Abstract
Type 2 diabetes mellitus (T2DM) is an endocrine illness associated with various changes in the immune system and adaptor protein levels. Cytokine dependent hematopoietic cell linker (CLNK) is an adapter protein that regulates immune receptor signaling and acts as a regulator of the receptor signaling of T-cells and natural killer cells. The role of CLNK in T2DM is not studied previously. In the present study, serum CLNK level was measured and correlated with some sociodemographic and insulin resistance (IR) parameters. To achieve these goals, we measured CLNK level and insulin parameters (glucose, insulin, HbA1c, in addition to the calculation of the functions of IR (HOMA2IR), insulin sensitivity (HOMA%S), and beta-cell function (HOMA%B)) in 60 T2DM patients and 30 controls. The results indicated a significant increase (p < 0.05) in serum CLNK in patients group in comparison with the controls. Multivariate generalized linear model (GLM) analysis revealed no significant effect of age, BMI, and sex on the CLNK level. The results of tests for between-subjects showed that the CLNK affects diagnosis significantly (F = 7.445, p = 0.008, partial η2 = 0.081) and its effect is approximately the same as the effect of insulin (F = 8.107, p = 0.006, partial η2 = 0.087). The correlation study showed a highly significant positive correlation between CLNK and the duration of disease (rho = 0.420, p < 0.001). It can be concluded that the increase CLNK in T2DM revealing the role of the adaptor proteins level in the progression of the disease and may act as a predictor for diabetes complications, which deserves more investigations.
Keywords: Diabetes Mellitus, Insulin resistance, Cytokines, Adaptor proteins, CLNK
Introduction
Diabetes mellitus (DM) is a prevalent disorder characterized by hyperglycemia and results from the interaction between environmental, genetic, and behavioral risk factors [1]. DM is a major public health concern with a growing prevalence around the world. In 2017, it was estimated that there were 451 million people aged over 18 years with DM worldwide, which expected to reach 693 million by the year 2045 [2]. It was estimated that almost half of all people living with DM are undiagnosed [2]. In Iraq, the prevalence of DM had risen significantly from 19.58/1000 in the year 2000 to 42.27/1000 in 2015 [3]. Diabetes contributes to 11.3% of deaths globally [4]. Type 2 DM (T2DM) is the most familiar form of DM designated by insulin resistance (IR), hyperglycemia, and relative insulin deficiency and insensitivity [5]. Insulin insensitivity is produced because of declining insulin production, IR, and final pancreatic β-cell malfunction results in a reduction in glucose transportation into the adipocytes, myocytes, and hepatocytes [6]. Another important factor in the etiology of T2DM is the chronic inflammation caused by an innate immune system dysregulation that acts as a potential link between diabetes and metabolic syndrome [7]. T2DM is associated with chronic inflammation in addition to metabolic dysregulation and there is also a link between metabolism and inflammation [8]. Chronic inflammation may be the cause and result of T2DM. Its related complications are due to an imbalance between proinflammatory and anti-inflammatory cytokines that in turn can affect the functions of the immune system [9]. Therefore, the study of other inflammation biomarkers in T2DM is important to diagnose and treat low-grade inflammation (LGI) which is responsible for most diabetic complications [10, 11]. For an instance, previous investigations have broadly revealed the relationship of high C-reactive protein (CRP) level with IR and progression of T2DM [12]. While interleukin (IL)-6 plays an exceptional role in the development of T2DM and elevated TNF-α levels could be a potential predictor of T2DM [13]. In addition to cytokines that released in response to certain stimuli and immune system dysregulation [14], adaptor proteins are another important constituents of the signaling transduction system both within and beyond the immune system [15]. Adaptor proteins are molecular platforms that other proteins are accumulated on [15]. These adapter proteins control signaling by stabilizing or constraining molecular interactions essential for suitable activation of specific enzymes and for implementing these key effector molecules correctly inside the cell [16]. Many adaptors are expressed in a variety of hematopoietic cell types [17, 18]. The term “hematopoietic cells” describes all mature cell types and their immature precursors, or hematopoietic stem cells, in the blood including hematopoietic stem cells, basophilic myelocytes, basophils, and B-cells [19]. Cytokine dependent hematopoietic cell linker (CLNK) is a type of adaptor protein Src homology-2 domain-containing leukocyte protein