Abstract
Persons with certain single nucleotide polymorphisms (SNPs) in the CACNA1D gene (encoding voltage-gated calcium channel subunit alpha 1-D) have increased risk of developing neuropsychiatric disorders such as bipolar, schizophrenia and autism. The molecular consequences of SNPs on gene expression and protein function are not well understood. Thus, the use of animal models to determine genotype-phenotype correlations is critical to understanding disease pathogenesis. Here, we describe the behavioural changes in larval zebrafish carrying an essential splice site mutation (sa17298) in cacna1da. Heterozygous mutation resulted in 50% reduction of splice variants 201 and 202 (haploinsufficiency), while homozygosity increased transcript levels of variant 201 above wild type (WT; gain-of-function, GOF). Due to low homozygote viability, we focused primarily on performing the phenotypic analysis on heterozygotes. Indeed, cacna1dasa17298/WT larvae displayed hyperlocomotion—a behaviour characterised in zebrafish as a surrogate phenotype for epilepsy, anxiety or psychosis-like behaviour. Follow-up tests ruled out anxiety or seizures, however, as neither thigmotaxis defects nor epileptiform-like discharges in larval brains were observed. We therefore focused on testing for potential “psychosis-like” behaviour by assaying cacna1dasa17298/WT larval locomotor activity under constant light, during light-dark transition and in startle response to dark flashes. Furthermore, exposure of larvae to the antipsychotics, risperidone and haloperidol reversed cacna1da-induced hyperactivity to WT levels while valproate decreased but did not reverse hyperactivity. Together, these findings demonstrate that cacna1da haploinsufficiency induces behaviours in larval zebrafish analogous to those observed in rodent models of psychosis. Future studies on homozygous mutants will determine how cacna1d GOF alters behaviour in this context.
Electronic supplementary material
The online version of this article (10.1007/s12035-020-02160-5) contains supplementary material, which is available to authorized users.
Keywords: Zebrafish, CACNA1D, Neurobehaviour, Neuropsychiatric disorders, Schizophrenia, Psychosis
Introduction
Schizophrenia (SCZ) is a highly heritable and polygenic neuropsychiatric disorder. The high prevalence of SCZ worldwide has made it a burgeoning public health concern. Genome-wide association studies (GWAS) have led to the initial identification of small nucleotide polymorphisms (SNPs) in about 108 risk loci linked to SCZ [1]. The majority of SNPs implicated in SCZ and other psychiatric disorders are located in noncoding (intergenic and intronic) regions of the genome [2]. To date, the functional significance of these noncoding variants remains unclear, thus obviating the need for establishment and validation of genetic animal models to elucidate underlying disease mechanisms associated with SCZ-risk genes. Nevertheless, SNPs in the intronic regions of genes are thought to result in changes in gene expression levels by altering splicing (via splice donor or acceptor sites) and transcription (via disruption of gene regulatory elements such as transcription factors, promoters, enhancers and/or suppressors) [3, 4]. For example, there is evidence that rs100637 (a SCZ-associated SNP, located in intron 3 of the CACNA1C gene) is associated with major depressive disorder (MDD), SCZ and attention deficit hyperactivity disorder, leads to changes in CACNA1C expression in both human carriers [5, 6] and induced human neurons [7]. In humans, broader phenotypes are present such as deficits in prepulse inhibition (PPI), latent inhibition and sleep disturbance in some SNP carriers, mimicking a broad spectrum of neuropsychiatric disorders [2, 8].
CACNA1D encodes the α1 subunit of the CaV1.3 voltage–gated L-type isoform. In the central nervous system (CNS) of humans, the CaV1.3 isoform is expressed in neuroendocrine cells, cerebral cortex, habenula, hippocampus, thalamus and basal ganglia, where it plays essential roles in consolidation of fear memory, drug-seeking behaviours and fine tuning of various elements of neuronal plasticity [9–12]. Mutations in CACNA1D have been implicated in neurodevelopmental and neuropsychiatric diseases [13–15]. For instance, persons with SNPs in CACNA1D have increased risk of developing bipolar disorder (BP), attention deficit hyperactivity disorder, SCZ, autism spectrum disorder (ASD) or MDD [1, 13, 16–21]. In one study, two single nucleotide variants in CACNA1D were found in separate cohorts of bipolar patients [22]. Although a study in the Han Chinese population did not find an association of CACNA1D with SCZ [23], other subsequent studies did identify such an association [8, 21]. Furthermore, whole-exome sequencing revealed variants in the CACNA1D coding region to be linked to ASD, depression, anxiety, fear and seizures [17, 18].
Homozygous CaV1.3 knockout mice are deaf and have impaired cardiac function [24], display antidepressant-like phenotypes [25] and have impaired consolidation of fear in the Pavlovian test [26]. In zebrafish (Danio rerio), cacna1d is duplicated into cacna1da and cacna1db, which encode CaV1.3a and CaV1.3b respectively [27]. The amino acid sequence of zebrafish Cacna1da and Cacna1db is 77% and 33% homologous to human CACNA1D respectively [28, 29]. Previous zebrafish studies have focused primarily on characterising the role of cacna1d in auditory and vestibular function [27, 30–32]. Homozygous cacna1da nonsense mutants display a classical auditory-vestibular phenotype reminiscent of “circler mutants” (i.e. circular swimming due to impaired balance) [27, 33, 34].
Interestingly, the essential splice site mutation described in this study was found to mimic both gene haploinsufficiency in the heterozygous state and gain-of-function (GOF) in the homozygous state. As homozygous mutant survival was low (i.e. very small sample size), we focused the majority of our analysis on heterozygotes, thus investigating the effects of cacna1da haploinsufficiency on larval zebrafish behaviour and electroencephalography (EEG) patterns. We profiled for anxiety-, seizure- or psychosis-like behaviours, by performing the following assays: PPI response, locomotor activity (constant light), light-dark transition test, thigmotaxis and startle response to dark flashes. We also tested the neuromodulatory activity of risperidone (RISP), an atypical antipsychotic drug [35]; haloperidol (HALO), a dopamine antagonist used as a typical antipsychotic drug [36]; and valproic acid (VPA), an anti-seizure drug and mood stabiliser [37, 38], to determine whether cacna1d mutant larvae would respond in a similar manner as SCZ, BP and/or epilepsy patients/animal models. Finally, EEG recordings were performed to establish whether the hyperlocomotion observed at earlier developmental time points resulted from epileptiform-like brain activity.
Materials and Methods
Ethical Considerations
Approval by the Norwegian Food Safety Authority via its experimental animal administration’s supervisory and application system (FOTS-ID 15469 and 23935) was obtained prior to animal experimentation. Also, compliance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and the European Community Council Directive of November 2010 for Care and Use of Laboratory Animals (Directive 2010/63/EU), as well as the ARRIVE guidelines, were adhered to during all experiments.
Zebrafish Strains and Husbandry
The sa17298 mutant line was generated by N-ethyl-N-nitrosourea (ENU) mutagenesis within the Zebrafish Mutation Project (Sanger, UK). The line carries a point mutation in cacna1da, which spans an essential splice site [for details see: [39]]. Fertilised sa17298 embryos were obtained from the Zebrafish International Resource Center (Eugene, Oregon, USA) and raised to adulthood, genotyped and the heterozygous animals outcrossed to AB wild-type (WT) zebrafish for three generations. Animals were raised under controlled conditions described by [40], in a 14-/10-h light/dark cycle at 28.5 °C.
Fertilised eggs from natural spawning of adult fish lines were collected, transferred to petri dishes (density N = 50–70), filled with embryo medium (17 mM NaCl, 2 mM KCl, 1.8 mM Ca(NO3), 2,0.12 mM MgSO4, 1.5 mM HEPES buffer pH 7.1–7.3 and 0.6 μM methylene blue). These petri dishes were stored in an incubator with a 14/-10-h light/dark cycle at 28.5 °C. The medium was refreshed daily until larvae reached 7-day post-fertilisation (dpf).
Genotyping
Adult fin clip tissue or whole larvae were snap frozen in liquid nitrogen. DNA from fin clip tissue or larval sample was extracted using PCR extraction buffer (10 mM Tris (pH 8.0), 2 mM EDTA, 0.2% Triton X-100) and proteinase K (200 μg/ml). DNA was amplified using DreamTaq DNA polymerase (EP0702, Thermofischer) according to the manufacturer’s instructions in a 20 μL final volume. The forward primer: 5′ TGTGCTGGTGTTGTGTGTG 3′ and reverse primer: 5′ TCAAGCCAGGAAGTACTGAAG 3′ were used with the following cycling conditions: Step 1: Initial denaturation 95 °C, 1 min; Step 2: Denaturation 95 °C, 30 s; Step 3: Annealing temperature 59 °C, 30 s; Step 4: Extension 72 °C, 1 min; Step 5: Repeat steps 2–4, 34 ×; Step 6: Final extension 72 °C, 1 min. This resulted in a 196 base pair (bp) amplicon. The PCR product was digested using the restriction enzyme, BstEII (R0162M, New England BioLabs). The bands were visualised using 2% agarose gel electrophoresis and Sybr Safe (S33102, Thermofischer) as the DNA intercalating agent. Observers conducting behavioural tests were blind to the genotype of the larvae. Hence, larvae were genotyped after each experiment.
Morphological Assessment
Larvae were photographed using a Leica M205 FA stereomicroscope and assembled using Adobe Photoshop 2020. All pictures were taken at 5 dpf at the same resolution for comparison.
Drugs
The following drugs were used: 5 μM RISP, 50 μM HALO and 100 μM VPA. All drugs were dissolved in DMSO at a final concentration of 0.5% v/v DMSO for the HALO and VPA groups but 0.1% v/v DMSO for the RISP-treated group. The appropriate vehicle controls were prepared by dissolving DMSO in zebrafish E3 medium. DMSO and HALO were purchased from Sigma-Aldrich, RISP from TOCRIS and VPA from Sanofi Aventis. The drug concentrations used were selected based on previously published studies [35, 36, 41].
Behavioural Tests and Material
Larvae were transferred to the behavioural analysis room at least 1 h prior to experimentation to allow animals to acclimate. Single larvae were gently transferred from the petri dishes to individual wells of a 24-well plate (diameter 16.2 mm) except when stated otherwise. Automated video-tracking of larval behaviour was carried out using the ZebraBox hardware and ZebraLab software (Viewpoint, Lyon, France). Light-dark test and thigmotaxis measurement were carried out using the same larvae by simply defining the inner and outer regions of each well of the tracking plate (the inner zone was of diameter 8 mm while the distance from the inner zone relative to the outer zone was 4 mm). A different set of larvae were used for the startle response test. Except for the startle response to dark flashes test, all other experiments were replicated two or three times and the results pooled together.
