Abstract
In various developed countries, diabetic nephropathy (DN) is the principal cause of end-stage kidney disease and a main reason of injury and mortality in individuals with renal morbidity worldwide. Polydatin (POL) has been evaluated as a potential antioxidant, anti-inflammatory and a nephroprotective agent. In spite of this, the possible benefits and protective effects of POL on early diabetic nephropathy are not quite clarified. For the effective clearance from the body besides safe drug delivery, biodegradable nanoparticles have interesting attraction. This work was designed to evaluate the positive effect and possible mechanisms of Polydatin-loaded Chitosan-Nanoparticles (POL-NPs) on early DN in streptozotocin–induced diabetic rats. Followed the induction of diabetes, rats classified into four groups, diabetic control and diabetic rats treated daily and orally with; POL, Polydatin-loaded chitosan-Nanoparticles (POL-NPs), plus normal control rats. Our findings showed that diabetic group presented a significant high level of the blood glucose, blood glycosylated hemoglobin (HbA1c), serum insulin, renal function related parameters, renal Advanced glycation-end products (AGEs) and lipid peroxidation level compared to normal control rats, while serum albumin level and the activities of renal antioxidant enzymes were significantly decreased. Moreover, in the kidney of diabetic rat mRNA expression of nuclear factor-kappa B (NF-κB) and cyclooxygenase-2 (Cox-2) were up-regulated. Besides, increase in serum levels of pro-inflammatory cytokines (TNF-α, IL-6 and IL-18) and decrease in anti-inflammatory cytokine (IL-10). POL and POL-NPs supplementation were significantly attenuate the above-mention results and returned the normal equilibrium between pro- and anti-inflammatory cytokines. In conclusion, POL and POL-NPs have antidiabetic effect, suppresses oxidative stress and mitigates renal inflammation through inhibition of NF-κB in diabetic kidney in early progressive DN.
Keywords: Diabetic nephropathy, Polydatin-loaded-chitosan nanoparticles, Hypoglycemia, Advanced glycation-end products, Oxidative stress, NF-κB, Inflammatory cytokines
Introduction
One of the chronic metabolic disorder is type 2 diabetes (T2D) which influencing a noteworthy number of worldwide population and the numbers is increasing uncontrollably. Hyperglycemia-induced toxicity causes a significant developing pathogenesis to various organs. Hyperglycemia has been reported to have a key role to induce oxidative stress, declined cellular antioxidants and produce proinflammatory cytokines [1]. It can manage many intracellular signal transduction pathways like NF-κB signaling pathway, followed by the regulation of oxidative stress and inflammation [2]. Furthermore, usually the transcription factor NF-κB was activated by reactive oxygen species (ROS), and involved in inflammation through COX-2 enzymes [3]. Therefore, considering the abovementioned, it is of interest to promote the regulation of the oxidative stress and the inflammatory responses to avoid the serious diabetic complications. One of the main complications in T2D is diabetic nephropathy (DN) [4]. DN is the principal origin of end-stage kidney disease in various developed countries and a main reason of mortality and morbidity in individuals with renal dysfunction over world [5]. Several studies evaluated that oxidative stress and NF-κB linked inflammatory responses on diabetic kidney participate together to progress DN [6, 7]. However, DN pathogenesis is still not fully appreciated. Therefore, it is important to get new strategies to avoid the occurrence and development of DN. Despite the therapeutic drugs which fight the development of DN are available, the herbal medicines are a new concern to avoid the origin of this complication. For the safety profile, the plant originated drugs always have benefits over present synthetic drugs for managing the complications of diabetes. Polydatin (POL), or pieceid, (3,4′,5-trihydroxystilbene-3-β-d-glucoside) was originally extracted from the rhizome and root of a Chinese herb called Polygonum cuspidatum. Traditionally, the herb is used to treat fever, pain, cough, hypertension [8]. It has many pharmacological activities, like anti-oxidative, anti- inflammatory [9], ameliorating renal injury [10] and consequently a protective effect on diabetic nephropathy [11]. POL improves kidney dysfunction of diabetic rats by inhibiting activation of the NF-κB signalling pathway, which results in DN resistance [11]. The possible beneficial and protective effects of POL on early DN are not fully clarified. The clinical applications of POL are limited, despite the promising pharmacological properties, due to low bioavailability because of chemical instability in aqueous alkaline medium, poor aqueous solubility and severe first-pass metabolism [12]. Several investigators are aiming to solve these problems through systems for drug delivery that protect from degradation, increase the water solubility of the loaded drug, target drug to specific sites, and do continued release patterns [13]. Because of the effective clearance from the body besides safe drug delivery, biodegradable nanoparticles have attracted broad interest. By our knowledge, no prior investigation studied whether POL-NPs had a therapeutic influence on early DN. Hence, this work was performed on experimental diabetic rats to study the mechanism and the ability of free POL and POL-NPs in attenuating hyperglycaemia and further evaluate its nephroprotective effect in early diabetic nephropathy focusing on (i) suppressing oxidative stress and renal inflammation via inhibition of NF-κB, (ii) its anti-inflammatory effect by keeping the normal equilibrium between pro- and anti-inflammatory cytokines and (iii) the marked antioxidative effects of free POL and POL-NPs.
Methodology
Chemicals and reagents
Polydatin (POL), nicotinamide and streptozotocin were bought from Sigma Aldrich Co. MO, USA. Using a modified ionic gelation method [14], Polydatin-loaded chitosan nanoparticles (POL-NPs) were synthesized. All details of Polydatin-loaded chitosan nanoparticles synthesis and characterization was mentioned by Abdel-Moneim et al. 2020 [15]. From standard commercial supplies, all other materials were got.
Animals
From the Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt) 32 male Wistar albino rats of 120–140 g body weight (b.wt.) were bought. Animals were housed at normal atmospheric conditions and in good aerated cages for normal 12 hours light/dark cycle. According to the guidance of the Institutional Animal Care and use Committee (IACUC) of Beni-Suef University (IACUC Permit Number: BSU-FS-2018-8) experimental animal were signed and managed. For 4 weeks after arrival, all the rats were fed with common diets, and allocated into two groups. The normal control group was still fed with common diets during the study; the other (diabetes group) was fed with diets high in fat [16].
