Abstract
Purpose
Saskatchewan has a high prevalence of diabetes. It is the largest, rurally populated, predominantly agricultural province in Canada. This research aims to determine the risk factors associated with the incidence and longitudinal changes in the prevalence of diabetes among Saskatchewan’s adult rural farm and non-farm residents.
Methods
The Saskatchewan Rural Health Study (SRHS) is a prospective cohort study conducted in two phases: a baseline survey (2010, 8261 participants) and a follow-up survey (2014, 4867 participants). Generalized estimation equations and survival analysis techniques were used to determine diabetes prevalence and incidence risk factors, respectively.
Results
Incidence of diabetes among rural residents was 2.75%. Positive family history, high BMI, sleep apnea and an abnormal Epworth Sleepiness Score (ESS) were significant predictors for diabetes incidence. A substantial increase (1.98%) of diabetes prevalence was observed after four years of follow-up. Risk factors of diabetes prevalence were increasing age, male, low income, positive family history, high BMI, hypertension and heart attack.
Conclusion
A mix of individual and contextual factors interacting in complex pathways were responsible for the high incidence and prevalence of diabetes among rural residents. The most original finding of that study was a positive association of sleep apnea, and ESS with incident diabetes warrants further research to identify a causal linkage. Increased diabetes risk among rural male insecticide users indicates an adverse consequence of unprotected chemical exposures in the agricultural field. Urgent population-based preventive measures should initiate to slow the increasing trend of diabetes prevalence among rural residents.
Keywords: Diabetes, Rural, Predictors, Agriculture, Incidence, Prevalence
Introduction
Diabetes is a global epidemic and imposes an enormous threat to human health and the economy of a country [1, 2], Canada is a prime example [3], with one out of three Canadians suffering from either diabetes or prediabetes [4]. The cost of managing diabetes in Canada heightens every year with the increasing prevalence of diabetes [5]. Diabetes adversely impacts not only the health of an individual but also causes work limitations, economic instability, diminished quality of life and life expectancy. Canadian adults who have diabetes are three times more likely to be admitted to the hospital due to cardiovascular complications compared to non-diabetic Canadians [6]. Diabetes is one of the leading causes of vision loss, strokes, heart attacks, kidney failure and non-traumatic limb amputations [6]. Nearly 36.5% of Canadians with diabetes suffer from at least two more other chronic diseases coexisting with diabetes [7].
Diabetes Canada states that Canada is burdened with a high rate of individual-level modifiable risk factors (for example, overweight/obesity, physical inactivity, and unhealthy diet) [6, 7], which were the major contributing factors for the high prevalence of diabetes in Canada. Indigenous populations are at high risk of developing diabetes, and the prevalence of diabetes is three to five times greater in First Nations populations than that found in the general population [8]. Accessing health care is more challenging for the rural diabetic population than the urban diabetic residents [6]; hence, rural Canadian populations are more vulnerable to developing diabetes-related complications.
Compared to the national average rural population (18.9%) [9], Saskatchewan is one of the highest rurally populated provinces (33%) [9]. The overall prevalence of diagnosed diabetes cases in Saskatchewan is 9% (106,000 diagnosed cases in 2020) [10]. In Saskatchewan, non-Indigenous males have a higher prevalence of diabetes as compared to non-Indigenous females [10], which is mirrored by the national male-female ratio of diabetes prevalence (8.4% in men and 6.3% in female) [11]. According to Diabetes Canada, Saskatchewan is facing an enormous challenge as it tries to meet the healthcare needs of people with diabetes [10]. Hence, knowledge about diabetes-related risk factors among rural residents is essential. Although some studies have been conducted to identify the predictors of the prevalence of diabetes among rural populations in Canada [12], most of them were based on cross-sectional studies [12–15]. Also, information regarding risk factors associated with the incidence of diabetes was limited across rural Canada. Therefore, the purpose of this study was to use the Saskatchewan rural health study (SRHS) longitudinal dataset to conduct an extensive analysis to assess the predictors associated with incidence and longitudinal changes in the prevalence of diabetes among rural residents in Saskatchewan, Canada.
By conducting this study, we aimed to identify the cumulative incidence of diabetes in rural residents. Additionally, the impact of unique agriculture-related risk factors on diabetes incidence was assessed after adjusting for known diabetes risk factors. We also determined the prevalence of diabetes among rural residents to find out if there were any significant changes in the prevalence of diabetes over time. Moreover, this paper provided information regarding unique risk factors of diabetes prevalence associated with rural farming practices. While identifying the prevalence of diabetes helped us to understand the burden of diabetes among rural residents, the incidence of diabetes helped us to understand the role of associated risk factors for developing new diabetes cases among rural residents.
Methods
Study design and population
The Saskatchewan Rural Health Study (SRHS) was a prospective cohort study conducted to identify the respiratory health-related problems primarily among the rural population of Saskatchewan. However, a wide range of information was also collected on non-respiratory chronic conditions, including diabetes. The SRHS consisted of a baseline survey conducted in 2010, with a follow-up survey in 2014. The details of the study design were given elsewhere [16, 17].
In brief, the study participants were initially selected from 39 of the 297 rural municipalities (RMs) and 16 of the 145 small towns of Saskatchewan. RMs and small towns were selected randomly from southeast, southwest, northeast, and northwest quadrants of the province. On behalf of the residents, the local councils for 32 (89%) out of 36 RMs and 15 (94%) out of 16 small towns gave their consent to participate in the study and provided a mailing address. Completed questionnaires were collected from 4624 households (8261 individuals, 4064 male and 4194 female) at baseline and at follow-up, 2797 households (4867 participants, 2364 male and 2502 female) by using mail-out survey methods. One hundred twenty-six new individuals were included in the follow-ups, who did not participate in the baseline survey. For the incidence study, we only considered respondents who participated in both baseline and follow-up (4741). Additionally, we removed the 411 participants who had diabetes at baseline. After four years of follow-up, 119 participants developed new diabetes cases among 4330 study participants and considered the incidence part of the study.
The data were collected from the rural Saskatchewan farm and small-town dwellers through a mailed out self-administered household and individual questionnaire to assess individual and contextual factors. To recruit study participants and maximize the response rate, researchers used the Dillman method, with all prospective participants receive a series of mail [16, 18].
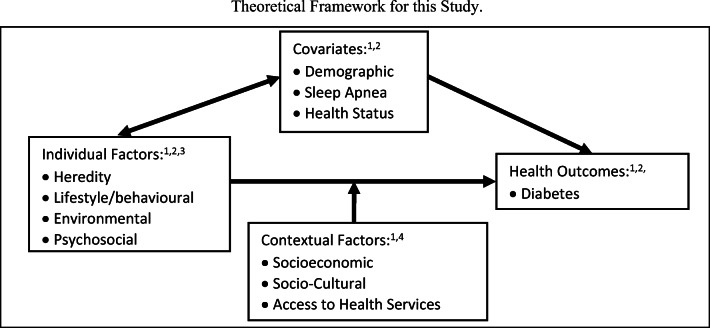
Theoretical framework
Health Canada’s “Population Health Framework (PHF)” was the conceptual guide of the SRHS. A modified version of the PHF has been used for this study [16, 17]. Briefly, the determinants of health were influenced at two levels; the first level includes individual factors, and the second level includes contextual factors. SRHS simultaneously evaluated individual and contextual factors associated with the health of the rural residents of Saskatchewan. Covariates included factors such as age, sex, marital status, family structure, health status and co-morbidities. We adjusted all analyses for known confounders. Details about the theoretical framework were listed in Appendix.
Variables
Primary health outcome
The primary outcome of interest for this paper was a physician, or primary caregiver diagnosed, self-reported diabetes status, which was captured from the SRHS baseline and follow-up survey questionnaires based on the question: “Has a doctor or primary caregiver ever said you have diabetes.” For both prevalence and incidence part of the study, our outcome variable of interest was the same “diabetes.” However, we had to re-define the “diabetes” outcome variable separately for prevalence and incidence study.
Prevalence: All the participants (eighteen years or older) from both baseline (8261) and follow-up (4867) participated in this study. At baseline, 759 participants had diabetes, and at follow-up, 540 participants had diabetes.
Incidence: For the incidence study, we only considered respondents who participated in both baseline and follow-up (4741 participants). Additionally, we removed the 411 participants who had diabetes at baseline. After four years of follow-up, 119 participants developed new cases of diabetes among 4330 study participants and considered for the incidence part of the study.
Contextual factors, individual factors and covariate
Contextual factors considered for this study were participant’s locations of residence, living quadrants, income adequacy, and household smoking. Family history of diabetes, behavioural risk factors, screen time, and participant’s education level considered as individual factors. Additionally, agricultural occupation-related exposure, such as pesticides, herbicides, fungicides, and insecticides, also acted as individual factors. Participants’ age, sex, marital status, general health, and co-morbidities were considered covariates.
Contextual factors
The contextual factors for our study were; (i) rural locations – participants residence either farm or non-farm location, (ii) quadrant- Location of the residents categorized into South West, South East, North East, and North West regions, (iii) income adequacy: This variable was created based on household income and categorized into > = $60,000 (reference category), $40,000 to $59,999, $20,000 to $39,999 and < $20,000, and (iv) household smoking: was based on smoking (cigarettes, cigars or pipes) habit of the respondents inside the home. It was a dichotomous variable and categorized into no (reference category) and yes.