Locomotor Activity and Thigmotaxis
Larvae were allowed to acclimate to the test chamber (ZebraBox) for 15 min in the dark, followed by 10 min of tracking in either 100% light or dark (0% light). For the light-dark transition test, after the acclimation, 10 min each of tracking in the following conditions: (1) 100% light followed by (2) dark in succession. The locomotor activity of larvae was measured as the total distance travelled in millimetres (mm) over a 10-min period.
For thigmotaxis, the following parameters were measured: (1) distance spent in inner zone, (2) distance spent in outer zone and (3) distance in entire arena. Thigmotaxis was calculated as percentages using the following formula [42]:
Startle Response to Dark Flashes
To trigger a startle response in larvae, 150-ms-long dark flashes were used, and this was repeated severally to assess the ability of the larvae to habituate. The startle response test was made up of three steps: (1) 15-min acclimatisation period in 100% illuminated test chamber, (2) 10-min baseline locomotor tracking in the fully illuminated test chamber, (3) followed by the presentation of 30 dark flashes repeated every 3 s [43].
Locomotor Activity of Drug-Treated Larvae
Larvae were pre-exposed 30 min to RISP, HALO or VPA and their respective vehicle control groups, transferred to the test chamber and tracking started immediately for 4 h at a 30 -min integration period. Only tracks after 2 h were used for analysis. For the 24-h RISP-treated group, larvae were pre-exposed to treatment 22 h then transferred to the test chamber and tracked immediately for 4 h with the tracks after 2 h used for analysis. Larvae were tracked in 100% light condition and the locomotor activity of larvae were measured as the total distance travelled in millimetres over a 30-min period. Vehicle control and drug-treated larvae were from the same clutch.
Acoustic Startle Response (ASR) and Prepulse Inhibition (PPI)
Acoustic stimuli were delivered using the ZebraBox Revo and the behavioural response analysed with EthoVision software as previously described [44] using larvae aged 6 dpf. All experiments were performed by placing larvae individually in each well of a custom-made plexiglass plate of 33 wells in a 96 format. Acclimation was for 5 min prior to the onset of experiment in a 100-Lx illuminated chamber. The startle stimulus was 100 ms at 660 Hz while the prepulse stimulus was 5 ms at 440 Hz. A 100-ms inter-stimulus interval (ISI) was used for PPI experiments.
Zebrafish EEG Recordings
The EEG recordings were performed as previously described by Afrikanova et al. [45]. Epileptiform-like discharges were detected by inserting a glass electrode filled with artificial cerebrospinal fluid (124 mM NaCl, 2 mM KCl, 2 mM MgSO4, 2 Mm CaCl2, 1.25 mM KH2PO4, 26 mM NaHCO3, 10 mM glucose) into the optic tectum of individual 6-dpf zebrafish larvae for 20 min (MultiClamp 700B amplifier, Digidata 1550 digitiser, Axon instruments, USA). The larvae were restrained with the aid of a thin layer of 2% low melting point agarose. The Clampfit version 10.6.2 software (Molecular Devices Corporation, USA) was used for processing the EEG recordings. The data were analysed manually by a trained observer, blind to the genotype of the larvae.
RNA Isolation and cDNA Synthesis
Total RNA was isolated from a pool of 6-dpf larvae (N = 25 larvae, 3 replicates/group) using the Invitrogen PureLink RNA Mini Kit (12183018A, Thermofischer). A Nanodrop 1000 spectrophotometer and agarose bleach gel described by [46] was used to assess the quality/integrity of the RNA. cDNA was synthesised from 1 μg RNA with oligo dT primers using the Invitrogen SuperScript IV Reverse Transcriptase (18090050, Thermofischer) according to the manufacturer’s instructions.
Reverse Transcriptase and Quantitative Polymerase Chain Reaction
rt-PCR was performed for 40 cycles using Taq-polymerase (EP0406, Thermofischer) in a 20 μl final reaction using 1 μl of cDNA template. PCR products were visualised on 2% agarose gels. The qPCR reactions were performed with Power SYBR Green Master Mix (4368702, Thermofischer) on the CFX384 Touch Real-Time PCR Detection System (1855485, Bio Rad). All primers were synthesised by Sigma with melting temperature > 60 °C. Samples were run in triplicates in a 20 μl final volume containing 10 μl 2 × sybr green, 1 μl 10 μM forward + reverse primer and 9 μl 1:180 cDNA dilution. The following cycling parameters were used: 95 °C for 10 min, and then 40 cycles of 95 °C for 15 s, 60 °C for 1 min. To check for the presence of non-specific products and/or primer dimers, a dissociation step was performed at the end of each amplification phase from 65 to 95 °C, at 0.5 °C increment for 5 s. The comparative ΔΔCT method was calculated using the Bio Rad CFX Manager 3.1. The endogenous genes glyceraldehyde-3-phosphate dehydrogenase (gapdh) and ribosoamal protein S18 (rps18) were used for data normalisation. See Suppl. Table 1 for the list of rt-/q-PCR primer sequences.
Statistical Analysis
Statistical analysis and figures generation were performed with the aid of GraphPad Prism 8.4.1 (San Diego, CA, USA). For behavioural experiments, a two-way analysis of variance (ANOVA) (factors: phase/treatment and genotype), followed by Tukey’s post hoc test, was used. For EEG experiment, Student’s t test was applied. Unpaired Student’s t test or its equivalent non-parametric test, Mann Whitney U, was performed where necessary. Statistical significance was established at p < 0.05. In figures, dots represent individual measurements.
Results
Genotyping and Molecular Consequences of the cacna1da Splice Site Mutation
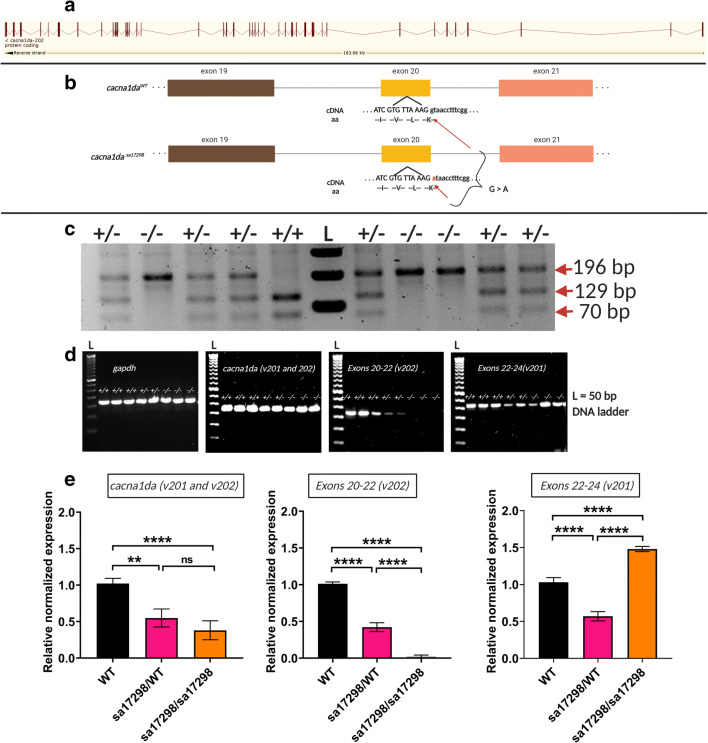
As mentioned earlier, the amino acid sequence of zebrafish Cacna1da and Cacna1db are 77% and 33% homologous to human CACNA1D protein respectively. cacna1da has two transcript variants while cacna1db has a single transcript [28, 29]. The cacna1da transcript variant 202 spans 47 exons (Fig. 1a), whereas variant 201 spans 48 exons (not shown). The sa17298 allele contains a single point mutation (G > A) at the first residue of intron 20–21 of the cacna1da-202 transcript variant (Fig. 1b). Importantly, it does not alter the sequence of variant 201. To identify mutants, PCR of larval and/or adult fin clip tissue was digested using BstEII restriction enzyme. The BstEII restriction site is absent in the mutant allele. Hence, 2% agarose gel electrophoresis of the digested product resulted in one band for homozygous sa17298 (~ 196 bp), two bands for WT sa17298 (~ 70 and 129 bp) and three bands for heterozygous sa17298 (~ 70, 129 and 196 bp) (Fig. 1c).
Fig. 1.
Zebrafish cacna1da mutant allele (sa17298) and its molecular consequence. a Schematic representation of the zebrafish cacna1da transcript. Red bars represent exons and lines represent introns. Image retrieved from ensembl.org. b Schematic representation of zebrafish cacna1da and sa17298 mutation. Nucleotides in upper case are within the exon while those in lower case are within the intron. c Sample gel electrophoresis of BstEII restriction digest and resulting PCR products to determine genotype of fish/larvae. BstEII does not cut homozygous sa17298, but cuts WT into two bands (70 and 119 bp) and heterozygous sa17298 into three bands (70, 119 and 196 bp). Note the multiple bands in the BstEII digested versus the undigested corresponding samples. L: 1 kilobase DNA ladder, +/+: cacna1daWT/WT, +/−: cacna1dasa17298/WT, −/−: cacna1dasa17298/sa17298. d cacna1da mRNA levels as measured by agarose gel electrophoresis (rt-PCR). e cacna1da mRNA levels as measured by qPCR. cacna1da mRNA expression is normalised against gapdh and rps18
To assess whether the splice site mutation resulted in exon skipping, rt-PCR, followed by agarose gel electrophoresis, was performed using primers spanning exons 20–22 of cacna1da transcript variant 202. No difference in product size was observed between WT and homozygous mutants, thus suggesting that the mutation did not result in exon skipping. Notably, however, a difference in signal intensity of the rt-PCR amplicons was observed (Fig. 1d). Given the difference in signal intensity obtained from WT and homozygotes, we next tested whether the mutation altered mRNA levels. We performed qPCR analysis using three cacna1da primer sets. The first primer set [cacna1da_qPCR] amplified a region with high complementarity between the 201 and 202 transcript variants, the second primer set [E20-22_v202] targeted only the 202 transcript while the third primer set [E22-24_v201] targeted only the 201 transcript (Suppl. Fig. 1). qPCR analysis revealed an overall reduction of cacna1da mRNA levels (ca. 50% and 40%) in heterozygous and homozygous mutants respectively. Primer-specific amplification of variant 202 showed a 50% and 90% decrease in transcript 202 levels in heterozygous and homozygous mutants respectively. Interestingly, when primers specifically targeting transcript variant 201 were used, homozygous mutants showed a 50% increase, while heterozygous mutants showed a 50% reduction in levels of variant 201 (Fig. 1e).