Induction of T2D in rats
After 4 weeks feeding with high-fat diets. In cold citrate buffer (pH 4.5), streptozotocin (50 mg/kg b.wt.) was dissolved and injected intraperitoneally immediately in overnight-fasted rats after 15 minutes of nicotinamide (NA) injection (110 mg/kg b. wt. intraperitoneally) which was prepared in normal physiological saline [17]. One weak post-injection, diabetic rats with fasting blood glucose level ≥ 200 mg/dl were included in the study. One of the of common animal model of DN is low-dose streptozotocin and high fat-fed treated rats [18, 19]. Partial lysis of pancreatic β -cell population and insulin resistance in the experimental rats are induced by Lower dose of STZ and high-fat diet respectively [20].
Design of the experiment
In the experiment, a total of thirty-two rats (24 diabetic rats, 8 normal rats) were used. The rats were allocated into 4 groups (N = 8) as follow:
The normal control rats: received a free tendency to water and food and don’t receive any treatment.
Diabetic control rats: were orally administered of distilled water.
Diabetic rats treated with POL (D + POL): were orally administered POL (50 mg/kg b. wt.)
Diabetic rats treated with POL-NPs (D + POL-NPs): were orally administered POL-NPs equivalent to 50 mg/kg b.wt. of POL [15].
All doses by gastric intubation were given daily for four weeks. According to the difference in body weight, the dose was adjusted every week.
Measurements of biochemical indexes
At the end of the experiment, on the day before sacrifice, fasting blood samples were taken from a lateral tail vein of overnight fasted rats (8–10 hours). Then all animals were supplemented with a single dose of glucose solution (3 g / kg b.wt.) through gastric intubation. After two hours of oral supplementation of glucose solution another blood sample was taken from all animals to estimate the level of postprandial blood glucose [21, 22]. Then the rats were anesthetized and sacrificed for the blood collection from the abdominal aorta. After 3000 rpm centrifuged for 10 minutes, the supernatant was collected from the blood sample. Level of fasting and postprandial blood glucose (FBG and PBG), kidney function tests as blood urea nitrogen (BUN), uric acid (UA), serum creatinine (SCr), and albumin were estimated using kits from Biodiagnostic Company (Dokki, Giza, Egypt). In addition, reagent kits purchased from Biosystems (Spain) and DRG (Germany) were used to evaluate blood glycosylated hemoglobin (HbA1c) percentage and serum insulin level (assayed by sandwich ELISA), respectively. Assessment of the renal Advanced glycation-end products (AGEs) was done by available ELISA Kits (CSB E09413r. China). And Rat Interleukin 10 ELISA Kits for determination of interleukin 10 (IL-10) were purchased from MyBioSource.com. USA. Interleukin 18 (IL-18) levels were assessed by using a rat IL‐18 ELISA kit (Wuhan Boster Bio‐technology Co., Ltd., China). Besides, tumor necrosis factor-alpha (TNF-α) and Interleukin 6 (IL-6) were assayed by ELISA Kits from RayBiotech.com. Parkway. All procedures were in accordance to the instructions provided by the manufacturer. Moreover, kidney homogenate was prepared by homogenizing kidney tissue in 10 ml sterilized saline solution (0.9% NaCl) to obtain 1% homogenate (w/v). Homogenates were centrifuged and supernatants were stored at -20oC for oxidative stress and antioxidant parameter estimations [23] of renal lipid peroxidation index (MDA), reduced glutathione (GSH), activities of superoxide dismutase (SOD) and catalase (CAT) according to the manufacturer’s instructions of the kits purchased from Biodiognostic, Egypt.
Quantitative real-time PCR
Total RNA was isolated from kidney tissues using Qiagen tissue extraction Kit (Qiagen, USA) according to instructions of the manufacturer. The purity (A260/A280 ratio) and the concentration of RNA were obtained using spectrophotometry (dual wavelength Beckman, spectrophotometer, USA). From total RNA (0.5-2 µg) was used for cDNA conversion using high capacity cDNA reverse transcription Kit (Fermentas, USA) according to the manufacturer’s protocols. Real-time qPCR amplification and analysis were performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). The qPCR assay with the primer sets was optimized at the annealing temperature. The relative quantification was calculated according to Applied Biosystem software according to the ∆∆Ct method and the values were normalized to β-actin. The RQ is the fold change compared to the calibrator (untreated sample). The following primers were used for qPCR amplification: NF-κB forward, 5′-CATTGAGGTGTATTTCACGG-3′, reverse, 5′-GGCAAGTGGCCATTGTGTTC-3′ and Cox-2 forward, 5′- GTGGGATGACGAGCGACTG-3′, reverse, 5′- CCGTGTTCAAGGAGGATGG-3′. β-actin forward, 5′-TACAACCTTCTTGCAGCTCCT-3′ and reverse, 5′-CCTTCTGACCCATACCCACC-3′ (NM_031144.3).
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 20 for Windows software system (SPSS Inc, Chicago, IL, USA). All statistical comparisons were made by means of one-way ANOVA test followed by Duncan′s method for post hoc analysis and the results were articulated as mean ± standard error (SE). P value < 0.05 was considered significant.
Results
Effect of POL and POL-NPs on blood glucose, HbA1c, serum insulin and AGEs levels
In diabetic group fasting, postprandial blood glucose, HbA1c and AGEs levels were significantly (P < 0.001) increased in contrast with serum insulin levels which markedly decreased in the diabetic group compared to normal control rats. POL and POL-NPs led to a significant (P < 0.001) decrease in FBG, PBS, HbA1c and AGEs levels while the insulin level was noticeably improved in treated groups when compared with the diabetic control group. Moreover, POL-NPs group showed a more significant antidiabetic effect compared to free POL group as illustrated in Table 1.