Individual factors
The individual factors for our study were; (i) family history: a positive family history of diabetes (father, mother, brother/sister), (ii) lifestyle or behaviour-related factors included smoking, alcohol use, body mass index, diet, exercise duration and screen time. Smoking was categorized into never smoker (reference category), ex-smoker and current smoker. Body mass index was categorized into normal (BMI < 25 kg/m2) (reference category), overweight (BMI 25–30 kg/m2) and obese (BMI >30 kg/m2). There were five categories of exercise duration: no exercise (reference category), less than 15 min, 15–30 min, 31–60 min, and more than 60 min per day, (iii) information on agricultural occupational exposures included pesticides, herbicides, fungicides, insecticides, and livestock. For every agriculture-related exposure, “no use” acted as a reference category, (iv) education level: Participant’s level of education was classified into less than high school (reference category), completed high school, completed university, and completed post-secondary education other than above.
Covariates
The covariates for our study were; (i) Age: Age of the respondents were classified into 18–45 years (reference category), 46–55 years, 56–65 years, and > 65 years, (ii) sex: Sex of the participants was categorized into a female (reference category) and male, (iii) marital status: Respondent’s marital status was classified into married/common-law/living together (reference category) and widowed/divorced/separated/single, never married, (iv) Co-morbidities related to diabetes, included cardiovascular diseases (stroke, blood pressure, heart attack and heart disease), respiratory diseases (tuberculosis, asthma, COPD, emphysema, and chronic bronchitis), others (cancer), (v) Variables related to sleep health: Snoring during sleep, Epworth Sleepiness Score: to assess the daytime sleepiness of the participants, we used the Epworth Sleepiness Score scale. This scale helps to diagnose sleep apnea clinically, (vi) self-perceived general health: participant’s perception of physical health based on their past medical history.
Statistical analysis
We used Stata 15 software for conducting all statistical analyses [19]. We calculated the frequencies of all individual, contextual and covariates at both baseline and follow-up. After that, we identified the crude prevalence of diabetes among rural residents in both baseline and follow-up separately. Additionally, we used Chi-square tests to determine the association of diabetes prevalence and each predictor at baseline and follow-up separately. In this step, we identified if there is any change in prevalence from baseline to follow-up.
To identify the longitudinal patterns in the prevalence and associated risk factors of diabetes over four years of the follow-up period, we considered complete data at baseline and the follow-up and restructured them into a single long file format. A series of bivariate unadjusted logistic regression analyses were conducted to determine the association of diabetes prevalence with each potential risk factor. Variables with a P value <0.20 were considered candidates for the multivariable model.
For further analysis, we used a multivariable logistic regression model, which was based on the generalized estimation equations (GEE) was used to identify the effects of both contextual and individual factors after adjustment for covariates of interest. All predictors significant with a p value <0.05 and those of scientific/clinical/biologic importance were retained in the final multivariable model. The clustering effects of more than one individual within a household were adjusted by GEE [12, 20]. We used the logit link function and exchangeable correlation structure for GEE analysis [20]. Associations between significant variables (both contextual and individual) and diabetes prevalence were presented by odds ratios (OR) and their 95% confidence intervals (CI). To account for clustering due to repeated observation, we used the jackknife variance estimate to obtain 95% CI. To identify the parsimonious GEE model and the best working correlation structure, quasi-likelihood under the independence model criterion (QIC) [21] goodness of fit statistics were applied.
Survival analysis techniques were used to determine the risk factors for the incidence of diabetes. We considered the baseline survey as the time of origin and used a follow-up survey for incident cases. During the follow-up survey, non-diabetic participants were considered as censored cases. Variables from the baseline survey were considered for Cox’s proportional hazards model. After bivariate analysis, variables with a P value <0.20 were considered for Cox’s proportional hazards model. After adjusting for all the predictors in multivariate Cox’s proportional hazards model, only variables with P value <0.05 were considered significant. The strength of the association between significant variables and diabetes incidence was demonstrated by hazard ratios (HR) and their 95% confidence intervals (CI). We included time-dependent covariates in the model for checking proportionality, which is considered as one of the primary assumptions of the Cox proportional hazard model [22]. Furthermore, to assess the goodness of fit of the final model, we used the Cox-Snell residuals and Nelson-Aalen cumulative hazard function [23].
Results
Prevalence of diabetes
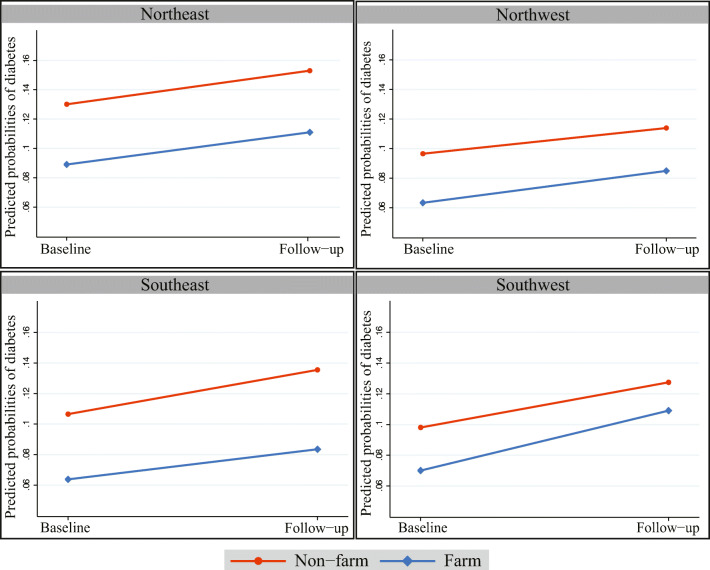
Table 1 showed the study participant’s characteristics – adults 18 years and older who participated at baseline (2010) and follow-up (2014) surveys. The crude prevalence of diabetes which was 9.31% (farm: 7.13% and non-farm:10.94%) at baseline and 11.29% (farm: 8.64% and non-farm:13.33%) at follow-up. Table 1 also highlighted that among four geographical locations, non-farm residents of the Northeast region showed the highest prevalence of diabetes in both baselines (13.35%) and follow-up (15.38%). Furthermore, obese non-farm males were at the highest risk of developing diabetes in both baselines (18.42%) and follow-up (18.51%) (data not shown).
Table 1.
Study population characteristics – adults 18 years and older who participated at baseline (2010) and follow-up (2014) surveys
| Parameters | Baseline | Follow-up | ||
|---|---|---|---|---|
| Farm | Non-farm | Farm | Non-farm | |
| Eligible household (based on household address) | 11,004 | 4454 | ||
|
Response rate (Based on household address), n (%) Baseline, n = 4624 Follow-up, n = 2797 |
4624 (42.0%) | 2797 (62.6%) | ||
|
Participating adults Baseline, n = 8206 Follow-up, n = 4844 |
3445 | 4761 | 2096 | 2748 |
| Male | 1792/4036 (44.40%) | 2244/4036 (55.60%) | 1079/2352 (45.88%) | 1273/2352 (54.12%) |
| Female | 1653/4170 (39.64%) | 2517/4170 (60.36%) | 1017/2492 (40.81%) | 1475/2492 (59.19%) |
|
Self-reported diabetes (Total) Baseline: 759/8154 (9.31%) Follow-up: 540/4782 (11.29%) |
243/3406 (7.13%) | 514/4698 (10.94%) | 178/2060 (8.64%) | 360/2700 (13.33%) |
|
Quadrant Diabetes, Yes/Total (%) | ||||
| South west | 37/547 (6.76%) | 97/969 (10.01%) | 32/311 (10.29%) | 72/521 (13.81%) |
| South east | 42/693 (6.06%) | 116/1057 (10.97%) | 30/405 (7.41%) | 86/585 (14.70) |
| North east | 102/1177 (8.67%) | 157/1176 (13.35%) | 64/693 (9.23%) | 104/676 (15.38%) |
| North west | 62/989 (6.27%) | 144/1492 (9.65%) | 47/602 (7.81%) | 93/847 (10.98%) |
Figure 1 showed that, a significant difference in the prevalence of diabetes between farm and non-farm residents irrespective of geographical location, with non-farm residents showing the highest prevalence. We observed a significant increase in diabetes prevalence in all four quadrants of Saskatchewan from baseline to follow-up.
Fig. 1.
Predicted Probabilities for Prevalence of diabetes by geographical locations and farm/non − farm residence at baseline and follow−up
Table 2 showed the unadjusted relationships between the individual, contextual factors, covariates and diabetes prevalence. In both baseline and follow-up surveys, non-farm residents (10.94% and 13.33% respectively) had a higher prevalence of diabetes compared to farm residents (7.13% and 8.64%). More people living at home (>2 people) reduced the prevalence of diabetes (5.15% at baseline and 5.71% at follow-up).
Table 2.