Morphological Analysis of sa17298 Mutants
When AB-outcrossed mutants were bred, the resulting heterozygous and homozygous mutants were morphologically indistinguishable from their WT siblings (Fig. 2). All experiments were carried out using the AB outcrossed fish.1 Based on genotyping results, less than 5% of homozygous sa17298 larvae survived until adulthood. No impairment in the touch-evoked response of heterozygous and homozygous sa17298 larvae was observed when compared to WT (data not shown).
Fig. 2.
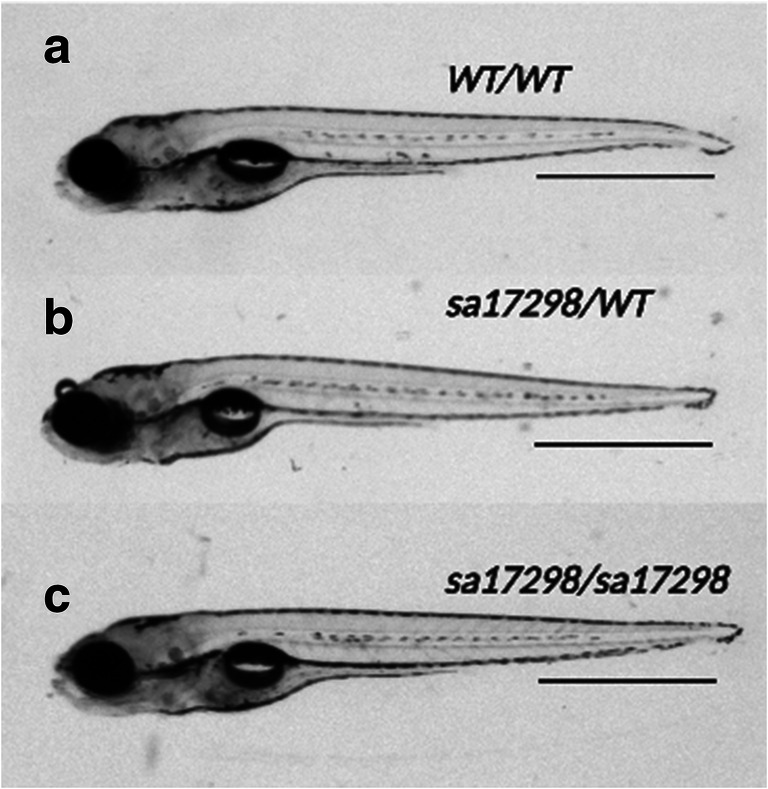
Morphology of a WT cacna1daWT/WT b heterozygous cacna1dasa17298/WT and c homozygous cacna1dasa17298/sa17298. Both heterozygous and homozygous mutants are morphologically indistinguishable from WT siblings. Scale bar (a–c): 1 mm
Acoustic Startle Response and Prepulse Inhibition
Four different auditory stimuli (40, 50, 60, 70 dB) at 660 Hz were tested to determine the suitable startle stimuli, and it was determined that 70 dB evoked the strongest startle response at p < 0.05 (Fig. 3a). When a 50-dB prepulse preceded the 70-dB stimulus, larval startle response was decreased (Fig. 3b). One-way ANOVA revealed no statistically significant difference in the PPI (%) between WT and either mutant genotype (i.e. heterozygotes and homozygotes) (Fig. 3c) [F (2, 16) = 1.403, p = 0.2745]. Notably, however, there was a tendency of homozygotes towards statistical significance (i.e. increased PPI).
Fig. 3.
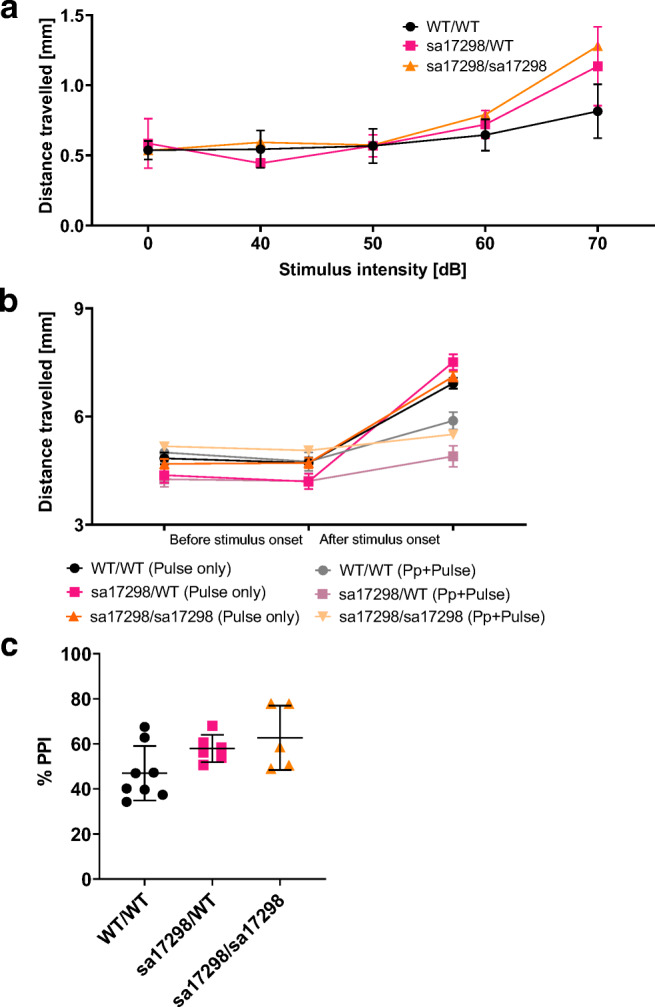
Acoustic startle response and PPI. a Graph of distance travelled when presented with different auditory stimuli. Data analysed using two-way ANOVA and presented as mean ± SEM. b Preceding a startle with a prepulse stimulus decreases the startle response of larvae. Data shown as mean ± SEM. c % PPI of larvae. One-way ANOVA revealed no overall significant difference among the groups. Tukey’s multiple comparison post hoc test however showed a strong tendency of homozygous mutant larvae towards significance (i.e. enhanced PPI). cacna1daWT/WT (N = 8), cacna1dasa17298/WT (N = 6) and cacna1dasa17298/sa17298 (N = 5), p > 0.05. Error bars represent mean ± SD. ASR, acoustic startle response; Pp, prepulse; PPI, prepulse inhibition. cacna1daWT/WT vs cacna1dasa17298/WT: p = 0.2002, cacna1daWT/WT vs cacna1dasa17298/sa17298: p = 0.0646, cacna1dasa17298/WT vs cacna1dasa17298/sa17298: p = 0.7702
Locomotor Assessment of sa17298 Larvae
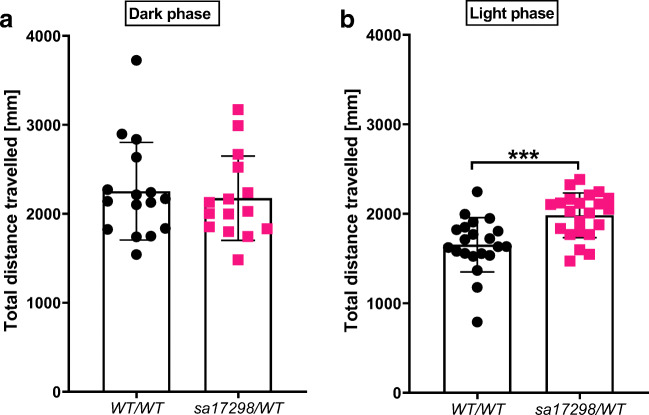
To determine if the sa17298 mutation modulates locomotor behaviour, one batch of larvae were tracked in dark conditions (0% light) while another group of larvae were tracked in 100% light. Mann Whitney U test showed no statistically significant difference between the genotypes [cacna1daWT/WT (Mdn = 2155, N = 16) and cacna1dasa17298/WT (Mdn = 2027, N = 15); U = 109, p = 0.6823] when tracked in the dark (Fig. 4a). On the other hand, when larvae where tracked in 100% light conditions, Mann Whitney U test indicated that the total distance travelled by cacna1daWT/WT (Mdn = 1632, N = 21) was significantly lower than cacna1dasa17298/WT (Mdn = 2039, N = 22); U = 92, p = 0.0005] (Fig. 4b).
Fig. 4.
Locomotor activity of WT and heterozygous sa17298 zebrafish larvae at 6 dpf, tracked independently under different illumination conditions. a Tracking in the dark reveals no difference in locomotor activity between cacna1daWT/WT (N = 16) and cacna1dasa17298/WT (N = 15). b Tracking in 100% light reveals a statistically significant difference in locomotor activity between cacna1daWT/WT (N = 21) and cacna1dasa17298/WT (N = 22). ***p < 0.001. Data analysed using Mann Whitney U test and represented as mean ± SD
Larval Behaviour in Response to Abrupt Change in Illumination
The locomotor behaviour of larval zebrafish differs depending on light-dark transitions [47]. Based on the observations made when two groups of larvae were independently tracked in either light or dark conditions, we hypothesised that changes in illumination in the tracking chamber would alter locomotor activity of sa17298 larvae. We analysed spontaneous swimming after a 15-min acclimation period. Swimming in each illumination state (light-dark) was recorded for 10 min (Fig. 5).
Fig. 5.