Table 1.
Effect of POL and POL-NPs on blood glucose, HbA1c, serum insulin and AGEs levels in STZ-induced diabetic rats
| Group | FBG (mg/dl) |
PBG (mg/dl) |
AGEs µg/ml |
HbA1c (%) |
Insulin µlU/ml |
|---|---|---|---|---|---|
| Parameter | |||||
| Control | 87.33 ± 1.65 | 110.67 ± 2.38 | 11.93 ± 0.88 | 4.19 ± 0.18 | 32.50 ± 1.02 |
| Diabetic | 259.33 ± 2.08*** | 371.33 ± 4.39*** | 53.50 ± 1.21*** | 7.80 ± 0.34*** | 14.89 ± 0.47*** |
| Diabetic + POL | 152.67 ± 3.06+++ | 153.00 ± 2.58+++ | 26.63 ± 1.11+++ | 6.71 ± 0.29+++ | 21.78 ± 0.68+++ |
| Diabetic + POL-NPs | 140.67 ± 3.04+++$$ | 141.67 ± 4.41+++$ | 17.40 ± 1.06+++$$$ | 6.50 ± 0.28+++ | 23.95 ± 0.75+++ |
Values were expressed as mean ± standard error. ***P < 0.001, compared with normal control group. +++P < 0.001, compared with diabetic group. $P < 0.05, $$P < 0.01, compared with diabetic group treated with free POL (Diabetic + POL). STZ: streptozotocin, POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, FBG: fasting blood glucose, PBG: postprandial blood glucose, HbA1c: blood glycosylated hemoglobin. AGEs: Advanced glycation-end products
Effect of POL and POL-NPs on renal function related parameters
As shown in Table 2 levels of serum UA, SCr and BUN were significantly (P < 0.001) increased in diabetic group compared with normal control rats while the serum albumin level was significantly decreased (P < 0.001). POL and POL-NPs administration significantly (P < 0.001) lowered their levels to nearly the normal value as compared with diabetic group. In contrast, POL and POL-NPs treatment significantly (P < 0.001) enhanced the reduced level of serum albumin compared with diabetic group.
Table 2.
Effect of POL and POL-NPs on renal function related parameters in STZ-induced diabetic rats
| Group | BUN (mg/dl) |
SCr (mg/dl) |
Uric acid (mg/dl) |
Albumin (g/dl) |
|---|---|---|---|---|
| Parameter | ||||
| Control | 14.33 ± 0.52 | 0.43 ± 0.03 | 4.21 ± 0.18 | 3.96 ± 0.17 |
| Diabetic | 26.66 ± 0.97*** | 0.69 ± 0.02*** | 6.24 ± 0.15 *** | 2.19 ± 0.10*** |
| Diabetic + POL | 19.17 ± 0.70+++ | 0.56 ± 0.08+++ | 5.10 ± 0.09+++ | 3.24 ± 0.12+++ |
| Diabetic + POL-NPs | 18.90 ± 0.64+++ | 0.54 ± 0.03+++ | 4.97 ± 0.20 +++ | 3.57 ± 0.13+++ |
Values were expressed as mean ± standard error. ***P < 0.001, compared with normal control group. +++P < 0.001, compared with diabetic group. STZ: streptozotocin, POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, SCr: serum creatinine, BUN: blood urea nitrogen
Effect of POL and POL-NPs on renal oxidative indexes
Table 3 illustrated the anti-oxidative effect of POL on the kidney of diabetic rats. The MDA level in the kidney of diabetic group was significantly higher than that in the normal control group (p < 0.001). This increase was inverted significantly (p < 0.001) by the treatments of POL and POL-NPs. The activity of SOD, CAT and GSH decreased significantly in the kidney tissue of diabetic rats as compared with the normal control rats (p < 0.001). POL and POL-NPs treatments led to a significant promotion (p < 0.001) of these enzyme’s activity as compared with the diabetic group. POL-NPs has the most significant (p < 0.01 for SOD & GSH and p < 0.001 for MDA & CAT) antioxidant effect compared to free POL group in all these parameters.
Table 3.
Effect of POL and POL-NPs on kidney oxidative stress and antioxidant enzymes in STZ-induced diabetic rats
| Group | LPO (nmol MDA/100 mg protein) |
SOD (U/g protein) |
CAT (U/mg protein) |
GSH (nmol/100 mg protein) |
|---|---|---|---|---|
| Parameter | ||||
| Control | 14.37 ± 1.10 | 7.33 ± 0.50 | 118.80 ± 0.94 | 76.63 ± 5.55 |
| Diabetic | 68.87 ± 3.08*** | 1.50 ± 0.16*** | 55.57 ± 1.47*** | 25.47 ± 1.44*** |
| Diabetic + POL | 29.27 ± 0.48+++ | 4.70 ± 0.73+++ | 95.33 ± 3.21+++ | 51.40 ± 1.67+++ |
| Diabetic + POL-NPs | 18.98 ± 0.60+++$$$ | 6.03 ± 0.50+++$$ | 108.13 ± 2.15+++$$$ | 67.13 ± 2.62+++$$ |
Values were expressed as mean ± standard error. ***P < 0.001, compared with normal control group. +++P < 0.001, compared with diabetic group. $$P < 0.01, $$$P < 0.001, compared with diabetic group treated with POL (Diabetic + POL). STZ: streptozotocin, POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, LPO: Lipid peroxidation, MDA: malonaldehyde, SOD: superoxide dismutase, CAT: catalase, GSH: glutathione
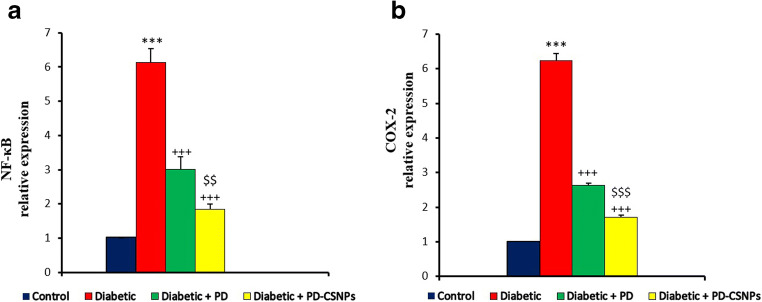
Effect of POL and POL-NPs on expression of NF-κB and Cox-2 in kidney
Compared to the normal control group, the expressions of NF- κB and COX-2 proteins were both up-regulation in the diabetic rats. After the treatments with POL and POL-NPs, the result presented a significant (p < 0.001) inversion of NF-κB and COX-2 protein expressions as compared with diabetic group. With more significant (p < 0.01 and p < 0.001 for NF-κB and Cox-2, respectively) positive effect of POL-NPs as compared with free POL group as shown in Fig. 1.