Univariate analysis of the association between risk factor (contextual and individual factors, and covariates) and diabetes
| Diabetes | |||
|---|---|---|---|
| Baseline | Follow-up | ||
| Yes/Total (%) | Yes/Total (%) | ORunadj (95% CI) | |
| Quadrant | |||
| South West | 134/1522 (8.80%) | 105/837 (12.54%) | 1.00 (ref) |
| South East | 159/1756 (9.01%) | 116/996 (11.65%) | 1.03 (0.81–1.30) |
| North East | 260/2368 (10.98%) | 169/1377 (12.27%) | 1.28 (1.03–1.59) |
| North West | 206/2495 (8.26%) | 140/1451 (9.65%) | 0.93 (0.74–1.17) |
| Location of home | |||
| Non-farm | 514/4694 (10.94%) | 360/2700 (13.33%) | 1.00 (ref) |
| Farm | 243/3406 (7.13%) | 178/2060 (8.64%) | 0.62 (0.55–0.71) |
| Family structure | |||
| # of people | |||
| = < 2 people | 635/5740 (11.06%) | 473/ 3756(12.59%) | 1.00 (ref) |
| > 2 people | 124/2410 (5.15%) | 56/981 (5.71%) | 0.42 (0.36–0.49) |
| Age of the participants | |||
| 18–45 years | 50/1932 (2.59%) | 17/642 (2.56%) | 1.00 (ref) |
| 46–55 years | 137/2036 (6.73%) | 67/968 (6.92%) | 2.73 (2.06–3.61) |
| 56–65 years | 214/1932 (11.08%) | 137/1813 (10.08%) | 4.47 (3.42–5.83) |
| >65 years | 3,582,251 (15.9%) | 319/1813 (17.6%) | 7.48 (5.79–9.66) |
| Sex | |||
| Female | 341/4146 (8.22%) | 235/2464 (9.54%) | 1.00 (ref) |
| Male | 418/4006 (10.43%) | 305/2317 (13.16%) | 1.35 (1.21–1.52) |
| Marital status | |||
| Married/Common law/living together | 614/6716 (9.14) | 435/4050 (10.74%) | 1.00 (ref) |
| Widowed/Divorced/separated/Single, never married | 139/1409 (9.87) | 103/720 (14.31%) | 1.19 (1.02–1.38) |
| Education level | |||
| Less than high school | 301/2096 (14.55%) | 208/1107 (18.79) | 1.00 (ref) |
| Completed high school | 240/2788 (8.61%) | 157/1584 (9.91%) | 0.52 (0.45–0.60) |
| Completed university | 49/935 (5.24%) | 49/611 (8.02%) | 0.35 (0.28–0.44) |
| Completed post-secondary education other than above | 158/2268 (6.97%) | 119/1414 (8.42%) | 0.42 (0.37–0.51) |
| Income adequacy | |||
| > = $60,000 | 212/3457 (6.13%) | 170/2247 (7.57%) | 1.00 (ref) |
| $40,000 to $59,999 | 154/1492 (10.32%) | 108/830 (13.01%) | 1.77 (1.50–2.09) |
| $20,000 to $39,999 | 183/1392 (13.15%) | 120/724 (16.57%) | 2.33 (1.98–2.73) |
| <$20,000 | 107/624 (17.15%) | 56/279 (20.07%) | 3.07 (2.52–3.74) |
| Exposure | |||
| Insecticide | |||
| No | 374/4410 (8.48%) | 219/2213 (9.9%) | 1.00 (ref) |
| Yes | 356/3601 (9.89%) | 299/2429 (12.31%) | 1.24 (1.10–1.39) |
| Pesticide | |||
| No | 585/6354 (9.21%) | 433/3748 (11.55%) | 1.00 (ref) |
| Yes | 166/1702 (9.75%) | 95/965 (9.84%) | 0.97 (0.84–1.12) |
| Fungicide | |||
| No | 460/5388 (8.54%) | 266/2763 (9.63%) | 1.00 (ref) |
| Yes | 270/2623 (10.29%) | 247/1847 (13.37%) | 1.34 (1.19–1.51) |
| Herbicide | |||
| No | 336/3957 (8.49%) | 200/1951 (10.25%) | 1.00 (ref) |
| Yes | 394/ 4054 (9.72%) | 318/2689 (11.83%) | 1.18 (1.05–1.33) |
| Livestock | |||
| No | 327/3900 (8.38%) | 193/1879 (10.27%) | 1.00 (ref) |
| Yes | 403/4111 (9.80%) | 327/2776 (11.78%) | 1.21 (1.07–1.35) |
| Household smoke | |||
| No | 638/6887 (9.26%) | 489/4290 (11.4%) | 1.00 (ref) |
| Yes | 118/1226 (9.62%) | 51/492 (10.37%) | 0.97 (0.82–1.15) |
| Family history | |||
| Dad diabetic | |||
| No | 464/6269 (7.40%) | 248/2929 (8.47%) | 1.00 (ref) |
| Yes | 187/1153 (16.22%) | 149/792 (18.81%) | 2.49 (2.16–2.86) |
| Mom diabetic | |||
| No | 433/6386 (6.78%) | 252/3040 (8.29%) | 1.00 (ref) |
| Yes | 238/1164 (20.45%) | 185/817 (22.64%) | 3.46 (3.03–3.96) |
| Siblings diabetic | |||
| No | 349/5827 (5.99%) | 188/2620 (7.18%) | 1.00 (ref) |
| Yes | 242/1063 (22.77%) | 214/876 (24.43%) | 4.52 (3.95–5.19) |
| Behavioural risk & protective factor | |||
| BMI | |||
| Normal (BMI < 25) | 103/2318 (4.44%) | 75/1349 (5.56%) | 1.00 (ref) |
| Overweight (BMI 25–30) | 236/3160 (7.47%) | 163/1860 (8.76%) | 1.69 (1.41–2.03) |
| Obese (BMI >30) | 371/2268 (16.36%) | 259/1324 (19.56%) | 4.12 (3.50–4.96) |
| Exercise duration/session | |||
| None | 372/3381 (11.0%) | 286/2152 (13.29%) | 1.00 (ref) |
| Less than 15 min | 63/449 (14.03%) | 47/324 (14.51%) | 1.22 (0.99–1.53) |
| 15–30 min | 196/2123 (9.23%) | 126/1181 (10.67%) | 0.8 (0.69–0.92) |
| 31–60 min | 75/1403 (5.35%) | 41/727 (5.64%) | 0.43 (0.34–0.52) |
| More than 60 min | 25/468 (5.34%) | 14/177 (7.91%) | 0.48 (0.34–0.67) |
| Smoking | |||
| Never Smoker | 306/4283 (7.14%) | 231/2508 (9.21%) | 1.00 (ref) |
| Ex-smoker | 363/2890 (12.56%) | 259/1818 (14.25%) | 1.77 (1.57–2.01) |
| Current smoker | 85/951 (8.94%) | 45/436 (10.32%) | 1.2 (0.98–1.47) |
| Screen time/ past three months | |||
| None | 10/150 (6.67%) | 6/113 (5.31%) | 1.00 (ref) |
| Less than 1 h | 23/188 (12.23%) | 16/123 (13.01%) | 2.21 (1.20–4.06) |
| From 1 to 2 h | 62/734 (8.45%) | 42/433 (9.70%) | 1.51 (0.88–2.6) |
| From 3 to 5 h | 171/1784 (9.59%) | 126/1050 (12.0%) | 1.81 (1.07–3.04) |
| From 6 to 10 h | 135/1749 (7.72%) | 94/1057 (8.89%) | 1.37 (0.81–2.31) |
| From 11 to 14 h | 85/1132 (7.51%) | 64/628 (10.19%) | 1.43 (0.84–2.43) |
| From 15 to 20 h | 105/1168 (8.99%) | 70/657 (10.65%) | 1.64 (0.96–2.78) |
| More than 20 h | 164/1204 (13.62%) | 119/675 (17.63%) | 2.74 (1.62–4.61) |
| Alcohol consumption / 12 month | |||
| Never | 209/1451 (14.4%) | 130/826 (15.74%) | 1.00 (ref) |
| Less than once a month | 207/1717 (12.06%) | 139/971 (14.32%) | 0.84 (0.72–0.99) |
| Once a month | 72/737 (9.77%) | 49/394 (12.44%) | 0.68 (0.55–0.85) |
| 2 to 3 times a month | 97/1463 (6.75%) | 91/833 (10.92%) | 0.52 (0.43–0.62) |
| Once a week | 57/909 (6.27%) | 31/494 (6.28%) | 0.38 (0.29–0.49) |
| 2 to 3 times a week | 44/1031 (4.27%) | 49/645 (7.6%) | 0.34 (0.26–0.43) |
| 4 to 6 times a week | 41/538 (7.62%) | 26/373 (6.97%) | 0.45 (0.34–0.59) |
| Every day | 32/317 (10.09%) | 22/233 (9.44%) | 0.62 (0.46–0.84) |
**Significant at the P value < 0.20 (marked as bold)
From Table 2 we also found that, the prevalence of diabetes significantly associated with known diabetes risk factors such as male sex (10.43% and 13.16%); widowed/divorced/separate/single (9.87% and 14.31%); less than high school educational attainment (14.55% and 18.79%); and low income (<$20,000) adequacy (17.15% and 20.07%) in both baseline and follow-up. The participants’ ages showed a dose-response relationship, and with an increase in age, diabetes prevalence significantly increased. Strong evidence of high diabetes prevalence was found among participants who were exposed to different chemical components used in the agricultural field when compared to non exposed participants. The prevalence of diabetes was higher among insecticide users (8.48% at baseline and 9.9% at follow-up); fungicide users (10.29% baseline and 13.37% follow-up; and herbicide users (9.72% baseline and 11.83% follow-up) compared to non-users. On univariate analysis, we found that common risk factors such as increasing age, male sex, low income, positive family history and behavioural and lifestyle risk factors (higher BMI and smoking) were significantly associated with higher diabetes prevalence. Farm residence, residents of the northwest quadrant, a higher number of residents living in the home, and over fifteen minutes of exercise per day act were associated with a lower prevalence of diabetes. Alcohol consumption acted as a protective factor.
In Table 3, we also observed that physician-diagnosed cardiovascular co-morbidities such as stroke, blood pressure, heart attack and heart disease are strongly associated with higher diabetes prevalence. Diagnosed cancer cases and respiratory co-morbidities such as tuberculosis, asthma, COPD, emphysema, and chronic bronchitis were relatively less strongly associated with diabetes than cardiovascular co-morbidities in univariate analysis.