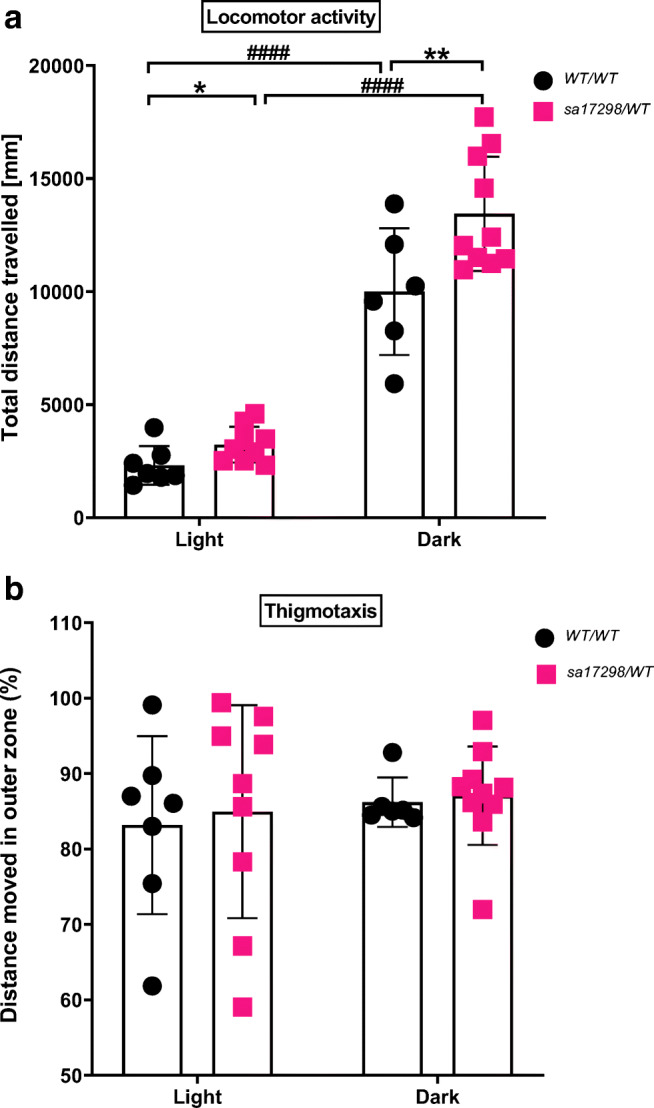
Behaviour of WT and heterozygous sa17298 zebrafish larvae at 6 dpf in the light-dark transition test. Each dot represents an individual larval measurement. Data analysed using non-RM two-way ANOVA followed by Tukey’s post hoc test. Data represented as mean ± SD. a Light-dark transition elicits an increase in locomotor activity in both genotypes with locomotor difference between cacna1daWT/WT and cacna1dasa17298/WT reaching statistical significance in both illumination states. * (p < 0.05), ** (p < 0.01): [cacna1daWT/WT vs cacna1dasa17298/WT] in the dark, #### (p < 0.0001): [cacna1daWT/WT vs cacna1daWT/WT] and [cacna1dasa17298/WT vs cacna1dasa17298/WT]. b Zone preference of WT and heterozygous sa17298 larvae (thigmotaxis) represented as % total distance moved in the outer zone in the light-dark transition test
In Fig. 5 a, larvae demonstrated an increased locomotor activity when transitioned from light to dark shown by a non-RM two-way ANOVA analysis [genotype F (1, 28) = 9.645, p < 0.01; illumination F (1, 28) = 162.3, p < 0.0001; interaction F (1, 28) = 3.241, p = 0.0826]. Tukey’s post hoc test revealed that both cacna1daWT/WT (p < 0.0001) and cacna1dasa17298/WT (p < 0.0001) travelled higher distances in the dark than in the light. Additionally, the post hoc test indicated statistical significance between cacna1daWT/WT and cacna1dasa17298/WT when they transitioned to the dark (p < 0.01), whereas Student’s t test showed statistical significant difference between cacna1daWT/WT and cacna1dasa17298/WT in the light phase (p < 0.05).
A previous study reported that changes in illumination altered the zone preference of larvae in the thigmotaxis test [42]. Therefore, we sought to determine if changes in illumination modulated the zone preference of cacna1daWT/WT and cacna1dasa17298/WT larvae. Larvae were assessed for the distance travelled (%) in the outer zones of a 24-well plate arena within 10 min.
As seen in Fig. 5b, there was no significant difference in the zone preference of cacna1daWT/WT and cacna1dasa17298/WT larvae using non-RM two-way ANOVA analysis in the light-dark transition [genotype F (1, 28) = 0.1310, p = 0.7201; illumination F (1, 28) = 0.4996, p = 0.4855; interaction F (1, 28) = 0.01618, p = 0.8997].
Assessment of Startle Response to Repetitive Dark Flashes
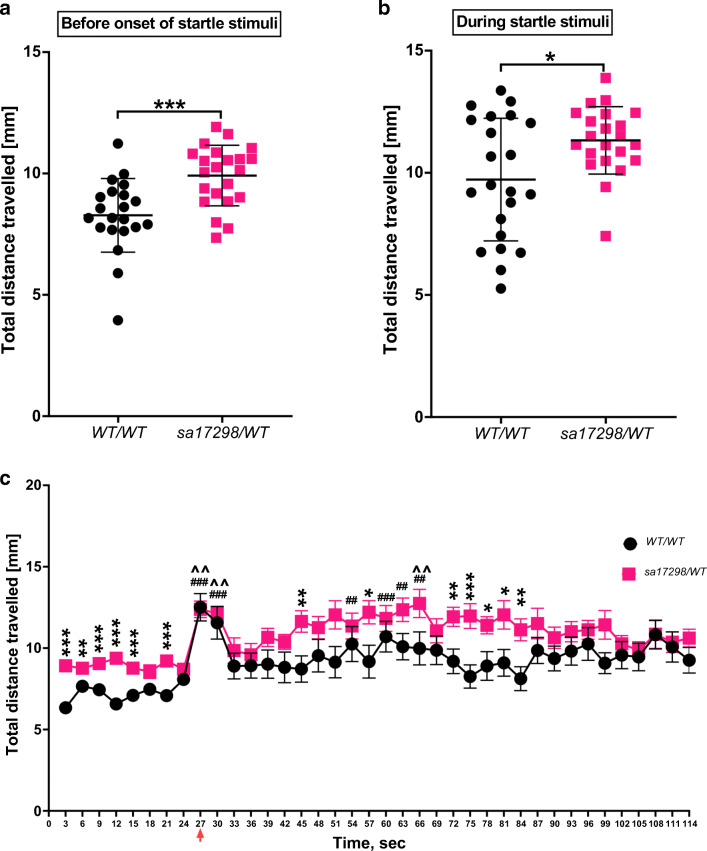
Zebrafish larvae respond to startle stimuli such as unexpected light or sound stimulus and quantifying changes in distance travelled have been established as a suitable readout of startle response [43, 48]. Following the “startle response to a dark flash” protocol of Norton, larvae were presented with 30 dark flashes repeated every 3 s [43].
The locomotor activity of the last 21 s of the 10-min basal locomotor activity was measured preceding the dark flashes that were every 3 s thirty times (Fig. 6a). In general, cacna1daWT/WT and cacna1dasa17298/WT larvae neither swum the same distance during 21-s basal locomotor activity [t (41) = 3.879, p = 0.0004, Fig. 6a] nor the 90-s startle experiment [t (41) = 2.627, p = 0.0120, Fig. 6b].
Fig. 6.
Startle response to dark flashes. a Baseline locomotor activity 21 s prior to onset of startle stimuli, ***p < 0.001. Data represented as mean ± SD. b Mean distance moved in response to startle stimuli. Unpaired Student’s t test showed cacna1daWT/WT moved less than cacna1dasa17298/WT during the 90-s startle stimulation, *p < 0.05. Data represented as mean ± SD. c Distance moved in response to startle stimuli represented as mean ± SEM. Paired Student’s t test analysis between cacna1daWT/WT and cacna1dasa17298/WT with statistical significance represented as *p < 0.05, #p < 0.01, ^p < 0.001. Red arrow: onset of startle stimulus (dark flashes)
The distance moved in response to the first and second dark flash was greater than the distance moved in response to subsequent stimuli. Whereas WT larvae showed signs of habituation after the 2nd stimulus, habituation was only visible after the 19th stimulus in heterozygous mutants. Multiple t test analysis showed that at several time points, cacna1dasa17298/WT larvae were significantly more active than cacna1daWT/WT at p < 0.05. Overall, cacna1daWT/WT displayed a robust startle response relative to baseline than cacna1dasa17298/WT [i.e. when we compared the last 21 s prior to stimulus onset with startle stimuli response] (Fig. 6c).
Effects of Neuroactive Drugs on the Locomotor Activity of WT and Heterozygous sa17298 Zebrafish Larvae
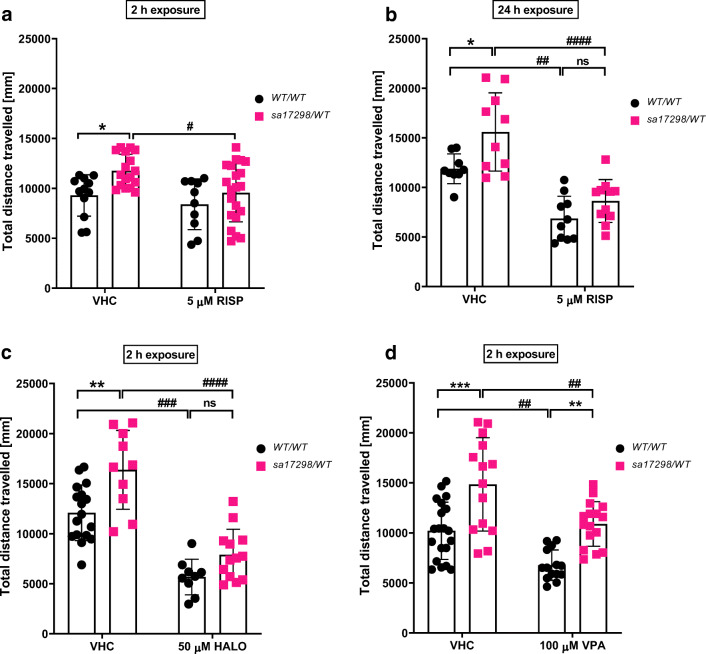
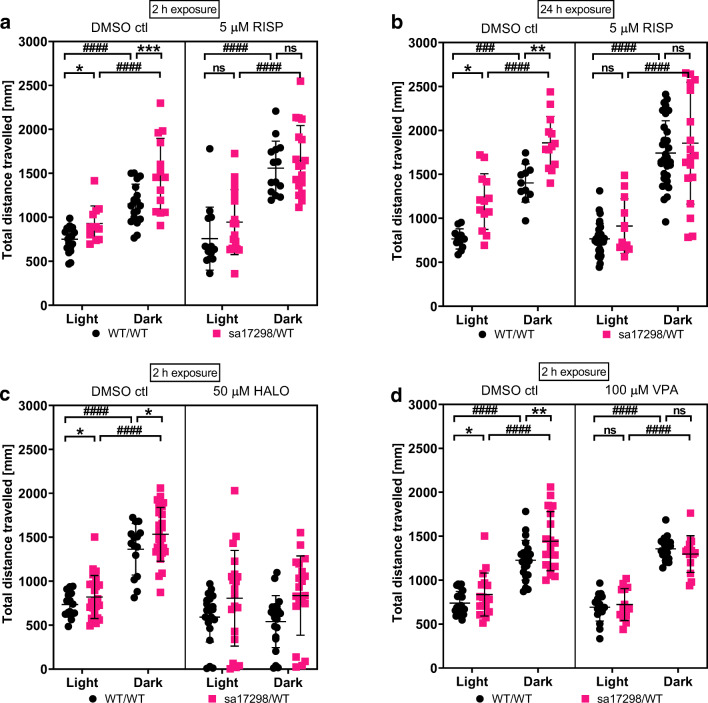
In zebrafish, hyperlocomotion is often characterised as a phenotype of epilepsy- [45, 49–52], anxiety- [51, 53] or psychosis-like behaviour [51, 54, 55]. To understand the possible cause of the hyperlocomotor behaviour, we observed in heterozygous sa17298/WT larvae, three neuroactive drugs i.e. RISP, HALO and VPA were evaluated for their ability to modulate the larval hyperactivity after 30 min of tracking. Non-RM two-way ANOVA revealed a significant effect of both genotype [F (1,56) = 8.010, p = 0.0064] and treatment [F (1,56) = 5.799, p = 0.0193] on locomotor activity when larvae were exposed to 5 μM RISP for 2 h. No significant interaction [F (1,56) = 1.066, p = 0.3064] was observed. However, a Tukey’s post hoc test showed that RISP was only effective in decreasing the locomotor activity of sa17298/WT (p < 0.05) but not WT/WT (p > 0.05) when compared with their untreated control (Fig. 7a).