Fig. 1.
Effect of POL and POL-NPs on expression of inflammation-associated proteins in STZ-induced diabetic rats. Values were expressed as mean ± standard error. ***P < 0.001, compared with normal control group. +++P < 0.001, compared with diabetic group. $$P < 0.01, $$$P < 0.001, compared with diabetic group treated with POL (Diabetic + POL). STZ: streptozotocin, POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, NF-κB: nuclear factor-kappa B, Cox-2: cyclooxygenase-2
Effects of POL and POL-NPs on the levels of pro- and anti -inflammatory cytokines
Table 4 explains the effects of POL and POL-NPs on serum pro- and anti -inflammatory cytokines (IL-6, TNF-α, IL-18 & IL-10). The diabetic rat’s serum showed a significant (p < 0.001) high levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-18) compared with normal control rats. POL and POL-NPs treatment significantly (p < 0.001) suppressed these serum pro-inflammatory cytokines level as compared to diabetic group. While anti-inflammatory cytokines IL-10 was significantly (p < 0.001) lowered in diabetic rats compared with normal control ones, which reversed significantly (p < 0.001) after administration of POL and POL-NPs compared with the diabetic group. Moreover, POL-NPs group showed a more significant (p < 0.001) anti-inflammatory effect compared to free POL group.
Table 4.
Effect of POL and POL-NPs on the levels of pro- and anti- inflammatory cytokines in STZ-induced diabetic rats
| Group | TNF-α pg/ml |
IL-6 pg/ml |
IL-18 pg/ml |
IL-10 pg/ml |
|---|---|---|---|---|
| Parameter | ||||
| Control | 35.60 ± 1.18 | 62.73 ± 0.81 | 27.33 ± 0.56 | 145.58 ± 1.50 |
| Diabetic | 113.80 ± 4.94*** | 136.40 ± 1.24*** | 108.10 ± 2.22*** | 77.03 ± 1.42*** |
| Diabetic + POL | 64.30 ± 1.69+++ | 83.97 ± 1.44+++ | 65.30 ± 0.70+++ | 116.20 ± 1.52+++ |
| Diabetic + POL-NPs | 43.40 ± 2.76+++$$$ | 69.57 ± 2.50+++$$$ | 45.80 ± 0.86+++$$$ | 132.50 ± 3.98+++$$$ |
Values were expressed as mean ± standard error. ***P < 0.001, compared with normal control group. +++P < 0.001, compared with diabetic group. $$$P < 0.001, compared with diabetic group treated with POL (Diabetic + POL). STZ: streptozotocin, POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, IL-18, IL-10, IL-6: interleukin-18, 10, 6 respectively, TNF-α: tumor necrosis factor-alpha
Discussion
Insulin resistance and hyperglycaemia is the primary origin of DN [24] which is the main reason of end stage renal failure [25]. The current study showed that oral supplement with free POL and POL-NPs for 4 weeks after induction of diabetes attenuated DN. The protective effects of free POL and POL-NPs was markedly evidenced by its antidiabetic action, inhibition of oxidative stress status by enhancing antioxidant enzymes and modulating pro-inflammatory cytokines with more positive effect of POL-NPs.
Our results of the diabetic control group showed that the anti-hyperglycaemic action of free POL and POL-NPs was demonstrated via (i) a significant decrease of the high level of FBG, PBG and HbA1c like other antioxidant herp as Hypericum perforatum [26]. (ii) a significant promotion in insulin secretion. These findings demonstrated the antidiabetic effect of POL as shown in previous studies [15, 27, 28]. Factors leading to that are the insulin resistance and/or deficiency, which sequentially leads to the activation of gluconeogenic and glycogenolytic pathways [29]. However, the hypoglycaemic effect was more pronounced by POL-NPs as compared to free POL. Our findings showed that diabetic group presented a significant high level of UA, SCr, and BUN and that was in line with many studies [16, 30] and a hight marked AGEs formation in the kidney tissue of diabetic group which indicated to the renal injury as a DN. Elevated levels of UA and BUN are associated to renal injury in uncontrolled diabetes because of muscle wasting and metabolic disorders which can also lead to an elevated release of purine, a main origin of UA [31]. Non-enzymatic glycation reaction is the reaction in which AGEs were formed nonenzymatically through an interaction between glucose as a reducing sugar by the chemical reactivity of its carbonyl group with an amino group of lipids and proteins [32]. Several studies stated that the formation of AGEs in kidney tissue has a main pathogenic role in DN development [33, 34]. The irreversible formation of AGEs disturbs lipids and proteins, thus initiating injury to the blood vessels and kidneys [35]. Thus, prevention of AGEs accumulation appears to be a potential therapeutic choice that could delay the progression of DN and alter the pathogenesis. The current study reveals that free POL and POL-NPs lowered BUN, UA and SCr levels, proposing an ameliorative effect on renal dysfunction, and show an inhibitory effect against the AGEs formation in the kidney of diabetic group. It could be because of the diminution in glucose level and consequently decrease the non-enzymatic glycation reaction. Furthermore, we also detected decreased serum albumin levels in the diabetic rats which could be as a result of elevated urinary excretion of albumin and glomerular basement membrane damage [31] which was normalised by free POL and POL-NPs treatment as well as showed by Chen et al. 2016 and 2013 [10, 36]. These results suggest that free POL and POL-NPs might exhibit a protective role against renal injury and DN through its anti-hyperglycaemic action and inhibiting AGEs formation with more positive effect of POL-NPs. Investigations on human DN have attributed ROS in kidney tissues to high blood glucose level in diabetic patients [6, 37]. As above mentioned in our results hyperglycaemia-induced accumulation of AGEs, which has been considered as the principal origin of ROS [4] because of ROS synthesis during oxidation of glucose and glycation of protein [38]. Additionally, in the kidneys of diabetic group, the high level of UA has been suggested to induce oxidative stress and inflammation [39].
