Abstract
Background
Diabetic peripheral neuropathy (DPN) is the most common and troublesome complication of diabetes mellitus. It affects almost half the population with diabetes and worsens quality of life of the patient. This study was aimed to determine the prevalence of peripheral neuropathy and associated pain in patients with diabetes mellitus.
Methods
This was a cross-sectional study conducted over a period of six months. Patient’s ≥ 18 years with confirmed diagnosis of diabetes mellitus were included in the study. Patients with hypothyroidism, medical illness such as cancer, liver or renal disease, cervical or lumbar spondylosis, pregnant patients with diabetes and patients receiving any treatment that might influence nerve function (e.g., cytotoxic or antiepileptic agents) were excluded from the study. DPN was diagnosed using 10 g monofilament test. The S-LANSS questionnaire was used to assess the associated painful symptoms. Association was calculated using chi-square test. A p- value of ≤0.05 was considered statistically significant. All the statistical analysis was performed using SPSS v22.
Result
The overall prevalence of DPN was found to be 28.85% from which 88% patients were found to have painful symptoms. A significant association of DPN was observed with the duration of diabetes (p = 0.004), poor glycaemic control (p = 0.03) and other diabetic complications such as nephropathy (p = 0.002). No association of neuropathy was found with retinopathy and hypertension. Duration of diabetes (>15 years), and HbA1c (>9%) was found to be positively associated the painful DPN.
Conclusion
The current study found a high prevalence of DPN and it was found to be significantly associated with duration of diabetes, poor glycaemic control and nephropathy.
Keywords: Diabetes complications, Diabetic neuropathy, DPN, India, T2DM
Introduction
Diabetes is now considered to be a prime cause for mortality and morbidity [1]. According to the latest facts and figure provided by International Diabetes Federation, 463 million people were living with diabetes globally and 374 million people are at an increased risk of developing type 2 diabetes mellitus (T2DM) [2]. In India around 77 million people are living with diabetes in 2019 and by 2045 this will rise to 134.2 million [2]. Diabetes is responsible for a range of complications including microvascular and macrovascular ramifications [3–5].
The most common and concerning complication of diabetes mellitus (DM) is diabetic neuropathy. It can be peripheral, focal, proximal or autonomic. Globally, more than 40 million people with diabetes live with neuropathy [6]. According to the American Diabetes Association (ADA), diabetic peripheral neuropathy (DPN) is defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” [7]. It affects specific regions of the nervous system [8], leading to morbidity, thereby increasing the economic burden for diabetes care [9]. It is a long-term complication of diabetes with up to 50% cases being asymptomatic [10] [11]. It is also responsible for 40–60% of lower extremity amputations [12].
DPN symptoms can be subjective and short-term [13], mimicking those of other diseases or may be silent and go undetected. It can either cause a loss of sensation or severely painful symptoms [14]. Therefore, clinical examination is the recommended method of diagnosis of peripheral neuropathy [15]. Common symptoms of DPN include significant deficits in tactile and pain sensitivity, kinesthesia, lower-limb proprioception, and vibration sense, caused by promotion neuronal apoptosis and inhibition of nerve regeneration in diabetes [16]. It is dominantly denoted by sensory changes in the “glove-and-stocking” distribution [17].
Pain is a common symptom experienced by patients and it adds to the humanistic and economic burden on the patients. Painful DPN can cause anxiety, depression, adversely affects the function and deteriorate the overall quality of life [18–20].
The true DPN prevalence is not known. Studies show variation from 10% to 90% in diabetic patients, depending on the criteria and methods used to define neuropathy [8]. DPN has been found between 26 to 31% in surveys in the Indian patients [21]. Treatment of DPN is challenging and the drugs available are scarce. Common treatment medications include tricyclic antidepressants, serotonin, and norepinephrine reuptake inhibitors, gabapentin, pregabalin and opioids.
Most studies of DPN have been performed in Western populations [22] and there is a scarcity of data from developing countries, especially from northern part of India, where a large proportion of the population walks bare foot [13]. In this study we aimed to estimate the prevalence of DPN and associated pain using confirmatory screening tools.
Method
Study design and setting
This was a cross sectional study conducted in the out-patient department of Hakeem Abdul Hameed Centenary (HAHC) Hospital, Jamia Hamdard, New Delhi, India. HAHC is a 470 bedded private academic teaching hospital with advanced tertiary care facilities. Study duration was of six months i.e. from December 2017 – May 2018. The study protocol was approved by the Research Project Advisory Committee (RPAC) of HAHC and Institutional Ethics Committee of Jamia Hamdard. The study was conducted in full compliance with the declaration of Helsinki guidelines [23] and written in accordance with strobe guidelines for reporting a cross-sectional study [24, 25]. The participants provided written informed consent. Participants were assured of their confidentiality and anonymity of their identity.