Fig. 7.
Effects of neuroactive drugs on the locomotor activity of 6-dpf WT and heterozygous sa17298 larvae. Larvae were exposed to different neuroactive drugs. Each dot represents individual larval measurement. Data analysed using non-RM two-way ANOVA followed by Tukey’s post hoc test. Data represented as mean ± SD. a 2-h RISP, b 24-h RISP c 2-h HALO and d 2-h VPA. HALO, haloperidol; RISP, risperidone; VPA, valproic acid. *p < 0.05, **p < 0.01, ***p < 0.001 [cacna1daWT/WT vs cacna1dasa17298/WT] in respective groups. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 [cacna1daWT/WT vs cacna1daWT/WT] and [cacna1dasa17298/WT vs cacna1dasa17298/WT]
To ascertain whether a prolonged exposure of zebrafish larvae to RISP would enhance RISP’s effectiveness in affecting locomotor activity, a set of larvae were incubated in RISP for 24 h and the locomotor activity measured subsequently. As seen in Fig. 7b, non-RM two-way ANOVA showed an overall statistically significant effect of RISP on larval locomotor activity after 24-h pre-exposure [genotype [F (1,36) = 10.77, p = 0.0023]; treatment [F (1,36) = 51.41, p < 0.0001]; interaction [F (1,36) = 1.351, p = 0.2527]]. Tukey’s post hoc test revealed a statistically significant decrease in locomotor activity in larvae treated with 5 μM RISP when compared with their untreated control at least p < 0.01 such that baseline locomotor activity became comparable between 5 μM [WT/WT vs sa17298/WT] at p = 0.4262.
Furthermore, two-way non-RM ANOVA of larvae exposed to 50 μM HALO for 2 h (Fig. 7c) yielded a statistically significant effect of genotype [F (1,45) = 15.03, p = 0.0003] and treatment [F (1,45) = 78.55, p < 0.0001] on locomotor activity with no significant interaction [F (1,45) = 1.518, p = 0.2243]. Subsequent Tukey’s post hoc analysis revealed a reduction in locomotor activity of the 50 μM treated larvae when compared to their respective within group control at least p < 0.001. There was no statistical significance observed between 50 μM [WT/WT vs sa17298/WT] at p = 0.3117.
In Fig. 7d, larvae pre-treated in VPA for 2 h displayed an overall significant effect of genotype [F (1,60) = 33.15, p < 0.0001] and treatment [F (1,60) = 23.57, p < 0.0001] on larval locomotor activity with no significant interaction [F (1,60) = 0.1149, p = 0.7358] observed when a two-way non-RM ANOVA was performed. Tukey’s post hoc analysis showed statistically significant effect of VPA to decrease larval locomotor activity across groups at least p < 0.01 when compared with untreated larvae of the same genotype. However, VPA was unable to reduce the hyperlocomotion of sa17298/WT larvae to a comparable level as the VPA-treated WT/WT group (p = 0.0023).
Effects of Neuroactive Drugs on the Behaviour of WT and Heterozygous sa17298 Zebrafish Larvae in the Light-Dark Test
The hyperlocomotor activity of heterozygous larvae relative to WT in the light and dark states was reversed after the exposure of larvae to all the treatment options i.e. 24-h exposure in 5 μM RISP and 2-h exposure in 5 μM RISP, 50 μM HALO and 100 μM VPA at p > 0.05. Larvae in either genotypes exposed to all the treatment groups but HALO were capable of eliciting a light-dark response by increasing their locomotion (p < 0.001) (See Fig. 8 and Suppl. Table 2).
Fig. 8.
Effects of neuroactive drugs on the behaviour of 6-dpf WT and heterozygous sa17298 larvae in the light-dark test. Larvae were exposed to different neuroactive drugs. Each dot represents individual larval measurement. Data analysed using two-way ANOVA followed by multiple comparison t test. Data represented as mean ± SD. a 2-h RISP, b 24-h RISP, c 2 h HALO and d 2-h VPA. ctl, control; HALO, haloperidol; RISP, risperidone; VPA, valproic acid. *p < 0.05, **p < 0.01, ***p < 0.001 [cacna1daWT/WT vs cacna1dasa17298/WT] in respective groups. ###p < 0.001, ####p < 0.0001 [cacna1daWT/WT vs cacna1daWT/WT] and [cacna1dasa17298/WT vs cacna1dasa17298/WT]
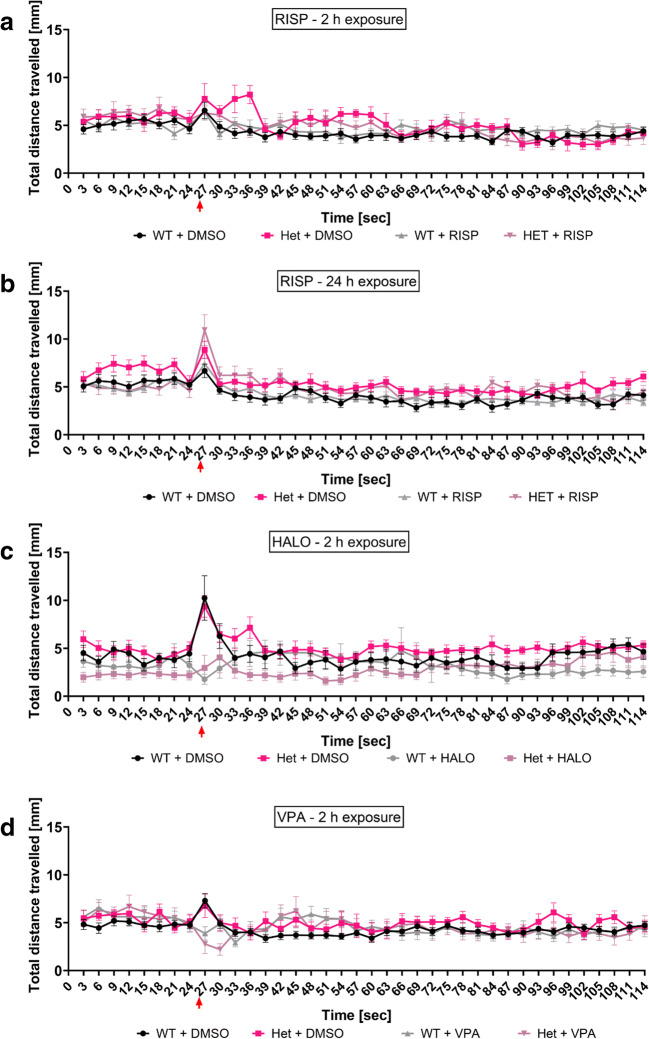
Effects of Neuroactive Drugs on the Behaviour of WT and Heterozygous sa17298 Zebrafish Larvae in the Startle Response to Dark Flashes
Larvae treated in vehicle control behaved similarly as their untreated (medium) counterparts previously described. In the 2-h exposure to RISP group, there was no difference in locomotor activity between WT and heterozygous mutant larvae prior to the onset of the dark flashes. After dark flashes onset, there was reduced startle response of heterozygous larvae in the first five dark stimuli with the next seven dark flashes resulting in comparable behaviour to the untreated group. In general, RISP at 2 h reduced heterozygous larval reactivity to the startle inducing dark flashes (p > 0.05, Fig. 9a). At 24-h exposure, RISP reversed the hyperlocomotor activity of heterozygous larvae prior to the onset of dark stimuli (p > 0.05, Fig. 9b). However, upon onset of dark flashes, heterozygous larvae behaved similar to their untreated siblings i.e. hyperactivity (p < 0.01, Fig. 9b). On the other hand, HALO was able to reverse the hyperlocomotor activity of heterozygous larvae both prior to and at the presentation of the startle inducing dark flashes (p > 0.05, Fig. 9c). Whereas, VPA treatment resulted in a delayed response of larvae to dark flashes and an overall reversal of hyperactivity of the heterozygous larvae (p > 0.05, Fig. 9d) (For modulatory activity of all drugs over time, see Suppl. Fig. 3 and Table 3).
Fig. 9.
Time series graph of the effects of neuroactive drugs on the behaviour of 6-dpf WT and heterozygous sa17298 larvae in the startle response to dark flashes test. Larvae were exposed to different neuroactive drugs. Each dot represents individual larval measurement. Data represented as mean ± SEM. a 2-h RISP, b 24-h RISP, c 2-h HALO, d 2-h VPA. HALO, haloperidol; RISP, risperidone; VPA, valproic acid
EEG Assessment
Student’s t test analysis of tectal EEG recordings of 6-dpf larvae indicated that there were no statistically significant differences with regard to the number of epileptiform-like discharges between cacna1daWT/WT (M = 2.088, N = 12) and cacna1dasa17298/WT (M = 4.333, N = 15); t (25) = 0.8741, p = 0.3904 (for example, see Suppl. Fig. 4).
Discussion
In this study, we describe for the first time neurobehavioural changes in larval zebrafish with an essential splice variant mutation (sa17298) in the cacna1da gene. Since our mutant (sa17298) harbours a single point mutation (G > A) at the donor splice site of intron 20–21 of the cacna1da-202 transcript variant, we anticipated that this could lead to dysregulation of cacna1da mRNA or protein expression, thus mimicking classical GOF or loss-of-function mutations. Agarose gel electrophoresis of rt-PCR products did not suggest exon skipping as a consequence of the mutation. The results showed significantly reduced “overall” cacna1da mRNA and 202 transcript levels in both heterozygous and homozygous mutants relative to WT. For the 201 transcript, while heterozygous mutants showed reduced levels, homozygous mutants on the contrary showed increased levels. We speculate that the sa17298 splice variant mutation leads to a premature termination codon in variant 202, as a result of intron retention, thereby resulting in nonsense-mediated decay, thus resulting in loss or significant reduction of this particular transcript and subsequent compensation through an increase in levels of transcript variant 201.