Table 3.
Univariate Analysis of the association between comorbidities and Diabetes among adult rural residents
| Diabetes | |||
|---|---|---|---|
| Baseline | Follow-up | ||
| Yes/Total (%) | Yes/Total (%) | ORunadj (95% CI) | |
| Comorbidity | |||
| Cardiovascular | |||
| Heart disease | |||
| No | 606/7470 (8.11%) | 408/4288 (9.51%) | 1.00 (ref) |
| Yes | 138/606 (22.77%) | 131/493 (26.57%) | 3.43 (2.95–3.99) |
| Heart attack | |||
| No | 673/7782 (8.65%) | 467/4504 (10.37%) | 1.00 (ref) |
| Yes | 80/345 (23.19%) | 72/277 (25.99%) | 3.16 (2.61–3.83) |
| High blood pressure | |||
| No | 239/5387 (4.44%) | 128/2807 (4.56%) | 1.00 (ref) |
| Yes | 516/2725 (18.94%) | 412/1975 (20.86%) | 5.25 (4.62–5.96) |
| Stroke | |||
| No | 704/7953 (8.85%) | 499/4643 (10.75%) | 1.00 (ref) |
| Yes | 49/181 (27.07%) | 40/138 (28.99%) | 3.66 (2.84–4.71) |
| Respiratory | |||
| Chronic bronchitis | |||
| No | 699/7797 (8.96%) | 496/4487 (11.05%) | 1.00 (ref) |
| Yes | 24/167 (14.37%) | 11/85 (12.94%) | 1.49 (1.04–2.15) |
| Emphysema | |||
| No | 718/7872 (9.12%) | 500/4523 (11.0%) | 1.00 (ref) |
| Yes | 5/94 (5.32%) | 7/54 (12.96%) | 0.81 (0.44–1.47) |
| Asthma | |||
| No | 674/7365 (9.15%) | 463/4252 (10.89%) | 1.00 (ref) |
| Yes | 85/789 (10.77%) | 77/530 (14.53%) | 1.29 (1.08–1.53) |
| COPD | |||
| No | 685/7761 (8.83%) | 490/4461 (10.98%) | 1.00 (ref) |
| Yes | 38/203 (18.72%) | 17/115 (14.78%) | 1.97 (1.46–2.64) |
| Cancer | |||
| No | 671/7465 (8.99%) | 457/4218 (10.83%) | 1.00 (ref) |
| Yes | 84/670 (12.54%) | 82/563 (14.56%) | 1.46 (1.22–1.73) |
| Sleep apnea | |||
| No | 654/7594 (8.64%) | 446/4371 (10.20%) | 1.00 (ref) |
| Yes | 85/481 (17.67%) | 93/410 (22.68%) | 2.46 (2.06–2.93) |
| Others | |||
| Snore | |||
| No | 124/2313 (5.36%) | 124/1507 (8.23%) | 1.00 (ref) |
| Yes | 542/5112 (10.60%) | 349/2780 (12.55%) | 1.83 (1.58–2.12) |
| Epworth Sleepiness Score | |||
| Normal | 532/6348 (8.38%) | 347/3619 (9.59%) | 1.00 (ref) |
| Abnormal | 143/1195 (11.97%) | 111/669 (16.59%) | 1.63 (1.4–1.89) |
| General health | |||
| Excellent | 7/725 (0.97%) | 6/362 (1.66%) | 1.00 (ref) |
| Very good | 96/2869 (3.35%) | 86/1570 (5.48%) | 3.53 (2.01–6.22) |
| Good | 362/3296 (10.98%) | 275/2119 (12.98%) | 11.01 (6.33–19.15) |
| Fair | 236/1036 (22.78%) | 144/613 (23.49%) | 24.74 (14.15–43.26) |
| Poor | 52/184 (28.26%) | 26/102 (25.49%) | 30.98 (16.91–56.77) |
**Significant at the P value < 0.20 (marked as bold)
Table 3 also demonstrated that having sleep apnea, snoring problems, and an abnormal Epworth Sleepiness Score (ESS) demonstrated a significant association with higher diabetes prevalence. After univariate analysis, from Tables 2 and 3, we found that most of the variables remained significant except pesticide use, household smoking, and emphysema (in all cases, p value > was 0.20). Therefore, we considered all variables except pesticide use, household smoking, and emphysema for the multivariate model.
Table 4 demonstrates the findings of the marginal logistic regression models based on GEE analysis. Most of the variables become non-significant after adjusting for all other variables in the multivariate model.
Table 4.
Multivariable logistic regression analysis of the dependency of the prevalence of diabetes on contextual factors, individual factors, and covariates
| (SE() | ORadj (95% CI) | |
|---|---|---|
| Residence type | ||
| Non-farm | – | 1.00 (ref) |
| Farm | −0.39 (0.12) | 0.68 (0.52–0.86) |
| Age of the participants | ||
| 18–45 years | 1.00 (ref) | |
| 46–55 years | 0.32 (0.20) | 1.37 (0.93–2.01) |
| 56–65 years | 0.54 (0.21) | 1.71 (1.13–2.60) |
| >65 years | 0.78 (0.23) | 2.17 (1.38–3.43) |
| Sex | ||
| Female | 1.00 (ref) | |
| Male | 0.43 (0.14) | 1.54 (1.18–2.02) |
| Income adequacy | ||
| > = $60,000 | 1.00 (ref) | |
| $40,000 to $59,999 | 0.15 (0.12) | 1.16 (0.92–1.47) |
| $20,000 to $39,999 | 0.13 (0.14) | 1.14 (0.87–1.50) |
| <$20,000 | 0.41 (0.21) | 1.51 (1.01–2.27) |
| Exposure | ||
| Insecticide | ||
| No | 1.00 (ref) | |
| Yes | −0.07 (0.16) | 0.93 (0.69–1.27) |
| Family History | ||
| Dad diabetic | ||
| No | 1.00 (ref) | |
| Yes | 0.79 (0.13) | 2.21 (1.72–2.83) |
| Mom diabetic | ||
| Yes | 1.00 (ref) | |
| No | 0.85 (0.13) | 2.34 (1.82–3.01) |
| Siblings diabetic | ||
| Yes | 1.00 (ref) | |
| No | 0.90 (0.12) | 2.46 (1.94–3.12) |
| Behavioural Risk & Protective Factor | ||
| BMI | ||
| Normal (BMI < 25) | 1.00 (ref) | |
| Overweight (BMI 25–30) | 0.15 (0.16) | 1.16(0.85–1.58) |
| Obese (BMI >30) | 0.64 (0.16) | 1.89 (1.38–2.59) |
| Smoking | ||
| Current smoker | 1.00 (ref) | |
| Ex-smoker | 0.17 (0.12) | 1.18 (0.93–1.51) |
| Never Smoker | 0.26 (0.19) | 1.30 (0.90–1.88) |
| Alcohol consumption/ 12 month | ||
| Never | 1.00 (ref) | |
| Less than once a month | −0.06 (0.15) | 0.94 (0.70–1.28) |
| Once a month | 0.05 (0.19) | 1.05 (0.72–1.52) |
| 2 to 3 times a month | −0.45 (0.17) | 0.64 (0.46–0.89) |
| Once a week | −0.59 (0.20) | 0.56 (0.37–0.83) |
| 2 to 3 times a week | −0.73 (0.20) | 0.48 (0.32–0.72) |
| 4 to 6 times a week | −0.66 (0.23) | 0.51(0.33–0.81) |
| Every day | −0.56 (0.26) | 0.57 (0.34–0.96) |
| Comorbidity | ||
| Cardiovascular | ||
| Heart attack | ||
| No | 1.00 (ref) | |
| Yes | 0.56 (0.22) | 1.75 (1.15–2.69) |
| High blood pressure | ||
| No | 1.00 (ref) | |
| Yes | 0.95 (0.12) | 2.57 (2.05–3.23) |
| General health | ||
| Excellent | 1.00 (ref) | |
| Very good | 1.03 (0.40) | 2.81 (1.29–6.14) |
| Good | 1.77 (0.40) | 5.91 (2.70–12.93) |
| Fair | 2.27 (0.41) | 9.68 (4.33–21.65) |
| Poor | 2.28 (0.47) | 9.74 (3.09–24.31) |
| Interaction | ||
| Sex X Smoking | ||
| Male # Ex-smoker | 0.63 (0.22) | 1.89 (1.22–2.90) |
| Male # Current smoker | 0.40 (0.33) | 1.48(0.78–2.84) |
| Sex X Insecticide | ||
| Male # Insecticide user | 0.43 (0.21) | 1.53 (1.02–2.29) |
**Significant at the P value < 0.05 (marked as bold)
However, the variables that remained statistically significant in the multivariate model (Table 4) were: older age (56–65 year, Odds ratio (OR) 1.71, 95% confidence interval (CI) [1.13–2.60], >65 year, OR 2.17, CI [1.38–3.43]), lower-income <$20,000 (OR 1.51, CI [1.01–2.27]), a positive family history (dad diabetic, OR 2.21, CI [1.72–2.83], mom diabetic, OR 2.34, CI [1.82–3.01], siblings diabetic, OR 2.46, CI [1.94–3.12]), obesity, (OR 1.89, CI [1.38–2.59]), and comorbidities, such as high blood pressure, (OR 2.57, CI [2.05–3.23]), and heart attack (OR 1.75, CI [1.15–2.69]). Farm residence (OR 0.68, CI [0.52–0.86]) and alcohol consumption were associated with decreased odds of diabetes. A significant interaction was observed between the sex of the rural participant and smoking (P value <0.05). We observed another significant interaction between the sex of the rural participant and insecticide use for diabetes prevalence (P value <0.05).