of 76 kDa (SLP-76) which exist in many lineages of hematopoietic cells including neutrophils, mast cells, macrophages, platelets, T-cells, and natural killer (NK) cells [16]. CLNK has been reported to be expressed in cytokine-dependent cell lines of both lymphoid and myeloid in addition to some mast cell lines [20]. Although it can be distinguished in most types of cells, its expression appears to be strictly dependent on continual exposure to cytokines such as IL-2 and IL-3 [21]. Furthermore, the temporary expression of CLNK results in increased immune-receptor signaling events in T-cells that are activated by cytokines [22]. CLNK can serve as a modulator to restrict excessive NK cell activation in the immune reaction process wherever the cytokines are out [23]. NK cells are inhabitant cells of adipose tissue and possess numerous immune regulatory capabilities including the release of immunoregulatory cytokines (IL-4 and IL-10), prompt dendritic cells, and expanding the recurrence of T regulatory cells. The number of NK cells in obese people is declining, indicating their contribution to local and systemic inflammation in obesity [24]. Obesity leads to IR which is the main feature of T2DM etiology and pathogenesis [25] that results from activated immune-inflammatory pathways [26] which activate a variety of kinases involved in the IR etiology [27]. There is a lack of studies that deal with the role of CLNK in T2DM disease. Therefore, the present study aims to examine the role of CLNK in the T2DM as a possible inflammatory marker or a potential predictor for some sociodemographic parameters and IR state parameters in patients with diabetes.
Subjects and methods
Subjects
The present case-control study involved 60 T2DM patients and 30 healthy controls age- and sex-matched collected by stratified random sampling. The samples were obtained from Al-Sadder Hospital in Najaf Governorate-Iraq from December 2019 to the end of January 2020. T2DM patients were diagnosed according to World Health Organization criteria [28]. They were assessed by full medical history by the physicians. All subjects were non-smokers. All subjects were fasted for at least 12 h before collection of blood specimen in the morning. The T2DM patients were on one tablet (5 mg) of glibenclamide drug daily. The control group was confirmed to be normal by clinical and biochemical tests. Written informed consent was taken from all subjects before participation in the current study. All procedures were under the established ethical standards. Approval for the study was obtained from the IRB of the University of Kufa (411/2019), which complies with the International Guidelines for Human Research protection as required by the Declaration of Helsinki, The Belmont Report, and International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Exclusion criteria
The present study excluded patients who had the following criteria: patients with serum FBG > 25 mM and fasting insulin > 57.6 mIU/L based on HOMA calculator software requirements, and patients with evident major diabetic complications, such as heart diseases, liver disease, and renal diseases. We also excluded patients who are receiving metformin and patients with albumin/creatinine ration above 30 mg/g. These exclusions were based on previously mentioned facts [29] that patients using metformin cannot be used for insulin sensitivity studies because of the well-known effect of metformin on IR [30] and insulin sensitivity [31]. Serum CRP titer was negative in all samples i.e. less than 6 mg/L. CRP test was used to exclude inflammation that causes changes in the levels of the acute phase reactant proteins.
Methods
Five milliliters of venous blood samples were drawn from patients and controls. The aspirated blood was divided into three aliquots, one added directly to a tube containing anticoagulants (K3EDTA) for measurement of HbA1c, one into a fluoride oxalate bottle for obtaining plasma for glucose. The other aliquot was transferred to a plain tube. After complete clotting, the blood was centrifuged 3000 rpm for 10 min, and then serum was separated to be stored at -80 C until analyzed. Commercial ELISA sandwich kits were used to measure serum levels of insulin (DRG® International Inc., USA) and CLNK (Bioassay Technology Laboratory®, China). Fasting plasma glucose was measured spectrophotometrically by an enzymatic method (endpoint) using ready for use kit supplied by Biolabo® Co., France. HbA1c percentage in the whole blood was determined by a fluorescence immunoassay (FIA) using the i-Chroma™ HbA1c test kit (BioLabs Diagnostics, Italy). Serum CRP was measured using a kit supplied by Spinreact®, Spain. The test is based on the principle of latex agglutination. Body mass index (BMI) was calculated by weight in kilograms divided by square of height in meters.