Our study revealed significant behavioural impairments in cacna1dasa17298/WT mutants when assayed for locomotor activity (under light conditions), during light-dark transition and in the startle response to dark flashes. However, we did not observe significant PPI deficits, thigmotaxis-related abnormalities or epileptiform-like discharges in larval brains as measured by EEG recordings.
The behavioural response of cacna1daWT/WT in the light-dark stimulus task is in agreement with other studies [41, 56–59], which showed an abrupt increase in locomotor activity of larvae during rapid switching from light to dark. Kedra et al. [56] revealed that homozygous tsc2-deficient larvae (model of tuberous sclerosis complex, in which 90% of patients display seizures) exhibited the same behavioural response as described here for cacna1dasa17298/WT mutants i.e. a pronounced increase in locomotor activity of tsc2−/− larvae compared with their control counterparts in the dark phase of the light-dark stimulus task. tsc2−/− larvae also displayed increased thigmotaxis as well as a preference to light phase in the light-dark preference task. Exacerbation of thigmotactic behaviour in zebrafish during dark phase is regarded as a marker of anxiety-like behaviour [42, 60], thus indicating an anxiety-like phenotype for tsc2−/− larvae. In contrast, we did not observe changes in thigmotaxis between cacna1da genotypes. Thus, it is unlikely that the increased locomotor activity of cacna1dasa17298/WT larvae in the dark phase of the light-dark stimulus task was a result of cacna1da-mediated anxiety-like behaviour.
Increased locomotor activity, as a readout of tonic-clonic-like seizures, was previously described in zebrafish genetic models of epilepsy [49, 61, 62], which correlated well with the occurrence of epileptiform-like discharges in larval brains as measured by EEG. In some human CACNA1D mutation carriers, different types of seizures have been observed (focal and/or generalised seizures) [17, 20, 63]. In our study, we observed epileptiform-like discharges in the EEG assay only in 3 out of 15 cacna1dasa17298/WT larvae. Thus, it is unlikely that the hyperlocomotion displayed by cacna1dasa17298/WT mutants is associated with seizures.
CACNA1D mutations have been implicated earlier in SCZ [8, 13, 21]. In behavioural pharmacology in rodents, the most common approach is to mimic psychotic-like positive symptoms by administering drugs that target N-methyl-D-aspartate (NMDA) (antagonism) or dopaminergic (agonism) receptors. These drugs induce psychosis in rodents with hyperactivity as a readout of this symptom (for review, see [64]). Interestingly, pharmacological studies revealed high correlation with regard to the ability of larval and adult zebrafish to mimic positive SCZ symptoms. For example, ketamine [65, 66] and MK-801 (also called dizocilpine) [54, 67] induce a robust increase in locomotion. Moreover, d-amphetamine or D2 receptor agonists elicit the same locomotor response in adult zebrafish [68, 69] as in rodents [70–74]. Previously, hyperlocomotion was observed in mouse genetic models of SCZ with mutations in Disc1 [75] and Nrg1 [76]. In larval zebrafish, this feature was also commonly observed in mutants harbouring mutations in different genes indicated by GWAS as SCZ susceptibility genes [77]. A study by Thyme et al. [77], which generated and analysed 132 different mutants of SCZ-risk genes, showed that hyperlocomotion was sometimes observed in a number of these fish.
To obtain further insight into the neurobehavioural changes in cacna1dasa17298/WT larvae, we evaluated sensorimotor gating and habituation in larvae by performing two tests—i.e. ASR and PPI tests, as well as the startle response to dark flashes assay. The startle response to dark flashes assay, in which animals habituate to dark flashes when exposed to continuous light stimulus, allows for the assessment of non-associative memory [43, 48, 78, 79], which is also impaired in most schizophrenic patients [80, 81]. The ASR and PPI are the most commonly used tasks in SCZ research with an altered PPI response observed in schizophrenia patients [82–85], rodent [86–88] and some zebrafish models of SCZ [77]. The difference between PPI and startle response to dark flashes is the type of stimulus—i.e. auditory versus visual. As CACNA1D mutations have been previously implicated in deafness in humans [89, 90], mice [24] and even zebrafish [34], we assessed the hearing sensitivities of larvae across genotypes using different stimulus intensities (sound) and found them to be comparable. Nevertheless, no difference in PPI between WT and heterozygous mutants was observed, albeit with tendency of homozygotes towards enhanced PPI. The very low survival rate of homozygotes proved prohibitive with regard to our ability to collect and assay a sufficiently large set of larvae to reach statistical significance in our experimental setup. To the best of our knowledge, there is a lack of data as to whether CACNA1D mutation carriers are blind or have other visual deficits. However, a study reported Cacna1d knockout mice to have slight visual impairment but found no evidence that this affected their behaviour in the Morris water maze test (a “visual” spatial learning and memory task) [25]. In the zebrafish, in situ hybridisation analysis revealed expression of cav1.3a mRNA in the eye (although faint) and ear, while cav1.3b expression was evident in the otic vesicle of 5-dpf larvae. qPCR analysis indicated expression of cav1.3a mRNA both in the eyes and ears of adult zebrafish [27]. Taking into account that interlarval behavioural patterns were consistent throughout all experiments, we postulate that mutant visual acuity is preserved and that the behavioural phenotypes observed are a result of changes in brain activity and not visual disturbances. Although it appears that the cacna1dasa17298/WT mutant response in this assay (startle response to dark flashes) might be related to cognitive symptoms of SCZ, this aspect of the mutant phenotype needs further investigation. Additionally, since the CACNA1D gene is duplicated in the zebrafish [27], the severity of the mutation may be attenuated through partial compensation by cacna1db. Thus, this may explain the absence of PPI deficits in the mutants.
We performed pharmacological profiling of WT and cacna1dasa17298/WT larvae in the locomotor (under constant illumination), light-dark and startle response to dark flashes tests after exposure to different neuroactive drugs: two drugs used in the management of SCZ (HALO and RISP) and one drug (VPA) indicated for the treatment of epilepsy, and as add-on therapy in BP, migraine, anxiety or SCZ. The behavioural response of our WT fish after incubation with all 3 drugs is in agreement with previous studies which showed that all of them decreased locomotor activity in both larval and adult animals [35, 36, 54, 58, 91–93]. It seems that the response of WT larvae (i.e. hypoactivity) is a common feature for antipsychotics, as other drugs from this group also decreased baseline activity in WT larvae (e.g. droperidol, phenothiazine or clozapine) [94, 95]. With regard to VPA exposure, our study indicated that this drug substantially decreased activity of cacna1dasa17298/WT larvae in all the three behavioural tests, although heterozygous mutants were significantly more active than their WT siblings in the locomotor test. The mechanism of action of VPA includes increasing levels of γ-aminobutyric acid (GABA) in the brain, inhibition of histone deacetylase and blockage of voltage-gated ion channels (sodium, potassium and calcium), such as CaV1.3 [96–98]. We therefore theorise that the effectiveness of VPA in decreasing locomotor activity in cacna1da mutants may act through a different mechanism other than seizure suppression.
In humans, CACNA1D mRNA has been detected in limbic and mesolimbic structures (i.e. amygdala, hippocampus, thalamus, hypothalamus, basal ganglia) [101, 102], which are enriched with dopaminergic neurons. Similarly, in mice, the Cacna1d gene is expressed in limbic and striatal areas [103–105]. Sidi et al. [27] observed that the expression of cav1.3a is detected as early as 30-h post-fertilisation (hpf) in the zebrafish brain (telencephalon, thalamic and hypothalamic diencephalon, midbrain, ventral hindbrain). From 48 hpf onwards, expression of cav1.3a becomes even more pronounced in almost the entire brain [27].
In conclusion, different zebrafish genetic models of epilepsy and to a lesser extent, SCZ, have emerged within the last 5 years (for comprehensive reviews see [106, 107]). Although the zebrafish brain is less complex than the mammalian one, fish are very sensitive to neuroactive compounds and their behavioural responses are easily tracked in 48-well plates. This allows for very rapid and efficient screening of compounds compared with rodents, thus enabling the identification of bioactive “hit” compounds for further investigation. Thus far, only zebrafish genetic models of epilepsy have been used successfully for discovering new therapeutic options in high-throughput screening assays [49, 108]. In SCZ research, there remains a need for identification of new antipsychotic agents with less side effects (especially those related to extrapyramidal symptoms) and effectiveness in alleviating negative and cognitive symptoms besides positive symptoms. Furthermore, with the advancements in genetic engineering methods in zebrafish [109–111], there is potential for using a precision medicine-based approach, where patient-specific mutations are introduced into zebrafish embryos to generate “customised” models for drug screening. This may aid scientists in tackling the pharmaco-resistance problem prevalent in SCZ patients, of which genetics plays a key role [see reviews [112, 113]. In relation to cacna1dasa17298/WT larvae, our data appears promising, but further in-depth phenotyping is warranted to fully validate it as a new model of Cacna1d-mediated SCZ.
Electronic Supplementary Material
(DOCX 5685 kb)
Authors’ Contributions
NSB, CVE and KG conceptualised the experiments. All authors contributed to preparation of the manuscript. NSB and CVE revised and edited the final version. NSB carried out all larval morphological assessment, behavioural phenotyping and corresponding data analysis. LDW assisted with behavioural experiments. KG performed EEG experiments and analysis. NSB and KG prepared the figures. All authors read and approved the manuscript.
Funding
Open Access funding provided by University of Oslo (incl Oslo University Hospital). This work was funded by the Research Council of Norway (ISP, BIOTEK2021/ DigiBrain) (for NSB and CVE) and partially supported by start-up funds from the Centre for Molecular Medicine Norway (for CVE). KG was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement No. 798703-GEMZ-H2020-MSCA-IF-2017).
Compliance with Ethical Standards
All experiments were approved by the Norwegian Food Safety Authority experimental animal administration’s supervisory and application system (FOTS-18/106800-1; ID 15469 and 23935).
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
It is important to note that gross morphological defects such as craniofacial abnormalities, smaller, fused or absent eyes, curved body axis, yolk sac necrosis and hyperpigmentation were visible as early as 2 dpf in a proportion of the offspring (~ 25%) when male and female adult heterozygous sa17298 (obtained directly from ZIRC) were in-crossed. These morphological defects were present in heterozygous and homozygous sa17298 populations as well as WT larvae (Suppl. Fig. 2). We therefore recommend that the line be outcrossed over multiple generations to remove other mutations that may confound the interpretation of results.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Linus De Witte, https://orcid.org/0000-0002-2374-637X.
