Folia Neuropathol. 1994;32:251–252. [PubMed] [Google Scholar]
- 59.Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 60.Geranmayeh F, Scheithauer BW, Spitzer C, et al. Microglia in gemistocytic astrocytomas. Neurosurgery. 2007;60:159–166. doi: 10.1227/01.NEU.0000249192.30786.67. [DOI] [PubMed] [Google Scholar]
- 61.Mieczkowski J, Kocyk M, Nauman P, et al. Down-regulation of IKKβ expression in glioma-infiltrating microglia/macrophages is associated with defective inflammatory/immune gene responses in glioblastoma. Oncotarget. 2015;6:33077–33090. doi: 10.18632/oncotarget.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 63.Szulzewsky F, Arora S, de Witte L, et al. Human glioblastoma-associated microglia/monocytes express a distinct RNA profile compared to human control and murine samples. Glia. 2016;64:1416–1436. doi: 10.1002/glia.23014. [DOI] [PubMed] [Google Scholar]
- 64.Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016 doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zagzag D, Salnikow K, Chiriboga L, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Investig. 2005;85:328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 66.Romani M, Pistillo MP, Carosio R, et al. Immune checkpoints and innovative therapies in glioblastoma. Front Oncol. 2018;8:1–8. doi: 10.3389/fonc.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christofides A, Kosmopoulos M, Piperi C. Pathophysiological mechanisms regulated by cytokines in gliomas. Cytokine. 2014;71:377–384. doi: 10.1016/j.cyto.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Iwami K, Natsume A, Wakabayashi T. Cytokine networks in glioma. Neurosurg Rev. 2011;34:253–263. doi: 10.1007/s10143-011-0320-y. [DOI] [PubMed] [Google Scholar]
- 69.Albulescu R, Codrici E, Popescu ID, et al. Cytokine patterns in brain tumour progression. Mediat Inflamm. 2013 doi: 10.1155/2013/979748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shay JES, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol. 2012;23:389–394. doi: 10.1016/j.semcdb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Mangraviti A, Raghavan T, Volpin F, et al. HIF-1α- Targeting acriflavine provides long term survival and radiological tumor response in brain cancer therapy. Sci Rep. 2017 doi: 10.1038/s41598-017-14990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 75.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 77.Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961;81:46–53. [PubMed] [Google Scholar]
- 80.Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23:597–602. doi: 10.1097/WCO.0b013e32833e5a5d. [DOI] [PubMed] [Google Scholar]
- 81.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 82.Giles AJ, Hutchinson MKND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018 doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badie B, Schartner JM, Paul J, et al. Dexamethasone-induced abolition of the inflammatory response in an experimental glioma model: a flow cytometry study. J Neurosurg. 2000;93:634–639. doi: 10.3171/jns.2000.93.4.0634. [DOI] [PubMed] [Google Scholar]
- 84.Suryadevara CM, Desai R, Abel ML, et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karachi A, Yang C, Dastmalchi F, et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019;21:730–741. doi: 10.1093/neuonc/noz015. [DOI] [PMC free article] [PubMed] [Google Scholar]