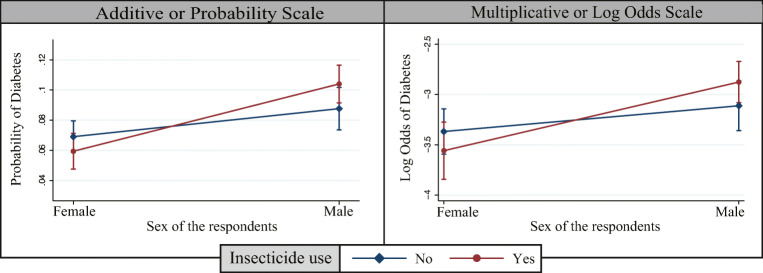
Figure 2 showed the interaction between the sex of the rural residents and insecticide use in both additive scale and multiplicative scale. In both scales, logit lines were not parallel; P value was <0.05 and no overlapping between male and female insecticide users, which indicates a significant interaction between sex of the rural residents and insecticide use. Male insecticide users had a higher probability of developing diabetes compared to non-insecticide users.
Fig. 2.
Interaction between the sex of the rural residents and insecticide use
Figure 3 showed the interaction between the sex of the rural residents and smoking status in both additive scale and multiplicative scale. In both scales, logit lines were not parallel; P value was <0.05 and no overlapping between male and female ex-smokers, which indicates a significant interaction between sex of the rural residents and smoking status. Male ex-smokers had a higher probability of developing diabetes compared to non-smokers.
Fig. 3.
Interaction between the sex of the rural residents and smoking status
Incidence of diabetes
The cumulative incidence of diabetes among rural residents of Saskatchewan was 2.75% from 2010 to 2014 (119 new cases). Results of unadjusted and adjusted hazard ratios based on Cox’s proportional hazards model are shown in Table 5. After conducting univariate analysis, we found that the significant variables associated with diabetes incidence were more than two people living in the house, an increase in age, the male sex, and low income. Among agricultural-related exposures, the factors found to significantly affect the incidence of diabetes were the use of insecticides, pesticides, fungicides and herbicides. Other statistically significant variables were a positive family history of diabetes and being overweight or obese. Additionally, comorbid conditions such as cardiovascular conditions (heart disease, heart attack, high blood pressure), respiratory conditions (chronic bronchitis, emphysema, asthma, COPD), and other conditions (cancer, sleep apnea) were significant in univariate analysis. Furthermore, Epworth Sleepiness Score, snoring, and general health status showed a positive association with diabetes incidence among the rural residents of Saskatchewan.
Table 5.
Cox regression analysis of diabetes during four years of follow-up according to a set of baseline characteristics (N = 4330)
| Crude HR (95% CI) |
Adjusted HR (95% CI) |
|
|---|---|---|
| Residence type | ||
| Non-farm | 1.00 (ref) | |
| Farm | 0.93 (0.64–1.34) | |
| Quadrant | ||
| South west | 1.00 (ref) | 1.00 (ref) |
| South east | 0.77 (0.45–1.33) | 0.71 (0.32–1.55) |
| North east | 0.74 (0.45–1.23) | 0.43 (0.19–0.95) |
| North west | 0.67 (0.40–1.13) | 0.36 (0.15–0.83) |
| People living at home | ||
| <= 2 people | 1.00 (ref) | |
| > 2 people | 1.88 (1.16–3.07) | |
| Age of the participants | ||
| 18–45 years | 1.00 (ref) | 1.00 (ref) |
| 46–55 years | 3.73 (1.55–8.98) | 2.48 (0.80–7.64) |
| > = 56 years | 5.55 (2.41–12.79) | 2.45 (0.79–7.65) |
| Sex | ||
| Female | 1.00 (ref) | 1.00 (ref) |
| Male | 1.76 (1.22–2.54) | 1.59 (0.73–3.50) |
| Marital Status | ||
| Married/Common law/living together | 1.00 (ref) | |
| Widowed/Divorced/separated/Single, never married | 1.18 (0.73–1.92) | |
| Education level | ||
| Less than high school | 1.00 (ref) | 1.00 (ref) |
| Completed high school | 0.59 (0.38–0.91) | 0.48 (0.24–0.98) |
| Completed university | 0.53 (0.28–1.00) | 0.85 (0.36–2.03) |
| Completed post-secondary education other than above | 0.38 (0.23–0.64) | 0.60 (0.27–1.33) |
| Income adequacy | ||
| > = $60,000 | 1.00 (ref) | |
| $40,000 to $59,999 | 1.28 (0.78–2.12) | |
| $20,000 to $39,999 | 1.55 (0.94–2.54) | |
| <$20,000 | 1.84 (0.93–3.66) | |
| Exposure | ||
| Insecticide | ||
| No | 1.00 (ref) | |
| Yes | 1.4 (0.98–2.02) | |
| Pesticide | ||
| No | 1.00 (ref) | |
| Yes | 1.38 (0.92–2.06) | |
| Fungicide | ||
| No | 1.00 (ref) | |
| Yes | 1.59 (1.18–2.29) | |
| Herbicide | ||
| No | 1.00 (ref) | |
| Yes | 1.48 (1.03–2.14) | |
| Livestock | ||
| No | 1.00 (ref) | |
| Yes | 1.11 (0.78–1.59) | |
| Household smoke | ||
| Yes | 1.00 (ref) | |
| No | 1.32 (0.81–2.15) | |
| Family History | ||
| Dad diabetic | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 1.67 (1.04–2.68) | 2.16 (1.09–4.27) |
| Mom diabetic | ||
| No | 1.00 (ref) | |
| Yes | 1.73 (1.08–2.76) | |
| Siblings diabetic | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 2.88 (1.88–4.42) | 1.97 (1.04–3.75) |
| Behavioural Risk & Protective Factor | ||
| BMI | ||
| Normal (BMI < 25) | 1.00 (ref) | 1.00 (ref) |
| Overweight (BMI 25–30) | 3.66 (1.72–7.77) | 3.32 (0.94–11.70) |
| Obese (BMI >30) | 7.28 (3.48–15.25) | 7.06 (2.02–24.65) |
| Exercise duration | ||
| None | 1.00 (ref) | |
| Less than 15 min | 1.31 (0.72–2.37) | |
| 15–30 min | 0.60 (0.39–0.94) | |
| 31–60 min | 0.21 (0.09–0.49) | |
| More than 60 min | 0.37 (0.11–1.14) | |
| Smoking | ||
| Never Smoker | 1.00 (ref) | |
| Ever smoker | 1.18 (0.82–1.69) | |
| Alcohol consumption/ 12 month | ||
| Never | 1.00 (ref) | |
| Less than once a month | 1.41 (0.80–2.47) | |
| Once a month | 0.89 (0.40–1.97) | |
| 2 to 3 times a month | 1.34 (0.74–2.42) | |
| Once a week | 0.48 (0.19–1.20) | |
| 2 to 3 times a week | 0.83 (0.41–1.69) | |
| 4 to 6 times a week | 0.50 (0.17–1.49) | |
| Every day | 0.78 (0.28–2.23) | |
| Comorbidity | ||
| Cardiovascular | ||
| Heart disease | ||
| No | 1.00 (ref) | |
| Yes | 3.22 (1.99–5.25) | |
| Heart attack | ||
| No | 1.00 (ref) | |
| Yes | 3.42 (1.87–6.28) | |
| High blood pressure | ||
| No | 1.00 (ref) | |
| Yes | 2.62 (1.82–3.78) | |
| Stroke | ||
| No | 1.00 (ref) | |
| Yes | 1.90 (0.69–5.25) | |
| Respiratory | ||
| Chronic bronchitis | ||
| No | 1.00 (ref) | |
| Yes | 2.15 (0.78–5.92) | |
| Emphysema | ||
| No | 1.00 (ref) | |
| Yes | 4.10 (1.47–11.42) | |
| Asthma | ||
| No | 1.00 (ref) | |
| Yes | 2.02 (1.22–3.36) | |
| COPD | ||
| No | 1.00 (ref) | |
| Yes | 3.39 (1.56–7.36) | |
| Cancer | ||
| No | 1.00 (ref) | |
| Yes | 1.67 (0.97–2.86) | |
| Sleep apnea | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 2.84 (1.68–4.79) | 2.24 (1.04–4.84) |
| Other | ||
| Snore | ||
| No | 1.00 (ref) | |
| Yes | 2.17 (1.29–3.64) | |
| Epworth Sleepiness Score | ||
| Normal | 1.00 (ref) | 1.00 (ref) |
| Abnormal | 2.24 (1.46–3.42) | 1.91 (1.03–3.53) |
| General health | ||
| Excellent | 1.00 (ref) | |
| Very good | 1.74 (0.61–4.97) | |
| Good | 3.37 (1.22–9.34) | |
| Fair | 7.67 (2.69–21.90) | |
| Poor | 6.33 (1.57–25.50) | |
| Interaction | ||
| Sex X Age | ||
| Male#46–55 years | 10.03 (0.92–104.44) | |
| Male# > =56 years | 8.21 (0.83–81.52) | |
**Crude HR: Significant at the P value < 0.20 (marked as bold)
**Adjusted HR: Significant at the P value < 0.05 (marked as bold)
Table 5 also represented that, non-significant variables such as residence type, marital status, smoking, household smoking, and stroke were not considered for the multivariable model as their P value was >0.20. After adjusting for all other variables, the factors that remained significant for diabetes incidence among rural residents were: a positive family history (diabetic father HR 2.16, CI [1.09–4.27], and sibling’s diabetic HR 1.97, CI [1.04–3.75]), obesity (HR 7.06, CI [2.02–24.65]), and a diagnosis of sleep apnea (HR 2.24, CI [1.04–4.84)]). All agriculture related exposure became non-significant after conducting a multivariable analysis. However, higher education (HR o.48, CI [0.24–0.98]) remained a protective factor for diabetes incidence. Furthermore, we observed a significant interaction between the sex and age of the residents (P values <0.05) for diabetes incidence. Male more than forty-five years old were at high risk of developing diabetes.