Study participants
The patients were first screened for the eligibility in the study. The eligible subjects were > 18 years of age of either sex and with confirmed type 1 or 2 diabetes mellitus. Diabetes mellitus was confirmed based on previous medical records, fasting plasma glucose, postprandial glucose, glycosylated hemoglobin (HbA1c) and current prescription or as per the ADA guidelines (random blood sugar ≥200 mg/dL) [26]. Only those patients were included in the study that provided written signed informed consent. Patients with a history of hypothyroidism, known medical illness such as cancer, cervical or lumbar spondylosis, alcohol related disorders, pregnant patients with diabetes and patients receiving any treatment that might influence nerve function (e.g., cytotoxic or antiepileptic agents) were excluded from the study.
Baseline demographic characteristics including age, sex, body mass index, systolic and diastolic blood pressure, and other relevant medical information of the eligible patients were captured in pre-designed case record form.
Study procedure
Patients were diagnosed on the basis of a clinical exam using a 10 g monofilament test. An Elli Lilly monofilament was used in this study. If the patient is unable to feel the pressure on the foot on testing through the monofilament, it depicts a loss of protective sensation. The device is now considered sensitive enough to assess neurological function and offers an inexpensive alternative screening method for identifying patients with moderate-to-severe neuropathy [27]. The exam was done on 10 standard positions- nine on the plantar surface of the foot and one on the dorsum surface of the foot, on both left and right feet three times. All the sites on the foot of the patient were examined and a score was allotted based on the presence or absence of sensation on the foot. If the subject felt the monofilament at all 10 positions, then the score was 10/10 signifying absence of neuropathy [28]. Two actual and one fake test were conducted to obtain accurate results. Diabetic retinopathy and nephropathy was diagnosed based on the clinical records.
Painful DPN was assessed using Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) questionnaire. We used the S-LANSS questionnaire as it was found to have an accuracy of 80% in type of pain identification when used in an interview format. This shows it to be a valid and attested tool to diagnose DPN [29–31].
Each question had a binary response (yes or no) to the presence of symptoms (5 items) or clinical signs (2 items). A score of ≥12 (out of a total possible 24) would be suggestive of neuropathic pain [32].
Statistical analysis
Descriptive statistics were used to present the data. Quantitative variables were presented using means and standard deviation and frequency and percentages were used to express categorical variables. Association between two categorical variables was computed by chi-square test and for quantitative variables t- test was used. A p value of ≤0.05 was considered statistically significant [33]. All statistical analysis was performed using SPSS v22.
Result
Pain prevalence and demographics
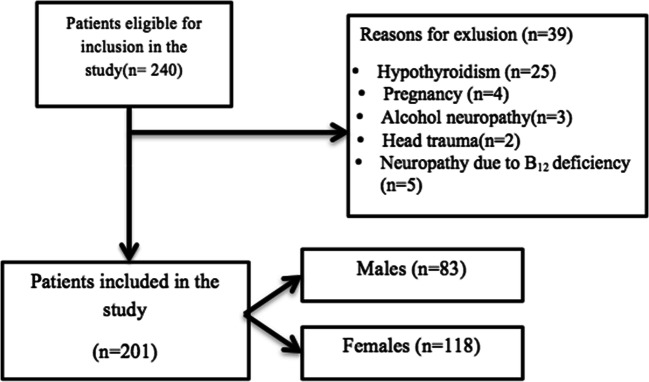
The result of this study was based upon 201 DM patients. A total of 240 DM patients were participated in the study and 201 completed the study of which 118 were female and 83 were male. The response rate was found to be 83.33%. Further, 6 patients had type 1 DM and 195 had type 2 DM. Figure 1 shows the detailed process of selection of participants in the study.
Fig. 1.
Flow process of selection of participants
The mean age of the participants was 61.50 ± 10.03 years. Table 1 shows the baseline demographics of the patients. The BMI of patients was 24.9 ± 3.3 kg/m2, whereas, the mean systolic and diastolic blood pressure of the patients was 138.52 ± 21.007 mmHg and 89 ± 12 mmHg respectively. Average duration of diabetes was 23 ± 6.7 years. Alpha Lipoic Acid + Methycobalamin was the most commonly prescribed to treat neuropathic pain at HAHC hospital. Other commonly prescribed drugs include pregabalin, duloxetine and dosulepin.