Insulin sensitivity percentage (HOMA%S), percentage of beta-cell function (HOMA%B), and IR HOMA2IR were measured from fasting insulin and serum glucose by HOMA2 Calculator downloaded freely from (https://www.dtuoxacuk/homacalculator/).
Statistical analysis
The distribution types of the variable’s results were examined using the Shapiro-Wilk test. The results were expressed as (mean ± standard deviation) or median (interquartile range). The Chi-square test was used for comparison between nominal variables. The pooled t-test has been used for the comparison of the measured parameters between the patients and control groups and between patients subgroups. Mann-Whitney U test was used for the comparison between nonparametric variables. Pearson’s correlation coefficients (r) or Spearman’s correlation coefficient (rho, ρ) were used to evaluate the correlations among parameters. We used multivariate general linear model (GLM) analysis to delineate the associations between diagnosis (having T2DM or healthy control) and the main five biomarkers (CLNK, HOMA%B, Insulin, HOMA2IR, and HOMA%S), while controlling for confounding variables including age, BMI, and sex. Consequently, we carried out tests for between-subject effects to delineate the associations between diagnosis and each of the biomarkers. The effect size was estimated using partial η2 values. We excluded the FBS and HbA1c from the multivariate GLM analysis because they already have a very high correlation with T2DM and will cause big collinearity of the tests. Power analysis showed that using an effect size of 0.3, α = 0.05, power = 0.8, and 2 groups the total sample size should be 90 samples. The difference between groups is considered statistically different when p < 0.05. All statistical analyses were performed using SPSS statistics version 25 (2017) by IBM-USA. While the figures constructed using the Excel program of Microsoft Office 2016.
Results
Socio-demographic and clinical characteristics
The clinical and socio-demographic characteristics of the patients with T2DM and control groups are presented in Table 1. The results are typical for T2DM patients where there is a significant increase in FPG and HbA1c (both p < 0.001), and HOMA2IR (p = 0.031). While the level of insulin is significantly decreased (p = 0.008) along with HOMA2%B (p < 0.001). HOMA%S showed no significant difference between the two groups. Serum CLNK was significantly elevated (p = 0.025) in patients group in comparison with the control group.
Table 1.
Socio-demographic, clinical and biomarker data in T2DM patients as compared with healthy controls
| Parameter | Control (n = 30) | Patients (n = 60) | p-value |
|---|---|---|---|
| Sex Male/Female | 20/10 | 31/29 | 0.176* |
| Age Year | 50.27 ± 8.25 | 53.08 ± 7.85 | 0.056 |
| BMI kg/m2 | 26.90 ± 2.54 | 28.65 ± 5.23 | 0.088 |
| Duration of illness Year | - | 9(3–13) | - |
| HbA1c % | 4.69 ± 0.38 | 8.57 ± 2.06 | < 0.001 |
| FPG mM | 5.20 ± 0.29 | 10.54 ± 4.58 | < 0.001 |
| Insulin pM | 88.57(52.26-122.96) | 73.53(35.99-103.91) | 0.008** |