Discussion
Prevalence
This longitudinal epidemiological study determined the risk factors associated with the incidence and prevalence of diabetes among adult self-declared Caucasian rural residents of Saskatchewan from 2010 to 2014. Most of the participants who reported doctor-diagnosed diabetes were: North East quadrant residents, male, older than 55 years, in the low-income category, and with education less than high school. There was a 1.98% increase in the prevalence of diabetes from baseline to follow-up. Non-farm residents had a higher prevalence of diabetes compared to farm. Significant risk factors for diabetes prevalence were an increase in age, male sex, low income, positive family history, and high BMI. Additionally, co-morbidities such as blood pressure and heart attack were also associated with high diabetes prevalence among rural residents. Furthermore, we found that factors significantly associated with the incidence of diabetes were positive family history, high BMI, abnormal Epworth Sleepiness Score and sleep apnea. However, all agricultural-related exposures failed to show any significant association with the incidence of diabetes among rural residents.
The prevalence of diabetes increased in all quadrants at follow-up, and it is evident that after four years of follow-up, the southwest region had the highest increase (3.80%) in diabetes prevalence. Male rural residents had a higher prevalence of diabetes than females, which was aligned with other study findings [10, 12]. Our results also aligned with the national [11] and global [24] male to female ratio of diabetes prevalence. However, according to a recent publication, women with diabetes are at a higher risk of developing cardiovascular complications than men [25].
This study demonstrates that non-farm residents had a higher prevalence of diabetes when compared to farm residents. Non-farm residents experienced a substantial rise in diabetes prevalence (10.94% at baseline to 13.33% at follow-up) after four years of follow-up, which is remarkably higher than the overall provincial prevalence (9%) of diabetes [10]. A previous study hypothesized multiple reasons behind the higher prevalence of diabetes among non-farm residents of a rural agricultural zone [12]. One reason could be an influx of farm residents into small towns, either due to retirement or pre-existing health conditions such as diabetes [12]. Authors also believed that, even with advanced automation in agricultural procedures, farmers still have to work outside and are more active than non-farmers [12].
According to the Diabetes Canada Clinical Practice Guidelines (2018), moderate to elevated physical activity can significantly reduce blood glucose levels, decrease blood cholesterol levels, and diminish insulin resistance in people with diabetes, which was consistent with our study findings [26]. Our study showed that moderate to elevated exercise is a protective factor and might reduce the prevalence of diabetes among rural residents of Saskatchewan. A recent study published by European Society of Cardiology claims that physical activity can significantly reduce diabetes-related mortality and morbidity, irrespective of having cardiovascular disease (CVD) [27].
Higher population density (>2 people) acted as a protective factor (OR 0.42, CI [0.36–0.49]) on the prevalence of diabetes. This finding concurred with other recent studies, and it might be because diabetes is highly prevalent in households with food insecurity [28], where nearly half of those food insecure households were comprised of primarily unattached individuals [29] and single persons living alone [30]. These papers also reinforce our study findings that marital status (single/divorced/separated/widowed) and poor socio-economic status (lack of post-secondary education/low income) increase the risk of developing diabetes.
Known diabetes risk factors such as respondent’s age, sex, income, positive family history remained significant after adjusting for all variables. Behavioural risk factors such as high BMI, physical inactivity, high screen time, and smoking were significant in the univariate model but became non-significant after multivariate analysis. However, smoking showed a significant interaction with the sex of the respondents. Male ex-smokers were at a higher risk of developing diabetes. A possible explanation could be that ex-smokers endure adverse metabolic changes such as weight gain and systemic inflammation, whereas current smokers do not, which may lead to an increase in the risk of diabetes [31]. This risk may peak as far as five to seven years after the respondent quits smoking [32]. Another behavioural risk factor was alcohol consumption (irrespective of dosages) acted as a protective factor for diabetes prevalence. It can significantly reduce diabetes risk, and the findings are supported by previous studies [33, 34]. The probable cause might be due to alcohol can reduce blood sugar levels [33, 34] and HbA1c [34].
As demonstrated in other publications, our univariate analysis showed that the use of insecticide, pesticide, and fungicide in agricultural fields was significantly associated with increased diabetes prevalence. Recent evidence-based studies illustrate that exposure to these chemical substances can increase the risk of diabetes by causing a reduction in insulin production [35], impaired glucose tolerance [35], insulin resistance [36], and circadian disruption [36]. Surprisingly, in multivariate analysis, after adjusting for other variables, all agricultural chemical exposures became non-significant. However, we observed a significant interaction between insecticide use in agricultural fields and the sex of the participants; male insecticide users were at a high risk of developing diabetes. It might be due to the habitual high risk-taking tendency of men [37] or that men are more prone to be in direct contact with those agricultural chemical substances [12]. Our findings also indicate that despite modernization in the agricultural sector, rural farming exposure might still be a risk factor for developing diabetes.
Declines in a participant’s perception of general health status also acted as a significant predictor and exponentially increased diabetes risk. As expected, cardiovascular co-morbidities such as heart attack and high blood pressure remained the most significant predictors of diabetes prevalence after multivariate analysis.
Incidence
This study is the first of its kind to measure the cumulative incidence (2.75%) of diabetes among rural residents of Saskatchewan. Based on self-report, 119 new diabetes cases were identified among 4330 rural residents after four years of follow-up. Information regarding the incidence of diabetes in rural Canada population is scarce. However, the Canadian Chronic Disease Surveillance System (CCDSS) states that the age-standardized incidence of diabetes remains nearly stable from 2000 to 2017 [38]. A cohort study was conducted over 7.5 years in Spain and found that the nationwide cumulative incidence was 6.4% [39], which is more than two-fold higher than our findings. Another 8-year cohort study conducted in Norway states that the cumulative incidence of that community was 6.1% [40]. The incidence rate difference in different parts of the world might be due to different demographics, urban/rural disparities, duration of the study, and study design.
We observed that being obese increased the incidence of diabetes among rural residents. Obese participants are twice as likely to develop diabetes when contrasted with overweight respondents. Univariate analysis found that positive family history also acted as a significant predictor for diabetes incidence. However, in the multivariate model, the diabetic mother variable became non-significant.
Common risk factors such as an increase in age, the male sex, and low-income became non-significant after adjusting for all variables. However, a significant interaction was observed between the age and sex of the participants; males over the age of forty-five were at a higher risk of developing diabetes than females. Occupational chemical exposures also became non-significant in the multivariate model, which is likely due to fewer study participants in each occupational chemical exposure group.
Only higher education remains a protective factor after multivariate analysis, as higher education can significantly reduce the incidence of diabetes. A recent study conducted among rural residents of China illustrates that lower health literacy levels are inversely associated with glycemic control and can aggravate diabetes risk [41]. Health literacy promotes healthy food eating practices and helps reduce behavioural risk factors.
Sleep apnea and its related symptom (Epworth Sleepiness Score) were significantly associated with the high incidence of diabetes, and results were consistent with previous research [42]. Insulin resistance and glucose intolerance are known mechanisms for developing diabetes among sleep apnea patients [42]. Obesity is a shared common risk factor between Diabetes and sleep apnea, which may be a key moderator/mediator for the effect of sleep apnea on developing diabetes. It is already proven that sleep apnea is associated with diabetes prevalence [42]. However, to our knowledge no study has been conducted to identify the effect of obesity via sleep apnea or abnormal Epworth Sleepiness Score (which measures excessive daytime sleepiness) on diabetes prevalence/incidence among rural Canadian residents.
Strength of the study
This longitudinal study was the first of its kind to estimate the cumulative incidence and associated risk factors among rural diabetic residents of Saskatchewan. The longitudinal nature of this study allows a better understanding of the causality of higher diabetes prevalence and incidence among the rural residents of Saskatchewan. It also provides valuable information on changes over time regarding diabetes. By analyzing a large number of farm/non-farm residents stratified by a widespread geographical location, we can better understand the role of a wide range of diabetes predictors in the province.
Limitation of the study
It is plausible that our research may have some limitations. We could not distinguish between type 1, type 2 and gestational diabetes due to limitations in the dataset. We failed to identify risk factors associated with different types of diabetes. However, type 2 diabetes mellitus is the most common form of diabetes and predominantly affects adult populations [24].
The information regarding occupational exposures (insecticide, fungicide, herbicide) such as intensity, chemical composition, method of applying it in the field, and use of protective gear was missing. Occupational exposures were self-reported, and no objective measurements were available for those exposures. The availability of objective measurements might help prove a strong linkage between specific occupational exposures and diabetes. We prove a strong longitudinal univariate association between insecticide use and diabetes. Furthermore, a significant interaction between male sex and insecticide use validates the biological plausibility of diabetes prevalence among insecticide users.
Our study fails to demonstrate the ethnic/racial distribution of diabetes, as most of the participants were Caucasian or of European heritage. The findings of our study might not be generalizable for the entire Saskatchewan rural population as there is an age distribution discrepancy between our study participants and Saskatchewan’s overall general population. Necessary caution should be exercised when generalizing our study findings against the total rural population of Saskatchewan. Details about generalizability limitations were described elsewhere [16, 17].