Table 1.
Baseline demographics variables of study population
| Variables | Mean ± SD |
|---|---|
| Age (years) | 61.50 ± 10.03 |
| Height (cm) | |
| Male | 169 ± 10.25 |
| Female | 160.59 ± 11.60 |
| Weight (kg) | |
| < 70 | 57.4 ± 7.3 |
| > 70 | 77 ± 20.8 |
| Duration of diabetes (years) | 23 ± 6.7 |
| SBP (mm Hg) | 138.52 ± 21.007 |
| DBP (mm Hg) | 89 ± 12 |
| HbA1c (%) | 9.04 ± 3.09 |
| FPG (mg/dl) | 167.91 ± 76.74 |
| PPG (mg/dl) | 217.41 ± 93.33 |
| BMI (kg/m2) | 24.9 ± 3.3 |
Data are mean ± SD
BMI, Body Mass Index; DBP, Diastolic Blood Pressure; FPG, Fasting Plasma Glucose; HbA1c, Glycosylated Hemoglobin; PPG, Post Prandial Glucose; SBP, Systolic Blood Pressure
Clinical diagnosis of diabetic peripheral neuropathy
Patients included in the study were assessed for DPN using the 10 g monofilament. Based on this test, 58 patients (28.85%) were diagnosed with neuropathy of which 25 were male and 33 were female. Most of the patients that were diagnosed with DPN were in the age group of 50–60 years. Majority of the patients having DPN had DM for >15 years.
We found significant association between the duration of diabetes and the presence of neuropathy (p = 0.004) and with the nephropathy (p = 0.002). Linear trend in increasing prevalence with the duration of diabetes was also observed. A significant association was found between the HbA1c level of the patient and that of neuropathy (p = 0.03). Likewise, significant association was observed between neuropathy and constipation (p = 0.043). No association of DPN was seen with the gender, age, and retinopathy or orthostatic hypotension (Table 2).
Table 2.
Comparison of variables in patients with and without DPN
| Variables | DM patient with neuropathy (n = 58) | DM patient without neuropathy (n = 143) | p value |
|---|---|---|---|
| Age (years) | 0.13 | ||
| <40 | 9 | 21 | |
| 40–50 | 17 | 45 | |
| 50–60 | 25 | 50 | |
| >60 | 7 | 27 | |
| Gender Distribution | 0.69 | ||
| Female | 33 | 85 | |
| Male | 25 | 58 | |
| Retinopathy | 5 | 5 | 0.32 |
| Nephropathy | 1 | 16 | 0.002* |
| Resting Tachycardia | 28 | 49 | 0.078 |
| Orthostatic Hypotension | 8 | 29 | 0.24 |
| Constipation | 14 | 56 | 0.043* |
| Gastroparesis | 21 | 60 | 0.62 |
| HTN Stage | |||
| Normal | 12 | 33 | |
| Pre HTN | 10 | 40 | |
| Stage I | 18 | 46 | |
| Stage II | 18 | 24 | |
| Duration of diabetes (years) | 0.004* | ||
| < 5 | 4 | 51 | |
| 6–10 | 4 | 22 | |
| 11–15 | 11 | 32 | |
| 16–20 | 14 | 17 | |
| >20 | 25 | 21 | |
| HbA1c (%) | 0.03* | ||
|
Poor control (HbA1c >7) |
47 | 100 | |
|
Good control (HbA1c < 7) |
11 | 43 | |
Association between two categorical variables was computed using chi-square test
HbA1c, Glycosylated Hemoglobin; HTN, Hypertension
*p < 0.05 statistical significance
Diagnosis and prevalence of pain using S-LANSS questionnaire
Prevalence of pain and painful symptoms in the diagnosed patients was assessed using the S-LANSS questionnaire. Pain was present in 88% (n = 51) patients who were diagnosed with DPN. Most patients felt either pins and needles, tingling or pricking sensation (Fig.2). More than half (67.24%) of the patients were also had burning pain. 90% patients reported sudden burst pain, and 84.48% reported increase in sensitivity at the site of pain. Duration of diabetes (>15 years), and HbA1c (>9%) was found to be positively associated the painful DPN. Non-significant effect of age, sex, and BMI was observed for painful DPN.
Fig. 2.