Camila V. Esguerra, Email: c.v.esguerra@ncmm.uio.no
References
- 1.Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol. 2015;29:85–96. doi: 10.1177/0269881114553647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawi Z, Tong J, Dark C, Yates H, Johnson B, Bellgrove MA. The role of cadherin genes in five major psychiatric disorders: a literature update. Am J Med Genet. 2018;177:168–180. doi: 10.1002/ajmg.b.32592. [DOI] [PubMed] [Google Scholar]
- 4.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, Shafer A, Neri F, Lee K, Kutyavin T, Stehling-Sun S, Johnson AK, Canfield TK, Giste E, Diegel M, Bates D, Hansen RS, Neph S, Sabo PJ, Heimfeld S, Raubitschek A, Ziegler S, Cotsapas C, Sotoodehnia N, Glass I, Sunyaev SR, Kaul R, Stamatoyannopoulos JA. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, Alliey-Rodriguez N, Cooper J, Romanos B, Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, Ongur D, McPhie D, Cohen B, Perlis R, Tsai LH. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2015;20:162–169. doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade A, Brennecke A, Mallat S, Brown J, Gomez-Rivadeneira J, Czepiel N, Londrigan L (2019) Genetic associations between voltage-gated calcium channels and psychiatric disorders. Int J Mol Sci:20. 10.3390/ijms20143537 [DOI] [PMC free article] [PubMed]
- 9.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 10.Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oguro-Okano M, Griesmann GE, Wieben ED, et al. Molecular diversity of neuronal-type calcium channels identified in small cell lung carcinoma. Mayo Clin Proc. 1992;67:1150–1159. doi: 10.1016/S0025-6196(12)61144-6. [DOI] [PubMed] [Google Scholar]
- 13.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabir ZD, Martínez-Rivera A, Rajadhyaksha AM. From gene to behavior: L-type calcium channel mechanisms underlying neuropsychiatric symptoms. Neurotherapeutics. 2017;14:588–613. doi: 10.1007/s13311-017-0532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinggera A, Striessnig J. Cav 1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J Physiol Lond. 2016;594:5839–5849. doi: 10.1113/JP270672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofer NT, Tuluc P, Ortner NJ, Nikonishyna YV, Fernándes-Quintero ML, Liedl KR, Flucher BE, Cox H et al (2020) Biophysical classification of a CACNA1D de novo mutation as a high-risk mutation for a severe neurodevelopmental disorder. Mol Autism 11. 10.1186/s13229-019-0310-4 [DOI] [PMC free article] [PubMed]
- 17.Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. CACNA1D De novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77:816–822. doi: 10.1016/j.biopsych.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinggera A, Mackenroth L, Rump A, Schallner J, Beleggia F, Wollnik B, Striessnig J. New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum Mol Genet. 2017;26:2923–2932. doi: 10.1093/hmg/ddx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinggera A, Negro G, Tuluc P, Brown MJ, Lieb A, Striessnig J. Gating defects of disease-causing de novo mutations in Cav1.3 Ca2+ channels. Channels (Austin) 2018;12:388–402. doi: 10.1080/19336950.2018.1546518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devor A, Andreassen O, Wang Y, et al. Genetic evidence for role of integration of fast and slow neurotransmission in schizophrenia. Mol Psychiatry. 2017;22:792–801. doi: 10.1038/mp.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross J, Gedvilaite E, Badner JA, Erdman C, Baird L, Matsunami N, Leppert M, Xing J, Byerley W. A rare variant in CACNA1D segregates with 7 bipolar I disorder cases in a large pedigree. Mol Neuropsychiatry. 2016;2:145–150. doi: 10.1159/000448041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan F, Li L, Qiao C, Chen G, Yan T, Li T, Zhang T, Liu X. Evaluation of genetic susceptibility of common variants in CACNA1D with schizophrenia in Han Chinese. Sci Rep. 2015;5:12935. doi: 10.1038/srep12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 25.Busquet P, Khoi Nguyen N, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2010;13:499–513. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- 26.McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of CaV1.3 knockout mice. Neurobiol Learn Mem. 2009;92:519–528. doi: 10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. Gemini encodes a Zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–4223. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.http://www.ensemble.org Gene: cacna1da (ENSDARG00000102773) - Orthologue alignment - Danio rerio - Ensembl genome browser 99. https://www.ensembl.org/Danio_rerio/Gene/Compara_Ortholog/Alignment?db=core;g=ENSDARG00000102773;g1=ENSG00000157388;hom_id=449865216;r=11:36793473-36963988. Accessed 23 Apr 2020
- 29.http://www.ensemble.org Gene: cacna1db (ENSDARG00000075917) - Orthologue alignment - Danio rerio - Ensembl genome browser 99. https://www.ensembl.org/Danio_rerio/Gene/Compara_Ortholog/Alignment?db=core;g=ENSDARG00000075917;g1=ENSG00000142609;hom_id=560456301;r=8:53204017-53260670. Accessed 23 Apr 2020
- 30.Nicolson T. Ribbon synapses in zebrafish hair cells. Hear Res. 2015;330:170–177. doi: 10.1016/j.heares.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheets L, Trapani JG, Mo W, Obholzer N, Nicolson T. Ribeye is required for presynaptic CaV1.3a channel localization and afferent innervation of sensory hair cells. Development. 2011;138:1309–1319. doi: 10.1242/dev.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheets L, Kindt KS, Nicolson T. Presynaptic CaV1.3 channels regulate synaptic ribbon size and are required for synaptic maintenance in sensory hair cells. J Neurosci. 2012;32:17273–17286. doi: 10.1523/JNEUROSCI.3005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- 34.Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/S0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 35.Prieto MJ, Gutierrez HC, Arévalo RA, Chiaramoni NS, Alonso SV. Effect of risperidone and fluoxetine on the movement and neurochemical changes of zebrafish. OJMC. 2012;02:129–138. doi: 10.4236/ojmc.2012.24016. [DOI] [Google Scholar]
- 36.Irons TD, Kelly P, Hunter DL, et al. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav. 2013;103:792–813. doi: 10.1016/j.pbb.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tremolizzo L, Doueiri M-S, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 38.White HS, Alex AB, Pollock A, Hen N, Shekh-Ahmad T, Wilcox KS, McDonough JH, Stables JP, Kaufmann D, Yagen B, Bialer M. A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage. Epilepsia. 2012;53:134–146. doi: 10.1111/j.1528-1167.2011.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanger Institute Zebrafish Mutation Project (2013) ZFIN Feature: sa17298. https://zfin.org/ZDB-ALT-131217-13434. Accessed 23 Apr 2020
- 40.Aleström P, D’Angelo L, Midtlyng PJ et al (2019) Zebrafish: housing and husbandry recommendations: laboratory animals. 10.1177/0023677219869037 [DOI] [PMC free article] [PubMed]
- 41.Gawel K, Turski WA, van der Ent W, Mathai BJ, Kirstein-Smardzewska KJ, Simonsen A, Esguerra CV. Phenotypic characterization of larval zebrafish (Danio rerio) with partial knockdown of the cacna1a gene. Mol Neurobiol. 2020;57:1904–1916. doi: 10.1007/s12035-019-01860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnörr S, Steenbergen P, Richardson M, Champagne D (2012) Measuring thigmotaxis in larval zebrafish - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0166432811008758?via%3Dihub. Accessed 5 Dec 2019 [DOI] [PubMed]
- 43.Norton WHJ. Measuring larval zebrafish behavior: locomotion, thigmotaxis, and startle. In: Kalueff AV, Stewart AM, editors. Zebrafish protocols for neurobehavioral research. Totowa, NJ: Humana Press; 2012. pp. 3–20. [Google Scholar]
- 44.Banono NS, Esguerra CV (2020) Pharmacological validation of the prepulse inhibition of startle response in larval zebrafish using a commercial automated system and software. JoVE:61423. 10.3791/61423 [DOI] [PubMed]
- 45.Afrikanova T, Serruys A-SK, Buenafe OEM, Clinckers R, Smolders I, de Witte PAM, Crawford AD, Esguerra CV. Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. PLoS One. 2013;8:e54166. doi: 10.1371/journal.pone.0054166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aranda PS, LaJoie DM, Jorcyk CL. Bleach gel: a simple agarose gel for analyzing RNA quality. ELECTROPHORESIS. 2012;33:366–369. doi: 10.1002/elps.201100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines. 2019;7:23. doi: 10.3390/biomedicines7010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Best JD, Berghmans S, Hunt JJFG, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- 49.Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4:2410. doi: 10.1038/ncomms3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gawel K, Kukula-Koch W, Nieoczym D, Stepnik K, van der Ent W, Banono NS, Tarabasz D, Turski WA, Esguerra CV. The influence of palmatine isolated from Berberis sibirica radix on pentylenetetrazole-induced seizures in zebrafish. Cells. 2020;9:1233. doi: 10.3390/cells9051233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H, Zebrafish Neuroscience Research Consortium Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 2013;10:70–86. doi: 10.1089/zeb.2012.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiraboschi E, Martina S, van der Ent W, et al. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome. Epilepsia. 2020;61:549–560. doi: 10.1111/epi.16456. [DOI] [PubMed] [Google Scholar]
- 53.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: A critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Seibt KJ, da Luz Oliveira R, Zimmermann FF, et al. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2010;214:417–422. doi: 10.1016/j.bbr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Seibt KJ, Piato AL, da Luz OR, et al. Antipsychotic drugs reverse MK-801-induced cognitive and social interaction deficits in zebrafish (Danio rerio) Behav Brain Res. 2011;224:135–139. doi: 10.1016/j.bbr.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 56.Kedra M, Banasiak K, Kisielewska K, Wolinska-Niziol L, Jaworski J, Zmorzynska J. TrkB hyperactivity contributes to brain dysconnectivity, epileptogenesis, and anxiety in zebrafish model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2020;117:2170–2179. doi: 10.1073/pnas.1910834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheldeman C, Mills JD, Siekierska A, Serra I, Copmans D, Iyer AM, Whalley BJ, Maes J, Jansen AC, Lagae L, Aronica E, de Witte PAM. mTOR-related neuropathology in mutant tsc2 zebrafish: Phenotypic, transcriptomic and pharmacological analysis. Neurobiol Dis. 2017;108:225–237. doi: 10.1016/j.nbd.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Li F, Lin J, Liu X, et al. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann Transl Med. 2018;6:173. doi: 10.21037/atm.2018.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Zhang Y, Lin J, et al (2016) Frontiers | social preference deficits in juvenile zebrafish induced by early chronic exposure to sodium valproate | Behavioral Neuroscience. https://www.frontiersin.org/articles/10.3389/fnbeh.2016.00201/full. Accessed 5 Dec 2019 [DOI] [PMC free article] [PubMed]