About 3520 participants did not participate in the follow-up survey. A comparison analysis was conducted to observe the difference between respondents who participated in both surveys and dropouts who participated only in the baseline. Dropouts tended to be town dwellers, residents of low socio-economic status, or respondents having had higher reported cases of respiratory disease. However, we did not find any statistically significant difference regarding lung function tests between respondents and dropouts. Additionally, there was a lack of sufficient information on dietary patterns, prescribed diabetic medication, and diabetes-related mortality/morbidity might be the limitation of the present study. Lastly, there may be a possible chance of bias as our outcome of interest was self-reporting diabetes.
Future direction
More intensive studies are required to prove a causal link between the onset of diabetes and agricultural occupational exposures, such as insecticide, fungicide, and pesticide use. A comprehensive questionnaire tailored to diabetes occurrence and risk factors specific to rural residence would help identify additional findings. Also, we would be able to pinpoint clinically diagnosed diabetic cases and reduce biases by engaging trained personnel and using hospital registries to monitor HbA1c and blood sugar levels. Data regarding new diabetes cases should be collected every year. Recording the responsible factors and the exact date of diabetes occurrence will allow us to calculate a distinct incidence rate as suggested by the Geneva Foundation for Medical Education and Research for long cohort studies [43]. A mediation analysis may also be conducted to evaluate the effect of sleep apnea on diabetes or vice versa, as obesity was a common risk factor. Strategies and recommendation for rural residents:
Agricultural Health and Safety Network (AHSN) at the Canadian Centre for Health and Safety (CCHS) has an outreach program and publish findings of research being conducted at the Canadian Centre for Health and Safety in Agriculture (CCHSA) in a regular newsletter on an on-going basis. We plan to communicate findings of this report with 26,000 Saskatchewan farmers to inform and educate them about the association between agricultural chemical and other rural exposures and prevalence of diabetes. The AHSN educates these farmers on how to handle chemical substances in the agricultural field safely.
Physical activity should be encouraged for farm and non-farm rural dwellers. This can be done via AHSN regular newsletter as well.
Educational interventions and lifestyle modification should be introduced among all rural residents, especially high-risk groups, including non-farm residents, low socioeconomic status, older age groups, males, smokers, and rural residents with high BMI.
Conclusion
A mix of individual and contextual factors was responsible for the high incidence and prevalence rate of diabetes among the rural residents of Saskatchewan, which demands urgent long term and population-based community health initiatives. We observed a steep rise in diabetes prevalence among Saskatchewan rural residents from baseline (2010) to follow-up (2014). Additionally, we found that the obese non-farm male residents were at the highest risk of developing diabetes when compared to the farm residents in rural Saskatchewan. Along with known modifiable risk factors (income, education, BMI level, smoking, heart attack and high blood pressure) and non-modifiable risk factors (age, sex, positive family history), agricultural chemical-related exposures were also responsible for the high prevalence of diabetes among rural residents. Significant predictors responsible for the high incidence of diabetes among rural residents of Saskatchewan after four years of follow-up study were high BMI and positive family history. A unique finding was that sleep apnea significantly increases the risk of developing new cases of diabetes among rural residents. Conversely, we found that sleep apnea was non-significant for high diabetes prevalence among the same population group. The common significant variable between incidence and prevalence of diabetes was obesity. Obesity is a mutually shared risk factor between sleep apnea and diabetes, and a mediation analysis could be conducted in the future to evaluate the impact of sleep apnea on diabetes or vice versa. We strongly recommend that information regarding healthy food choices and lifestyle practices, as well as suggestions for personal safety measurements regarding the long-term effects of agricultural chemical exposures should be made readily available to everyone to increase awareness and reduce diabetes occurrence and its complications in Canada.
Acknowledgments
The Saskatchewan Rural Health Study Team consists of: James Dosman, MD (Designated Principal Investigator, University of Saskatchewan (UofS), Saskatoon, SK Canada); Punam Pahwa, PhD (Co-Principal Investigator, UofS, Saskatoon SK Canada); John Gordon, PhD (Co-Principal Investigator, UofS, Saskatoon SK Canada); Yue Chen, PhD (University of Ottawa, Ottawa Canada); Roland Dyck, MD (UofS, Saskatoon SK Canada); Louise Hagel (Project Manager, UofS, Saskatoon SK Canada); Bonnie Janzen, PhD (UofS, Saskatoon SK Canada); Chandima Karunanayake, PhD (UofS, Saskatoon SK Canada); Shelley Kirychuk, PhD (UofS, Saskatoon SK Canada); Niels Koehncke, MD (UofS, Saskatoon SK Canada); Joshua Lawson, PhD, (UofS, Saskatoon SK Canada); William Pickett, PhD (Queen’s University, Kingston ON Canada); Roger Pitblado, PhD (Professor Emeritus, Laurentian University, Sudbury ON Canada); Donna Rennie, RN, PhD, (UofS, Saskatoon SK Canada); Ambikaipakan Senthilselvan, PhD (University of Alberta, Edmonton, AB, Canada). We are grateful for the contributions of all the participants who donated their time to complete and return the survey.
All sources of support requiring acknowledgement
Canadian Institutes of Health Research.
Appendix
Fig. 4.
Theoretical framework for this study. Legend of Assessment Methods: 1. Survey questionnaire (farm and small-town cohorts); 2 Clinical assessment (sub-sample of both cohorts); 3. Environmental assessments; 4. Health care utilization records.
Authors contribution
SA conducted all the analysis, interpretation, discussion and writing of this manuscript under the supervision of PP. RD, BJ, CK, and JD reviewed the manuscript and assisting in preparing the manuscript by providing feedback.
Funding
was received from Canadian Institutes of Health Research “Saskatchewan Rural Health Study”, Canadian Institutes of Health Research MOP-187209-POP-CCAA-11829.
Data availability
Available and will provide upon request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Available and will provide upon request.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Md Saiful Alam, Email: mda523@mail.usask.ca.
Roland Dyck, Email: roland.dyck@usask.ca.
Bonnie Janzen, Email: bonnie.janzen@usask.ca.
Chandima Karunanayake, Email: cpk646@mail.usask.ca.
James Dosman, Email: james.dosman@usask.ca.
Punam Pahwa, Email: pup165@mail.usask.ca.
References
- 1.Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. European Journal of Preventive Cardiology [Internet]. 2019 Dec;26(2_suppl):7–14. Available from: . 10.1177/2047487319881021.http://journals.sagepub.com/doi/10.1177/2047487319881021 [DOI] [PubMed]
- 2.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. The Lancet Diabetes & Endocrinology [Internet]. 2017 Jun;5(6):423–30. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213858717300979. 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed]
- 3.Houlden RL. Introduction : 2018 clinical practice guidelines. Canadian Journal of Diabetes [Internet]. 2018 Apr;42:S1–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1499267117308110. 10.1016/j.jcjd.2017.10.001.
- 4.Powderley K (2019) One in three Canadians is living with diabetes or prediabetes, yet knowledge of risk and complications of disease remains low [Internet]. Diabetes Canada. [cited 2019 Nov 7]. Available from: https://www.diabetes.ca/media-room/press-releases/one-in-three-canadians-is-living-with-diabetes-or-prediabetes,-yet-knowledge-of-risk-and-complicatio
- 5.Bilandzic A, Rosella L (2017) The cost of diabetes in Canada over 10 years: applying attributable health care costs to a diabetes incidence prediction model. Health Promotion and Chronic Disease Prevention in Canada [Internet]. 2017 Feb;37(2):49–53. Available from: https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-37-no-2-2017/cost-diabetes-canada-over-10-years-applying-attributable-health-care-costs-diabetes-incidence . 10.24095/hpcdp.37.2.03 [DOI] [PMC free article] [PubMed]
- 6.Diabetes in Canada: Backgrounder. Ottawa: Diabetes Canada; 2020.
- 7.Public Health Agency of Canada, Diabetes in Canada: Facts and figures from a public health perspective. Ottawa, 2011.
- 8.Harris SB, Bhattacharyya O, Dyck R, Hayward MN, Toth EL. Type 2 diabetes in aboriginal peoples. Canadian Journal of Diabetes [Internet]. 2013 Apr;37:S191–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1499267113000476. 10.1016/j.jcjd.2013.01.046. [DOI] [PubMed]
- 9.Canada’s rural population since 1851 [Internet]. Statistics Canada. 2011 [cited 2019 Nov 3]. p. 1–6. Available from: https://www12.statcan.gc.ca/census-recensement/2011/as-sa/98-310-x/98-310-x2011003_1-eng.cfm
- 10.Diabetes in Saskatchewan: Backgrounder.Ottawa: Diabetes Canada; 2020.
- 11.Health Fact Sheets Diabetes, 2017 [Internet]. Statistics Canada. 2018 [cited 2019 Jul 23]. Available from: https://www150.statcan.gc.ca/n1/pub/82-625-x/2018001/article/54982-eng.htm.