In the current investigation of the kidneys of diabetic rats, we detected an elevation in the level of renal lipid peroxidation index (MDA) associated with the reduced activity of CAT, GSH and SOD enzymes, free radical scavenging system, which indicated to the oxidative stress status. Supplement with free POL and POL-NPs significantly prevented oxidation of lipids as a scavenging agent for free radicals [2] by increasing the antioxidant enzymes (SOD, CAT and GSH) levels in the diabetic rats and decreased the MDA level. Our results are under previously stated about POL antioxidant properties’ [40–42] and other antioxidant agents as sesame oil [43], which by inhibiting the production of ROS can abolish many organ injuries. These results suggest that POL and POL-NPs might be a protective agent against DN through reducing the generation of ROS by its hypoglycaemic action, preventing AGEs formation and decreasing serum UA level that including in the enhancement of kidney function, besides enhance antioxidant enzyme activity.
Many clinical and experimental reports have established the principal role of many inflammatory components in the progress of DN. Similarly, in human DN renal inflammation is proposed to be NF-κB correlated and pro-inflammatory cytokines can start the event [44, 45]. Here, we investigated the effect of POL and POL-NPs on NF-κB pathway of DN. In the status of oxidative stress, ROS activate the transcription factor NF-κB, which is involved in inflammation via COX-2 enzymes [3]. Also, UA could regulate various intracellular signal transduction ways as NF-κB signalling pathway, followed by regulating oxidative stress and inflammatory responses [46, 47].
Furthermore, the expression of genes encoding cytokines and mediators, such as IL-8, TNF-α and IL-6 can be regulated by NF-κB [48]. One of the inducible form of prostaglandin synthase enzymes is COX-2, which can be induced by TNF-α to trigger the inflammatory condition [49]. Our results showed that NF-κB and COX-2 upregulation was detected in the kidney of diabetic rats. This may be owing to the severe oxidative conditions induced by the hyperglycaemia in the renal tissue. In accordance with our results, other studies on human DN reported that in diabetic kidney NF-κB is highly upregulated [50]. The alteration of NF-κB and COX-2 expression via POL and POL-NPs showed their essential role in the amelioration of oxidative status and inflammation in the kidney of diabetic rats. Our results are consistent with these of Chen et al. 2013 [36] who revealed that POL decrease the expressions of NF-κB and COX-2 proteins in pathogenesis of urate nephropathy. Moreover, POL-NPs was observed to be more efficient in lowering the up- regulation of NF-κB and COX-2 and thus participates in the management of DN.
In this study, we detected abnormally elevated IL-6, IL-18 and TNF-α levels and reduced anti-inflammatory cytokine IL-10 level in diabetic rats. In contrast, our results showed that POL and POL-NPs administration pronounced the decreased levels of pro-inflammatory cytokines (IL-6, IL-18 and TNF-α) and enhanced the level of IL-10 with more positive effect of POL-NPs. In the same context with our results, studies on human DM demonstrated higher levels of TNF-α, IL-18 [51] and IL-6 [52] than the normal in the patient’s serum, and reduced IL-10 level [53, 54] especially in those with DN. The pathogenetic ability of TNF-α is for its potential to cause direct renal cell injury [55]. The classical markers as IL-6 and TNF-α are regarded as a pro-inflammatory cytokine that can lead to insulin resistance [56–58]. Therefore, inflammation is a related factor in the diabetes pathogenesis, with considerable data that T2D includes an inflammatory molecule [59]. The reduction effect of POL and POL-NPs on the level of TNF-α explained the prevention of COX-2 expressions, and the improvement of the inflammatory responses in the diabetic rats.
IL-18 is a new actor with a proinflammatory activity among the group of interleukins, which has a role in DN [60]. Not much is recognized about the association between IL-18 and DM, except for some reports about IL-18 and type 1 DM [61]. In contrast, there are little studies of IL-18 with T2DM. The present study may be considered one of the first reports showed that IL-18 levels were elevated in the DN of T2DM. It has been showed that IL-18 participates more strongly to the DN progression than other complications of diabetes [53]. IL-18 could have a role in the progress of DN, since it is elevated with the existence of albuminuria, so it could refer to early DN. Infiltrating macrophages could be responsible for elevated levels of IL-18 [51]. IL-10 is one cytokine that known as a cytokine synthesis inhibitory factor (CSIF) [62] or an “anti-cytokine” [63] because it inhibits the formation of many pro-inflammatory cytokines as TNF-α [64].
Recognition of therapeutic agents that can downregulate proinflammatory responses and mediators may be a hopeful strategy in the management of complications of DM. POL could be one of the anti-inflammatory agents which is useful in diabetes [65]. It is notable that our investigation is the first to study the effect of POL-NPs on inflammatory cytokines in serum of DN rats. A number of reports have established that POL influences the oxidative stress and consequently inflammatory respond [9, 27]. Furthermore, our results showed that POL-NPs could attenuate the DN through ameliorating the inflammatory responses by downregulation of NF-κB and consequently COX-2 besides restore the normal equilibrium between pro- and anti-inflammatory cytokines through modulating of the pro-inflammatory response in the kidney, leading to enhance renal injury.