Presence of symptoms associated with pain using S-LANSS
Concomitant conditions
Of 201 patients, five patients had concomitant retinopathy whereas only one patient had nephropathy. Other complications observed were foot lesions, resting tachycardia, orthostatic hypotension, constipation and gastroparesis (Fig. 3).
Fig. 3.

DPN patients with concomitant diseases
Discussion
Peripheral neuropathy due to DM is the most prevalent form of neuropathy. It is one of the most common complications that affect a large portion of diabetes patients. Its early symptoms are often very subtle and can easily be overlooked if not asked specific questions about them. There are also very limited treatment options and strategies available for it. There is a lack of awareness among the Indian diabetic population about this complication. DPN has been found to be a crucial cause of age-related frailty and probability of fall in elderly. It also limits mobility, impairs sleep and reduces the overall quality of life of the patient. There is a shortage of data on the prevalence of DPN, especially in the Indian population. Recovery from this complication can be achieved if detected early. To date, limited information is available from the north Indian population. This was the first cross sectional study conducted at HAHC hospital to determine the prevalence of peripheral neuropathy and associated pain in patients with diabetes.
The current study was based on 201 diabetic patients of which 28.85% (n = 58) patients were diagnosed with peripheral neuropathy using the monofilament test. This particular test was used for the primary diagnosis of neuropathy because it was found to have sensitivity and specificity of up to 95% and 82% respectively [34]. The prevalence of neuropathy using this screening tool in a study conducted in Mangalore in 2015 was found to be 32.3% [35], which is in accordance with the prevalence of 28.85% as found in our study. Our finding was in alignment with other published studies from southern parts of India. The prevalence was ranged from 26% to 31% in surveys on Indian patients [13]. In a study conducted in rural south India reported the prevalence of DPN around 39% among T2DM patients [36]. The possible difference in prevalence of DPN as compared to our findings could be due to the inclusion of only T2DM patients, diabetes duration, and patient selection criteria [36]. In an another study conducted in south India by Ashok et al, the prevalence was found to be 29% [37].
A significant number of neuropathy patients also suffer from painful symptoms. Therefore, we also assessed painful neuropathy in these patients using the S-LANSS tool. We used this screening tool to assess pain as it has been found to be a valid, reliable, and self-complete instrument to identify symptoms associated with painful DPN [32]. It has better sensitivity as compared to other neuropathic pain screening instruments like the DN4 [38]. The most common features of neuropathic pain in our study were pricking and tingling sensations. Some patients also reported burning pain. These painful symptoms were found to arise suddenly in patients and were most common in the night leading to discomfort and difficulty in sleeping. The prevalence of pain in patients diagnosed with neuropathy was found to be 87.93% using S-LANSS questionnaire. In a similar study by Liberman et al using the S-LANSS questionnaire, he observed an 86.5% prevalence of painful DPN in the Israeli population with diabetes [39].
We found a significant association between the duration of diabetes and glycaemic control with the presence of neuropathy, wherein, a linear trend of increasing prevalence with the duration of diabetes was also observed. Aslam et al observed a similar finding in his study [38]. Studies in Arab populations have also linked poor glycemic control to the presence of diabetic neuropathy [40]. The possible reason is due to the positive association of HbA1c levels and duration of diabetes with DPN [41]. A significant association of neuropathy with the other diabetic complications such as nephropathy and autonomic complications such as constipation was also observed. This result is similar to that obtained by Jambert et al who also found association between neuropathy and renal function [42].
on-significant effect of age, sex, and BMI was observed for painful DPN in our study. This is contrary to the published studies as obesity (BMI >30 Kg/m2) was considered as the most significant predictors of painful DPN. The main reason for this difference is the inclusion of non-obese patients in our study [43].
A number of drugs have been proposed for the management of pain in diabetic neuropathy. Pregabalin and Duloxetine are the only ones that have received regulatory approval for treatment in the US, Canada and Europe. In our study we found that the combination of α Lipoic Acid + Methycobalamin was most commonly prescribed to treat neuropathic pain. Other commonly prescribed drugs include pregabalin, duloxetine and dosulepin. In a study conducted by Mijnhout et al in 2010, they found a clinically significant reduction in neuropathic pain with a three week treatment of α Lipoic Acid given intravenously [44]. A pilot study conducted by Vasudevan found that methylcobalamine, α-Lipoic Acid and pregabalin combination provides pain relief and improves sleep interference. He also concluded that addition of methylcobalamine and α-Lipoic Acid to pregbalin improved nerve function [45].