- 12.Dyck R, Karunanayake C, Pahwa P, Hagel L, Lawson J, Rennie D, et al. Prevalence, risk factors and co-morbidities of diabetes among adults in rural Saskatchewan: the influence of farm residence and agriculture-related exposures. BMC Public Health [Internet]. 2013 Dec 5;13(1):7. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-710.1186/1471-2458-13-7 [DOI] [PMC free article] [PubMed]
- 13.Oster RT, Toth EL. Differences in the prevalence of diabetes risk-factors among first nation, Métis and non-aboriginal adults attending screening clinics in rural Alberta, Canada. Rural and remote health [internet]. 2009;9(2):1170. Available from. http://www.ncbi.nlm.nih.gov/pubmed/19496641. [PubMed]
- 14.Dinca-Panaitescu S, Dinca-Panaitescu M, Bryant T, Daiski I, Pilkington B, Raphael D. Diabetes prevalence and income: results of the Canadian community health survey. Health Policy [Internet]. 2011 Feb;99(2):116–23. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168851010002241. 10.1016/j.healthpol.2010.07.018. [DOI] [PubMed]
- 15.Fuller D, Neudorf J, Lockhart S, Plante C, Roberts H, Bandara T, et al. Individual- and area-level socioeconomic inequalities in diabetes mellitus in Saskatchewan between 2007 and 2012: a cross-sectional analysis. CMAJ Open [Internet]. 2019 Jan 21;7(1):E33–9. Available from: http://cmajopen.ca/lookup/doi/10.9778/cmajo.20180042. 10.9778/cmajo.20180042. [DOI] [PMC free article] [PubMed]
- 16.Pahwa P, Karunanayake CP, Hagel L, Janzen B, Pickett W, Rennie D, et al. The Saskatchewan rural health study: an application of a population health framework to understand respiratory health outcomes, BMC Research Notes [Internet]. 2012 Dec 1;5(1):400. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-5-400:. 10.1186/1756-0500-5-400. [DOI] [PMC free article] [PubMed]
- 17.Pahwa P, Rana M, Pickett W, Karunanayake CP, Amin K, Rennie D, et al. Cohort profile: the Saskatchewan Rural Health Study—adult component. BMC Research Notes [Internet]. 2017 Dec 11;10(1):732. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-017-3047-110.1186/s13104-017-3047-1 [DOI] [PMC free article] [PubMed]
- 18.Faubion CW, Andrew JD, Book Review: Dillman DA (2000) Mail and internet surveys: The tailored design method (2nd ed.). New York: Wiley 464 pp. Rehabil Couns Bull [Internet]. 2001 Apr 13 [cited 2019 May 18];44(3):178–80. Available from: http://journal.
- 19.StataCorp . Stata statistical software: release 15. College Station: StataCorp LLC; 2017. [Google Scholar]
- 20.Hanley JA. Statistical analysis of correlated data using generalized estimating equations: an orientation. American Journal of Epidemiology [Internet]. 2003 Feb 15;157(4):364–75. Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwf215. 10.1093/aje/kwf215. [DOI] [PubMed]
- 21.Cui J. QIC program and model selection in GEE analyses. The Stata Journal: Promoting communications on statistics and Stata [Internet]. 2007 Jun 19;7(2):209–20. Available from: http://journals.sagepub.com/doi/10.1177/1536867X0700700205. 10.1177/1536867X0700700205.
- 22.Kleinbaum DG, Edition T. Extended Cox Model. 3rd ed. Springer-Verlag New York; 2012. 463–555 p.
- 23.Survival analysis with STATA [Internet]. UCLA: Institute for digital research and education. [cited 2019 Aug 23]. Available from: https://stats.idre.ucla.edu/stata/seminars/stata-survival/
- 24.IDF Diabetes Atlas, 9th edn. Brussels, Belgium [Internet]. International Diabetes Federation. 2019. Available from: https://www.diabetesatlas.org.
- 25.Women most affected by vascular complications of diabetes [Internet]. European Society of Cardiology (ESC). 2019 [cited 2020 Jan 23]. Available from: https://www.escardio.org/The-ESC/Press-Office/Press-releases/Women-most-affected-by-vascular-complications-of-diabetes
- 26.Sigal RJ, Armstrong MJ, Bacon SL, Boulé NG, Dasgupta K, Kenny GP, et al. Physical activity and diabetes. Canadian Journal of Diabetes [Internet]. 2018 Apr;42:S54–63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1499267117308183. 10.1016/j.jcjd.2017.10.008. [DOI] [PubMed]
- 27.Kemps H, Kränkel N, Dörr M, Moholdt T, Wilhelm M, Paneni F, et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: What to pursue and how to do it. A Position Paper of the European Association of Preventive Cardiology (EAPC). European Journal of Preventive Cardiology [Internet]. 2019 May 14;26(7):709–27. Available from: http://journals.sagepub.com/doi/10.1177/2047487318820420. 10.1177/2047487318820420 [DOI] [PubMed]
- 28.Gucciardi E, Vahabi M, Norris N, Del Monte JP, Farnum C. The intersection between food insecurity and diabetes: a review. Current Nutrition Reports [Internet] 2014 Dec 2;3(4):324–332. Available from: http://link.springer.com/10.1007/s13668-014-0104-4 . 10.1007/s13668-014-0104-4 [DOI] [PMC free article] [PubMed]
- 29.Tarasuk, V, Mitchell, A, Dachner, N. (2016). Household food insecurity in Canada, 2014.Toronto: Research to identify policy options to reduce food insecurity (PROOF).Retrieved from https://proof.utoronto.ca/.
- 30.Lim H-S, Lee M-N. Comparison of health status and nutrient intake by household type in the elderly population. Journal of Bone Metabolism [Internet]. 2019;26(1):25. Available from: https://synapse.koreamed.org/DOIx.php?id=10.11005/jbm.2019.26.1.25 . 10.11005/jbm.2019.26.1.25 [DOI] [PMC free article] [PubMed]
- 31.Shriner RL. Smoking Cessation and the Risk for Type 2 Diabetes Mellitus. Annals of Internal Medicine [Internet]. 2010 Jun 1;152(11):755. Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-152-11-201006010-00020 . 10.7326/0003-4819-152-11-201006010-00020 [DOI] [PubMed]
- 32.Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. New England Journal of Medicine [Internet]. 2018 Aug 16;379(7):623–32. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1803626. 10.1056/NEJMoa1803626. [DOI] [PMC free article] [PubMed]
- 33.Kerr WC, Ye Y, Williams E, Lui CK, Greenfield TK, Lown EA. Lifetime alcohol use patterns and risk of diabetes onset in the national alcohol survey. Alcoholism: Clinical and Experimental Research [Internet]. 2018 Dec 10;acer.13924. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/acer.13924 . 10.1111/acer.13924 [DOI] [PMC free article] [PubMed]
- 34.Wiss DA. The relationship between alcohol and Glycohemoglobin: a biopsychosocial perspective. BioResearch Open Access [Internet]. 2019 Oct 1;8(1):146–54. Available from: https://www.liebertpub.com/doi/10.1089/biores.2019.0009. 10.1089/biores.2019.0009. [DOI] [PMC free article] [PubMed]
- 35.Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia [Internet]. 2018 Jul 9;61(7):1495–502. Available from: http://link.springer.com/10.1007/s00125-018-4621-3. 10.1007/s00125-018-4621-3. [DOI] [PMC free article] [PubMed]
- 36.Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environmental Health and Preventive Medicine [Internet]. 2018 Dec 27;23(1):3. Available from: https://environhealthprevmed.biomedcentral.com/articles/10.1186/s12199-018-0692-5 . 10.1186/s12199-018-0692-5 [DOI] [PMC free article] [PubMed]
- 37.Lord JR. Psychology the Science of Mind. Vol. 76, Journal of Mental Science. Nelson Education; 1930. 543–545 p.
- 38.LeBlanc AG, Gao YJ, McRae L, Pelletier C. At-a-glance - Twenty years of diabetes surveillance using the Canadian Chronic Disease Surveillance System. Health Promotion and Chronic Disease Prevention in Canada [Internet]. 2019 Nov;39(11):306–9. Available from: https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-39-no-11-2019/twenty-years-diabetes-surveillance.html. 10.24095/hpcdp.39.11.03 [DOI] [PMC free article] [PubMed]
- 39.Rojo-Martínez G, Valdés S, Soriguer F, Vendrell J, Urrutia I, Pérez V, et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort diabetes study. Scientific Reports [Internet]. 2020 Dec 17;10(1):2765. Available from: http://www.nature.com/articles/s41598-020-59643-7 . 10.1038/s41598-020-59643-7 [DOI] [PMC free article] [PubMed]
- 40.Naseribafrouei A, Eliassen BM, Melhus M, Svartberg J, Broderstad AR. Estimated 8-year cumulative incidence of diabetes mellitus among Sami and non-Sami inhabitants of Northern Norway - The SAMINOR Study. BMC Endocrine Disorders [Internet]. 2019 Dec 24;19(1):66. Available from: https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-019-0399-7 . 10.1186/s12902-019-0399-7 [DOI] [PMC free article] [PubMed]
- 41.Hu Z, Zhu X, Kaminga AC, Xu H. Associated risk factors and their interactions with type 2 diabetes among the elderly with prediabetes in rural areas of Yiyang City. Medicine [Internet]. 2019 Nov;98(44):e17736. Available from http://journals.lww.com/10.1097/MD.0000000000017736: . 10.1097/MD.0000000000017736 [DOI] [PMC free article] [PubMed]
- 42.Doumit J, Prasad B. Sleep apnea in type 2 diabetes. Diabetes spectrum [Internet]. 2016 Feb 16;29(1):14–9. Available from: http://spectrum.diabetesjournals.org/lookup/doi/10.2337/diaspect.29.1.14:. 10.2337/diaspect.29.1.14. [DOI] [PMC free article] [PubMed]
- 43.O. Meirik. Cohort and case control studies [Internet]. Geneva foundation for medical education and research. 2019 [cited 2020 Feb 16]. Available from: https://www.gfmer.ch/Books/Reproductive_health/Cohort_and_case_control_studies.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available and will provide upon request.