Conclusions
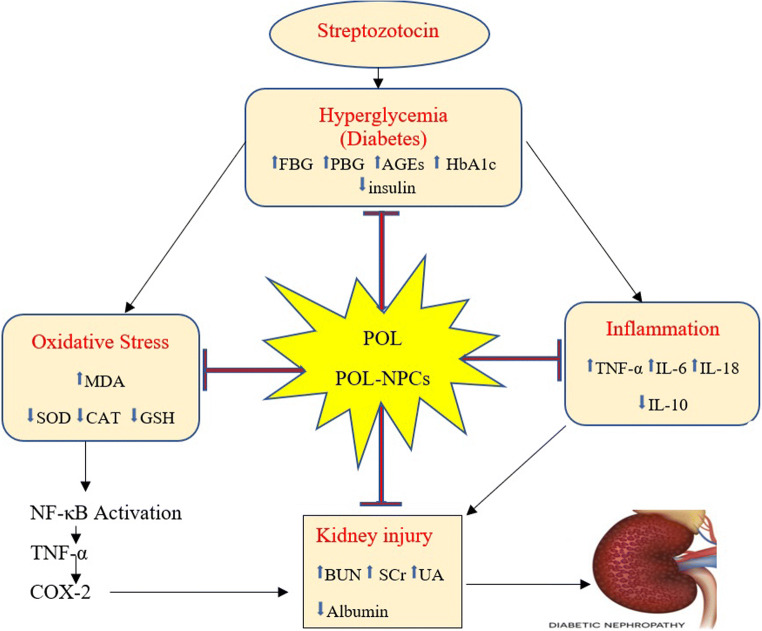
The present study exhibited that POL-NPs new formula is biocompatible and can successively ameliorate nephropathy in diabetic rats than free POL. The nephroprotective effect of POL-NPs shows the effectiveness of CSNPs as an efficient nanocarrier for assisted POL delivery. The study also revealed that the nephroprotective effects of POL-NPs on DN may be because of (i) its antidiabetic action through promotion insulin secretion, regulation of blood glucose and HbA1c, (ii) enhancing oxidative stress status by its antioxidant effect and reducing AGEs formation, (iii) its role as an anti-inflammatory agent and (iv) The most positive effect of POL-NPs may be attributed to improved absorption and prolonged-release properties as shown in Fig. 2. Therefore, POL-NPs formula could represent a substitute to the usual form of POL for treatment. Our findings will stimulate further interest in the protective role of POL-NPs in renal disease.
Fig. 2.
A graph shows the possible mechanism of POL and POL-NPs in ameliorating early diabetic nephropathy via antidiabetic, antioxidant, anti-inflammatory effects and alleviating’s kidney injury by inhibition of NF-κB pathway. POL: polydatin, POL-NPs: polydatin-loaded chitosan nanoparticles, FBG: fasting blood glucose, PBG: postprandial blood glucose, HbA1c: blood glycosylated hemoglobin, AGEs: Advanced glycation-end products, MDA: malonaldehyde, SOD: superoxide dismutase, CAT: catalase, GSH: glutathione, IL-18, IL-10, IL-6: interleukin-18, 10, 6 respectively, TNF-α: tumor necrosis factor-alpha, SCr: serum creatinine, BUN: blood urea nitrogen, UA: uric acid, NF-κB: nuclear factor-kappa B, Cox-2: cyclooxygenase-2
Compliance with ethical standards
Research involving Animals (rats) only.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mahmoud AM, Mohamed B, Ashour A, Abdel-Moneim OMA. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26(6):483–90. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Huang QH, Xu LQ, Liu YH, Wu JZ, Wu X, Lai XP, et al. Polydatin protects rat liver against ethanol-induced injury: involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 pathway. Evidence-Based Complement Altern Med. 2017;2017:14. 10.1155/2017/7953850. [DOI] [PMC free article] [PubMed]
- 3.Park CH, Noh JS, Yamabe N, Kang KS, Tanaka T, Yokozawa T. Beneficial effect of 7-O-galloyl-d-sedoheptulose on oxidative stress and hepatic and renal changes in type 2 diabetic db/db mice. Eur J Pharmacol. 2010;640(1–3):233–42. doi: 10.1016/j.ejphar.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur J Pharmacol. 2016;791:8–24. doi: 10.1016/j.ejphar.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Zhao T, Gong Y, Dong X, Zhang W, Sun S, et al. Attenuation of diabetic nephropathy by Chaihuang-Yishen granule through anti-inflammatory mechanism in streptozotocin-induced rat model of diabetics. J Ethnopharmacol [Internet]. 2014;151(1):556–64. doi: 10.1016/j.jep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Wan TT, Li XF, Sun YM, Li YB, Su Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother. 2015;74:145–7. doi: 10.1016/j.biopha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Luo L, Ke Z, Liu C, Yin L, Yao Y, et al. Polydatin protects against acute cholestatic liver injury in mice via the inhibition of oxidative stress and endoplasmic reticulum stress. J Funct Foods. 2019;55:175–83. [Google Scholar]
- 9.Du QH, Peng C, Zhang H, Polydatin A review of pharmacology and pharmacokinetics. Pharm Biol. 2013;51(11):1347–54. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Huang K, Hao J, Huang J, Yang Z, Xiong F, et al. Polydatin attenuates AGEs-induced upregulation of fibronectin and ICAM-1 in rat glomerular mesangial cells and db/db diabetic mice kidneys by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol [Internet]. 2016;427:45–56. 10.1016/j.mce.2016.03.003. [DOI] [PubMed]
- 11.Xie X, Peng J, Huang K, Huang J, Shen X, Liu P, et al. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mol Cell Endocrinol. 2012;362(1–2):183–93. doi: 10.1016/j.mce.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W, Li X, Zhang C, Chen W, Yuan H, Xu S. International journal of drug development preparation and in vivo-in vitro evaluation of polydatin-phospholipid complex with improved dissolution and bioavailability. Int J Drug Dev Res. 2017;9(1):39–43. [Google Scholar]
- 13.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–93. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 14.Nagpal K, Singh SK, Mishra DN. Optimization of brain targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int J Biol Macromol. 2013;59:72–83. doi: 10.1016/j.ijbiomac.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Moneim A, El-Shahawy A, Yousef AI, Abd El-Twab SM, Elden ZE, Taha M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int J Biol Macromol [Internet]. 2020;154:1496–504. doi: 10.1016/j.ijbiomac.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Z, Wang Y long, Yue Y, Zhang Y, tang, Chen C ping, Wan L sheng, et al. Preventive effects of polysaccharides from Liriope spicata var. prolifera on diabetic nephropathy in rats. Int J Biol Macromol [Internet]. 2013;61:114–20. 10.1016/j.ijbiomac.2013.06.047. [DOI] [PubMed]
- 17.Soufi FG, Mohammad-nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress - Nuclear factor κB - Apoptosis pathway. Pharmacol Reports. 2012;64(6):1505–14. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- 18.Sugano M, Yamato H, Hayashi T, Ochiai H, Kakuchi J, Goto S, et al. High-fat diet in low-dose-streptozotocin-treated heminephrectomized rats induces all features of human type 2 diabetic nephropathy: A new rat model of diabetic nephropathy. Nutr Metab Cardiovasc Dis. 2006;16(7):477–84. doi: 10.1016/j.numecd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Meng G, Zhu H, Yang S, Wu F, Zheng H, Chen EE, et al. Attenuating effects of Ganoderma lucidum polysaccharides on myocardial collagen cross-linking relates to advanced glycation end product and antioxidant enzymes in high-fat-diet and streptozotocin-induced diabetic rats. Carbohydr Polym [Internet]. 2011;84(1):180–5. 10.1016/j.carbpol.2010.11.016.