This study has few limitations also. The cross sectional nature of the study and its small sample size were its major limitation. Prevalence of DPN was established on the basis of the monofilament test. NCV could not be performed due to financial strains. However, monofilament test is most commonly used for the primary diagnosis of neuropathy and has a high sensitivity and specificity of up to 95% and 82% respectively. Another limitation could be recalling bias in responding to the S-LANSS questionnaire albeit the patients were asked to respond based on the recent pain perception. We recommend that a large epidemiological study should be performed in the North Indian population to understand the epidemiological aspects of DPN with a focus on the humanistic and economic aspects.
High prevalence of peripheral neuropathy and associated pain was observed in north Indian DM patients. There is a need to emphasize the timely diagnosis and early management of DPN.
Conclusion
This study found a high prevalence of peripheral neuropathy in patients with DM. A significant association of DPN was observed with the duration of diabetes, glycaemic control and diabetic complications such as nephropathy. High prevalence of painful DPN was also observed.
Compliance with ethical standards
Conflict of interest
All authors declare absence of competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zychowska M, Rojewska E, Przewlocka B, Mika J. Mechanisms and pharmacology of diabetic neuropathy–experimental and clinical studies. Pharmacol Rep. 2013;65:1601–1610. doi: 10.1016/S1734-1140(13)71521-4. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Facts and Figures. International Diabetes Federation. Available at: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed on 20th April 2020.
- 3.Hussain S, Habib A, Najmi AK. Anemia prevalence and its impact on health-related quality of life in Indian diabetic kidney disease patients: evidence from a cross-sectional study. Journal of Evidence-Based Medicine. 2019;12:243–252. doi: 10.1111/jebm.12367. [DOI] [PubMed] [Google Scholar]
- 4.Hussain S, Habib A, Singh A, Akhtar M, Najmi AK. Prevalence of depression among type 2 diabetes mellitus patients in India: a meta-analysis. Psychiatry Res. 2018;270:264–273. doi: 10.1016/j.psychres.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Hussain S, Jamali MC, Habib A, et al. Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers. Clinical Epidemiology and Global Health. 2020. 10.1016/j.cegh.2020.05.016.
- 6.Abbott CA, Malik RA, van Ross ER, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplin S. ADA position statement on diabetic neuropathy. Prescriber. 2017;28:39–40. doi: 10.1002/psb.1561. [DOI] [Google Scholar]
- 8.Vinik AI, Nevoret M-L, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin. 2013;42:747–787. doi: 10.1016/j.ecl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJ, Kratz K, Karnes J, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort the Rochester diabetic neuropathy study. Neurology. 1993;43:817–7. [DOI] [PubMed]
- 10.Aslam A, Singh J, Rajbhandari S. Pathogenesis of painful diabetic neuropathy. Pain Res Treat. 2014;2014:1–7. doi: 10.1155/2014/412041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks JB. The forgotten complication. Clinical Diabetes. 2005;23:3–4. doi: 10.2337/diaclin.23.1.3. [DOI] [Google Scholar]
- 13.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai urban rural epidemiology study (CURES-55) Diabet Med. 2008;25:407–412. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 14.Dros J, Wewerinke A, Bindels PJ, van Weert HC. Accuracy of monofilament testing to diagnose peripheral neuropathy: a systematic review. The Annals of Family Medicine. 2009;7:555–558. doi: 10.1370/afm.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Chen R, Zhang Y, et al. Scoring systems to screen for diabetic peripheral neuropathy. Cochrane Database of Systematic Reviews. 2014.