- 60.Lundegaard PR, Anastasaki C, Grant NJ, Sillito RR, Zich J, Zeng Z, Paranthaman K, Larsen AP, Armstrong JD, Porteous DJ, Patton EE. MEK inhibitors reverse cAMP-mediated anxiety in zebrafish. Chem Biol. 2015;22:1335–1346. doi: 10.1016/j.chembiol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Kecskés A, Copmans D, Langlois M, Crawford AD, Ceulemans B, Lagae L, de Witte PAM, Esguerra CV. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS One. 2015;10:e0125898. doi: 10.1371/journal.pone.0125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pena IA, Roussel Y, Daniel K et al (2017) Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency. Genetics genetics.300137.2017. 10.1534/genetics.117.300137 [DOI] [PMC free article] [PubMed]
- 63.Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones C, Watson D, Fone K. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michelotti P, Quadros VA, Pereira ME, Rosemberg DB. Ketamine modulates aggressive behavior in adult zebrafish. Neurosci Lett. 2018;684:164–168. doi: 10.1016/j.neulet.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 66.De Campos EG, Bruni AT, De Martinis BS. Ketamine induces anxiolytic effects in adult zebrafish: a multivariate statistics approach. Behav Brain Res. 2015;292:537–546. doi: 10.1016/j.bbr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Chen J, Patel R, Friedman TC, Jones KS. The behavioral and pharmacological actions of NMDA receptor antagonism are conserved in zebrafish larvae. Int J Comp Psychol. 2010;23:82–90. [PMC free article] [PubMed] [Google Scholar]
- 68.Kyzar E, Stewart AM, Landsman S, Collins C, Gebhardt M, Robinson K, Kalueff AV. Behavioral effects of bidirectional modulators of brain monoamines reserpine and d-amphetamine in zebrafish. Brain Res. 2013;1527:108–116. doi: 10.1016/j.brainres.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irons TD, MacPhail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 70.Lautar SL, Rojas C, Slusher BS, Wozniak KM, Wu Y, Thomas AG, Waldon D, Li W, Ferraris D, Belyakov S. DPP IV inhibitor blocks mescaline-induced scratching and amphetamine-induced hyperactivity in mice. Brain Res. 2005;1048:177–184. doi: 10.1016/j.brainres.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 71.Alttoa A, Eller M, Herm L, Rinken A, Harro J. Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: Differences in the role of locus coeruleus. Brain Res. 2007;1131:138–148. doi: 10.1016/j.brainres.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 72.Gormley S, Rompré P-P. Blockade of mGLUR5 receptors differentially alters amphetamine-induced enhancement of locomotor activity and of brain stimulation reward. J Psychopharmacol. 2011;25:393–401. doi: 10.1177/0269881110367460. [DOI] [PubMed] [Google Scholar]
- 73.Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008;7:906–914. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 76.Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: Implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–687. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 77.Thyme SB, Pieper LM, Li EH, et al. Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell. 2019;177:478–491.e20. doi: 10.1016/j.cell.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolman MA, Jain RA, Liss L, Granato M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci. 2011;108:15468–15473. doi: 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Randlett O, Haesemeyer M, Forkin G, et al. Distributed plasticity drives visual habituation learning in larval Zebrafish. Curr Biol. 2019;29:1337-1345.e4. doi: 10.1016/j.cub.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braff DL. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 81.Avery SN, McHugo M, Armstrong K, Blackford JU, Woodward ND, Heckers S. Disrupted habituation in the early stage of psychosis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2019;4:1004–1012. doi: 10.1016/j.bpsc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13:1383–1395. doi: 10.1017/S1461145710000787. [DOI] [PubMed] [Google Scholar]
- 83.Quednow BB, Frommann I, Berning J, Kühn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 84.Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kühn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry. 2006;59:536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 85.Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 86.Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- 87.Kanahara N, Shimizu E, Ohgake S, Fujita Y, Kohno M, Hashimoto T, Matsuzawa D, Shirayama Y, Hashimoto K, Iyo M. Glycine and d-serine, but not d-cycloserine, attenuate prepulse inhibition deficits induced by NMDA receptor antagonist MK-801. Psychopharmacology. 2008;198:363–374. doi: 10.1007/s00213-008-1151-6. [DOI] [PubMed] [Google Scholar]
- 88.Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, Weinberger DR. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 2012;17:85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nürnberg P, Striessnig J, Bolz HJ. Loss of Cav1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 90.Liaqat K, Raza SI, Lee K, et al. Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance. J Hum Genet. 2019;64:153–160. doi: 10.1038/s10038-018-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giacomini NJ, Rose B, Kobayashi K, Guo S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol. 2006;28:245–250. doi: 10.1016/j.ntt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Idalencio R, Kalichak F, Rosa JGS, Oliveira TA, Koakoski G, Gusso D, Abreu MS, Giacomini ACV, Barcellos HHA, Piato AL, Barcellos LJG. Waterborne Risperidone decreases stress response in zebrafish. PLoS One. 2015;10:e0140800. doi: 10.1371/journal.pone.0140800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magno LDP, Fontes A, Gonçalves BMN, Gouveia A. Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): waterborne administration. Pharmacol Biochem Behav. 2015;135:169–176. doi: 10.1016/j.pbb.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E, Taylor J, Lakhani P, Lensen D, Evron T, Lorello PJ, Huang XP, Kolczewski S, Carey G, Caldarone BJ, Prinssen E, Roth BL, Keiser MJ, Peterson RT, Kokel D. Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds. Nat Chem Biol. 2016;12:559–566. doi: 10.1038/nchembio.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R. D4 dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007;6:155–166. doi: 10.1111/j.1601-183X.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 96.Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, Altman RB, Klein TE. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013;23:236–241. doi: 10.1097/FPC.0b013e32835ea0b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johannesen KM, Gardella E, Linnankivi T, Courage C, de Saint Martin A, Lehesjoki AE, Mignot C, Afenjar A, Lesca G, Abi-Warde MT, Chelly J, Piton A, Merritt JL, II, Rodan LH, Tan WH, Bird LM, Nespeca M, Gleeson JG, Yoo Y, Choi M, Chae JH, Czapansky-Beilman D, Reichert SC, Pendziwiat M, Verhoeven JS, Schelhaas HJ, Devinsky O, Christensen J, Specchio N, Trivisano M, Weber YG, Nava C, Keren B, Doummar D, Schaefer E, Hopkins S, Dubbs H, Shaw JE, Pisani L, Myers CT, Tang S, Tang S, Pal DK, Millichap JJ, Carvill GL, Helbig KL, Mecarelli O, Striano P, Helbig I, Rubboli G, Mefford HC, Møller RS. Defining the phenotypic spectrum of SLC6A1 mutations. Epilepsia. 2018;59:389–402. doi: 10.1111/epi.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franceschetti S, Hamon B, Heinemann U. The action of valproate on spontaneous epileptiform activity in the absence of synaptic transmission and on evoked changes in [Ca2+]0 and [K+]0 in the hippocampal slice. Brain Res. 1986;386:1–11. doi: 10.1016/0006-8993(86)90135-6. [DOI] [PubMed] [Google Scholar]
- 99.Leysen JE, Janssen PMF, Schotte A, Luyten WHML, Megens AAHP. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology. 1993;112:S40–S54. doi: 10.1007/BF02245006. [DOI] [PubMed] [Google Scholar]
- 100.Kalichak F, Idalencio R, da Rosa JGS, Barcellos HHA, Fagundes M, Piato A, Barcellos LJG. Psychotropic in the environment: risperidone residues affect the behavior of fish larvae. Sci Rep. 2017;7:14121. doi: 10.1038/s41598-017-14575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CAK, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Tissue-based map of the human proteome. Science. 2015;347:1260419–1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 102.Tissue expression of CACNA1D - Summary - The HUMAN PROTEIN ATLAS. https://www.proteinatlas.org/ENSG00000157388-CACNA1D/tissue. Accessed 25 Jun 2020
- 103.Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Brain activation pattern induced by stimulation of L-type Ca2+−channels: contribution of CaV1.3 and CaV1.2 isoforms. Neuroscience. 2006;139:1005–1015. doi: 10.1016/j.neuroscience.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 104.Gene Detail: Allen brain atlas: mouse brain. http://mouse.brain-map.org/gene/show/12074. Accessed 25 Jun 2020
- 105.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Feng Yuan X, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 106.Gawel K, Banono NS, Michalak A, Esguerra CV. A critical review of zebrafish schizophrenia models: time for validation? Neurosci Biobehav Rev. 2019;107:6–22. doi: 10.1016/j.neubiorev.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Gawel K, Langlois M, Martins T, van der Ent W, Tiraboschi E, Jacmin M, Crawford AD, Esguerra CV. Seizing the moment: zebrafish epilepsy models. Neurosci Biobehav Rev. 2020;116:1–20. doi: 10.1016/j.neubiorev.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 108.Eimon PM, Ghannad-Rezaie M, De Rienzo G, et al. Brain activity patterns in high-throughput electrophysiology screen predict both drug efficacies and side effects. Nat Commun. 2018;9:219. doi: 10.1038/s41467-017-02404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suster ML, Abe G, Schouw A, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish. Nat Protoc. 2011;6:1998–2021. doi: 10.1038/nprot.2011.416. [DOI] [PubMed] [Google Scholar]
- 110.Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- 111.Hwang WY, Fu Y, Reyon D, Gonzales APW, Joung JK, Yeh JRJ. Targeted mutagenesis in zebrafish using CRISPR RNA-guided nucleases. In: Lundgren M, Charpentier E, Fineran PC, editors. CRISPR. New York, NY: Springer New York; 2015. pp. 317–334. [DOI] [PubMed] [Google Scholar]
- 112.Arranz MJ, Rivera M, Munro JC. Pharmacogenetics of response to antipsychotics in patients with schizophrenia. CNS Drugs. 2011;25:933–969. doi: 10.2165/11595380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 113.Vita A, Minelli A, Barlati S, Deste G, Giacopuzzi E, Valsecchi P, Turrina C, Gennarelli M. Treatment-resistant schizophrenia: genetic and neuroimaging correlates. Front Pharmacol. 2019;10:402. doi: 10.3389/fphar.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 5685 kb)