- 20.Khanra R, Bhattacharjee N, Dua TK, Nandy A, Saha A, Kalita J, et al. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed Pharmacother [Internet]. 2017;94:726–41. 10.1016/j.biopha.2017.07.112. [DOI] [PubMed]
- 21.Hosni AA, Abdel-Moneim AA, Abdel-Reheim ES, Mohamed SM, Helmy H. Cinnamaldehyde potentially attenuates gestational hyperglycemia in rats through modulation of PPARγ, proinflammatory cytokines and oxidative stress. Biomed Pharmacother. 2017;88:52–60. doi: 10.1016/j.biopha.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Mone A, Hosni A, Abdel-Rehe ES, Ismail A. Role of PPARγ/Anti-inflammatory Axis in the Protective Effect of Ellagic Acid Against FSD/STZ-induced Gestational Diabetes in Rats. Asian J Biol Sci. 2020;13(2):228–36. [Google Scholar]
- 23.Bakry LN, El-Hameed AMA, El-Twab SMA, Ahmed OM, A-MA The preventive effects of Cynara scolymus leaf and flower extracts on diethylnitrosamine/ acetylaminoflourene induced nephrotoxicity in male wistar rats. Adv Anim Vet Sci. 2020;8(2):74–81. [Google Scholar]
- 24.Khanra R, Dewanjee S, K Dua T, Sahu R, Gangopadhyay M, DeFeo V, et al. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015;13(1):6. [DOI] [PMC free article] [PubMed]
- 25.Wang X, Zhang X, He Y, Yang G, Lu J, Li J, et al. Protective effects of tranilast on diabetic nephropathy in rats. Med J Wuhan Univ. 2013;34(1):42–5. [Google Scholar]
- 26.El-hameed AMA, Eskandrani AA, Elroby FA. Assessment of the ameliorative effect of Hypericum perforatum on olanzapine-induced hepatic oxidative stress and metabolic abnormalities in experimental male albino rats. 2020. 10.1080/16583655.2020.1834757.
- 27.Li T, Cai S, Zeng Z, Zhang J, Gao Y, Wang X, et al. Protective effect of polydatin against bum-induced lung injury in rats. Respir Care. 2014;59(9):1412–21. doi: 10.4187/respcare.02831. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Kar M, Roy A, Chakraborti AS. Effect of nonenzymatic glycation on functional and structural properties of hemoglobin. Biophys Chem. 2005;113(3):289–98. doi: 10.1016/j.bpc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Pari L, Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16(4):257–74. doi: 10.1515/jbcpp.2005.16.4.257. [DOI] [PubMed] [Google Scholar]
- 30.Abouzed TK, del Mar Contreras, Sadek M, Shukry KM, Abdelhady MH, Gouda D. WM, et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res Clin Pract. 2018;140:253–64. [DOI] [PubMed]
- 31.Achilles CM, Reynolds JS, Achilles SH. Problem analysis [Internet]. Problem analysis. 2014. 10.1016/j.biochi.2015.03.025%0A, 10.1038/nature10402%0A, 10.1038/nature21059%0A, 10.1038/nrmicro2577%0A, http://journal.stainkudus.ac.id/index.php/equilibrium/article/view/1268/1127%0A.