- 16.Bansal V, Kalita J, Misra U. Diabetic neuropathy. Postgrad Med J. 2006;82:95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo M, D'silva LJ, Martin K, et al. Pilot study of exercise therapy on painful diabetic peripheral neuropathy. Pain Med. 2015;16:1482–1489. doi: 10.1111/pme.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett AM, Lucero MA, Le T, et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8:S50–S62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 19.Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes, metabolic syndrome and obesity: targets and therapy. 2013;6:79. doi: 10.2147/DMSO.S37415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 21.Paul U, Sinharay K, Bhattacharyya A, Pal SK. Update in diabetic neuropathy with special reference to Indian scenario. J Indian Med Assoc. 2012;110:616–622. [PubMed] [Google Scholar]
- 22.Carrington AL, Shaw JE, Van Schie CH, et al. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. 2002;25:2010–2015. doi: 10.2337/diacare.25.11.2010. [DOI] [PubMed] [Google Scholar]
- 23.Association WM World medical association declaration of Helsinki. Ethical principles for medical research involving human subjects. Bulletin of the World Health Organization. 2001;79:373. [PMC free article] [PubMed] [Google Scholar]
- 24.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Hussain S, Habib A, Najmi AK. Limited knowledge of chronic kidney disease among type 2 diabetes mellitus patients in India. Int J Environ Res Public Health. 2019;16:1443. doi: 10.3390/ijerph16081443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association AD Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2001;24:S5–S20. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Parisi MCR, Giannella D, Fernandes TD, Rezende KF, Nery M. Diabetic foot screening: study of a 3000 times cheaper instrument. Clinics. 2011;66:1105–1107. doi: 10.1590/S1807-59322011000600032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2013;36:1604–1606. doi: 10.2337/dc12-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yunus Y, Rajbhandari S. Insensate foot of diabetic foot ulcer can have underlying silent neuropathic pain. Int Wound J. 2011;8:301–305. doi: 10.1111/j.1742-481X.2011.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho SI, Lee CH, Park G-H, Park CW, Kim HO. Use of S-LANSS, a tool for screening neuropathic pain, for predicting postherpetic neuralgia in patients after acute herpes zoster events: a single-center, 12-month, prospective cohort study. J Pain. 2014;15:149–156. doi: 10.1016/j.jpain.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? PAIN®. 2013; 154: 690–99. [DOI] [PMC free article] [PubMed]
- 32.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Hussain S, Habib A, Hussain MS, Najmi AK. Potential biomarkers for early detection of diabetic kidney disease. Diabetes Res Clin Pract. 2020;161:108082. doi: 10.1016/j.diabres.2020.108082. [DOI] [PubMed] [Google Scholar]
- 34.Bijli AH, Rasool A, Wani AH, et al. Footboards: indigenous and novel method of screening for diabetes peripheral neuropathy–a pilot study. Indian journal of endocrinology and metabolism. 2017;21:293. doi: 10.4103/ijem.IJEM_549_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Souza M, Kulkarni V, Unnikrishnan Bhaskaran HA, et al. Diabetic peripheral neuropathy and its determinants among patients attending a tertiary health care Centre in Mangalore, India. Journal of public health research. 2015;4. [DOI] [PMC free article] [PubMed]
- 36.Darivemula S, Nagoor K, Patan SK, et al. Prevalence and its associated determinants of diabetic peripheral neuropathy (DPN) in individuals having type-2 diabetes mellitus in rural South India. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2019;44:88. [PMC free article] [PubMed] [Google Scholar]
- 37.Ashok S, Ramu M, Deepa R, Mohan V. Prevalence of neuropathy in type 2 diabetic patients attending a diabetes Centre in South India. Japi. 2002;50:546–550. [PubMed] [Google Scholar]
- 38.Aslam A, Singh J, Rajbhandari S. Prevalence of painful diabetic neuropathy using the self-completed Leeds assessment of neuropathic symptoms and signs questionnaire in a population with diabetes. Can J Diabetes. 2015;39:285–295. doi: 10.1016/j.jcjd.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Liberman O, Peleg R, Shvartzman P. Chronic pain in type 2 diabetic patients: a cross-sectional study in primary care setting. Eur J Gen Pract. 2014;20:260–267. doi: 10.3109/13814788.2014.887674. [DOI] [PubMed] [Google Scholar]
- 40.Halawa MR, Karawagh A, Zeidan A, Mahmoud AEDH, Sakr M, Hegazy A, et al. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin. 2010;26:337–43. [DOI] [PubMed]
- 41.Nisar MU, Asad A, Waqas A, et al. Association of diabetic neuropathy with duration of type 2 diabetes and glycemic control. Cureus. 2015;7. [DOI] [PMC free article] [PubMed]
- 42.Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, et al. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res. 2011;39:366–77. [DOI] [PubMed]
- 43.Garoushi S, Johnson MI, Tashani OA. A cross-sectional study to estimate the point prevalence of painful diabetic neuropathy in eastern Libya. BMC Public Health. 2019;19:78. doi: 10.1186/s12889-018-6374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mijnhout G, Alkhalaf A, Kleefstra N, Bilo HJ. Alpha lipoic acid: a new treatment for neuropathic pain in patients with diabetes. Neth J Med. 2010;68:158–162. [PubMed] [Google Scholar]
- 45.Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition.[MAINTAIN]: Results of a pilot study. Annals of Indian Academy of Neurology. 2014;17:19. doi: 10.4103/0972-2327.128535. [DOI] [PMC free article] [PubMed] [Google Scholar]