- 32.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol - Ren Physiol. 2005;289:F645–59. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 33.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14(SUPPL. 3):S254–8. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 34.Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, et al. Glucose, glycation, and RAGE: Implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1383–95. doi: 10.1097/01.asn.0000065100.17349.ca. [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis pre- dominantly through transforming growth factor beta-independent pathway. J Pathol. 2004;165:2033–43. doi: 10.1016/s0002-9440(10)63254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L, et al. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol [Internet]. 2013;52:28–35. doi: 10.1016/j.fct.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–33. [PubMed] [Google Scholar]
- 38.Jakuš V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53(2):131–42. [PubMed] [Google Scholar]
- 39.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(SUPPL.):67–72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao-Sheng H, Zong-Wei W, Ming-Ping L, Qiao-Mei L, Qing-Guang W. Protective effects of polydatin on CCl4-induced injury of primary cultured rat hepatocytec. Chinese Pharmacol Bull. 1998;14(6):543–5. [Google Scholar]
- 41.Zhang H, Yu CH, Jiang YP, Peng C, He K, Tang JY, et al. Protective effects of polydatin from polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS One. 2012;7(9):e46574. doi: 10.1371/journal.pone.0046574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao Q, Wang S, Miao S, Wang J, Xie Y, Yang Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: Roles of protein kinase C and mito K ATP activation. Phytomedicine. 2011;19(1):8–12. doi: 10.1016/j.phymed.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Abd El-Hameed AM, Mahmoud HS. Cypermethrin induced apoptosis and testicular toxicity by upregulation of p53 in the brain and testis of male rats is alleviated by Sesame oil. J Taibah Univ Sci [Internet]. 2020;14(1):1342–9. doi: 10.1080/16583655.2020.1822057. [DOI] [Google Scholar]
- 44.Lenz O, Fornoni A, Ijaz A, Tejada T. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev. 2008;4(1):10–7. doi: 10.2174/157339908783502361. [DOI] [PubMed] [Google Scholar]
- 45.Balakumar P, Arora MK, Reddy J, Anand-Srivastava MB. Pathophysiology of diabetic nephropathy: Involvement of multifaceted signalling mechanism. J Cardiovasc Pharmacol. 2009;54(2):129–38. doi: 10.1097/FJC.0b013e3181ad2190. [DOI] [PubMed] [Google Scholar]
- 46.Han HJ, Min JL, Yun JL, Jang HL, Il SY, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-κB. Am J Physiol - Ren Physiol. 2007;292(1):F373–F381. doi: 10.1152/ajprenal.00104.2006. [DOI] [PubMed] [Google Scholar]
- 47.Quan H, Peng X, Liu S, Bo F, Yang L, Huang Z, et al. Differentially expressed protein profile of renal tubule cell stimulated by elevated uric acid using SILAC coupled to LC-MS. Cell Physiol Biochem. 2011;27(1):91–8. doi: 10.1159/000325209. [DOI] [PubMed] [Google Scholar]
- 48.Lee JI, Burckart GJ. Nuclear factor kappa B: Important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38(11):981–93. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 49.Akarasereenont P, Bakhle YS, Thiemermann C, Vane JR. Cytokine-mediated induction of cyclo‐oxygenase‐2 by activation of tyrosine kinase in bovine endothelial cells stimulated by bacterial lipopolysaccharide. Br J Pharmacol. 1995;115(3):401–8. doi: 10.1111/j.1476-5381.1995.tb16347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, et al. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(10):2505–12. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 51.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, et al. Elevated levels of interleukin-18 and tumor necrosis factor-α in serum of patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Metabolism. 2003;52(5):605–8. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 52.Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(3 SUPPL. 1):S78–82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- 53.Elsherbiny NM, Al-Gayyar MMH, Abd El Galil KH. Nephroprotective role of dipyridamole in diabetic nephropathy: Effect on inflammation and apoptosis. Life Sci [Internet]. 2015;143:8–17. 10.1016/j.lfs.2015.10.026. [DOI] [PubMed]
- 54.Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41(5):2629–39. doi: 10.3892/ijmm.2018.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borgohain MP, Chowdhury L, Ahmed S, Bolshette N, Devasani K, Das TJ, et al. Renoprotective and antioxidative effects of methanolic Paederia foetida leaf extract on experimental diabetic nephropathy in rats. J Ethnopharmacol [Internet]. 2017;198:451–9. 10.1016/j.jep.2017.01.035. [DOI] [PubMed]
- 56.Day CP, Grove J, Daly AK, Stewart MW, Avery PJ, Walker M. Tumour necrosis factor-alpha gene promoter polymorphism and decreased insulin resistance. Diabetologia. 1998;41(4):430–4. doi: 10.1007/s001250050926. [DOI] [PubMed] [Google Scholar]
- 57.Fernández-Real JM, Broch M, Vendrell J, Gutiérrez C, Casamitjana R, Pugeat M, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49(3):517–20. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 58.Alarcon-Aguilar FJ, Fortis-Barrera A, Angeles-Mejia S, Banderas-Dorantes TR, Jasso-Villagomez EI, Almanza-Perez JC, et al. Anti-inflammatory and antioxidant effects of a hypoglycemic fructan fraction from Psacalium peltatum (H.B.K.) Cass. in streptozotocin-induced diabetes mice. J Ethnopharmacol. 2010;132(2):400–7. doi: 10.1016/j.jep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Crook M. Type 2 diabetes mellitus: A disease of the innate immune system? An update. Diabet Med. 2004;21(3):203–7. doi: 10.1046/j.1464-5491.2003.01030.x. [DOI] [PubMed] [Google Scholar]
- 60.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep. 2006;6(6):463–8. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 61.Nicoletti F, Conget I, Di Marco R, Speciale AM, Morinigo R, Bendtzen K, et al. Serum levels of the interferon-γ-inducing cytokine interleukin-18 are increased in individuals at high risk of developing Type I diabetes. Diabetologia. 2001;44(3):309–11. doi: 10.1007/s001250051619. [DOI] [PubMed] [Google Scholar]
- 62.Feghali CA, Wright TM. Introduction 3. Discussion 3.1 Cytokines involved in acute inflammation 3.1.1 Interleukin-1 3.1.2 Tumor necrosis factor 3.1.3 Interleukin-6 3.1.4 Interleukin-11 3.1.5 Interleukin-8/chemokines 3.1.6 Eotaxin 3.1.7 Interleukin-16 3.1.8 Interleukin-17 3.1.9 C. Front Biosci [Internet]. 1997;2:12–26. https://www.bioscience.org/1997/v2/d/feghali1/htmls/feghali.pdf.
- 63.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Parasitol Today. 1991;7(3):49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 64.Wagener FADTG, Dekker D, Berden JH, Scharstuhl A, Van Der Vlag J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis. 2009;14(12):1451–8. doi: 10.1007/s10495-009-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lou T, Jiang W, Xu D, Chen T, Fu Y. Inhibitory Effects of Polydatin on Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflammation. 2015;38(3):1213–20. doi: 10.1007/s10753-014-0087-8. [DOI] [PubMed] [Google Scholar]