| HOMA2%B | 103.30(79.25-124.25) | 42.70(19.8–72.20) | < 0.001** |
| HOMA2%S | 75.10(64.00-99.45) | 49.40(24.10-106.65) | 0.058** |
| HOMA2IR | 1.33(0.99–1.56) | 2.09(0.94–4.15) | 0.031** |
| CLNK ng/ml | 4.27(2.59–5.08) | 4.89(3.67–5.93) | 0.025** |
-Results expressed as mean ± SD or median (interquartile range)
*Calculated by χ2, **Calculated by Mann-Whitney U test, while other p-values were carried out by using pooled T-test
Abbreviations: FBS: Fasting plasma glucose, BMI: Body mass index, HOMA2R: Homeostatic model assessment of insulin resistance, HOMA2%B: Homeostatic model assessment of beta cells function percentage, HOMA2%S: Homeostatic model assessment of insulin sensitivity percentage, CLNK: Cytokine Dependent Hematopoietic Cell Linker
Multivariate GLM analysis
The data were analyzed by multivariate generalized linear model (GLM) analysis with the biomarkers (HOMA%B, Insulin, HOMA2IR, HOMA%S, and CLNK) as dependent variables and diagnosis and the covariates (age, BMI, and sex) as an explanatory variable to examine their effect on the measured parameters were shown in Table 2. We found a highly significant effect of diagnosis (F = 25.048, p < 0.001) on the measured parameters with a big effect size of 60.7%, while age, BMI, and sex were not significant (p < 0.05). We also examined the effects of measured parameters on the diagnosis by using tests for between-subjects. The highest parameter that affects diagnosis significantly is HOMA%B (F = 30.14, p < 0.001, partial η2 = 0.262), followed by HOMA2IR (F = 13.164, p < 0.001, partial η2 = 0.134), Insulin (F = 8.107, p = 0.006, partial η2 = 0.087), and CLNK (F = 7.445, p = 0.008, partial η2 = 0.081). While HOMA%S has no significant effect on the diagnosis (p < 0.05). These results indicated that diagnosis (i.e., being patients) have the same effects on the variation of serum CLNK or insulin levels.
Table 2.
Results of multivariate GLM analysis with the biomarkers as dependent variables and diagnosis as explanatory variable while adjusting for extraneous variables
| Tests | Dependent Parameters | Explanatory variables | F | df | P | Partial η2 |
|---|---|---|---|---|---|---|
| Multivariate |
HOMA%B, Insulin, HOMA2IR, CLNK, HOMA%S. |
Diagnosis | 25.048 | 5/81 | < 0.001 | 0.607 |
| Sex | 0.735 | 5/81 | 0.599 | 0.043 | ||
| Age | 0.501 | 5/81 | 0.774 | 0.030 | ||
| BMI | 1.027 | 5/81 | 0.408 | 0.060 | ||
| Tests of Between-Subjects Effects | HOMA%B | Diagnosis | 30.140 | 1 | < 0.001 | 0.262 |
| HOMA2IR | Diagnosis | 13.164 | 1 | < 0.001 | 0.134 | |
| Insulin | Diagnosis | 8.107 | 1 | 0.006 | 0.087 | |
| CLNK | Diagnosis | 7.445 | 1 | 0.008 | 0.081 | |
| HOMA%S | Diagnosis | 0.056 | 1 | 0.601 | 0.003 |
Abbreviations: BMI: Body mass index, HOMA2R: Homeostatic model assessment of insulin resistance, HOMA2%B: Homeostatic model assessment of beta cells function percentage, HOMA2%S: Homeostatic model assessment of insulin sensitivity percentage, CLNK: Cytokine dependent hematopoietic cell linker
Correlation of CLNK with other parameters
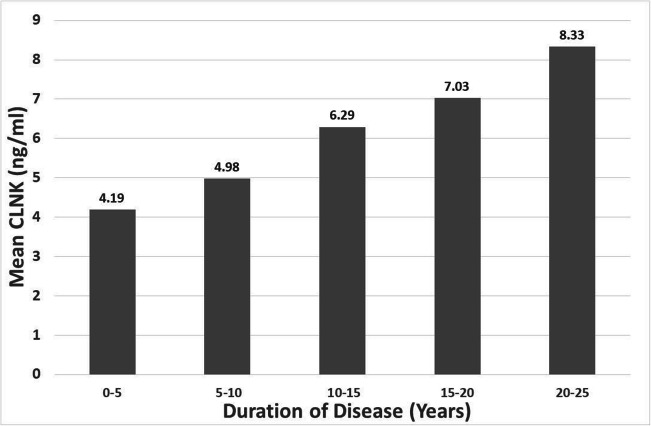
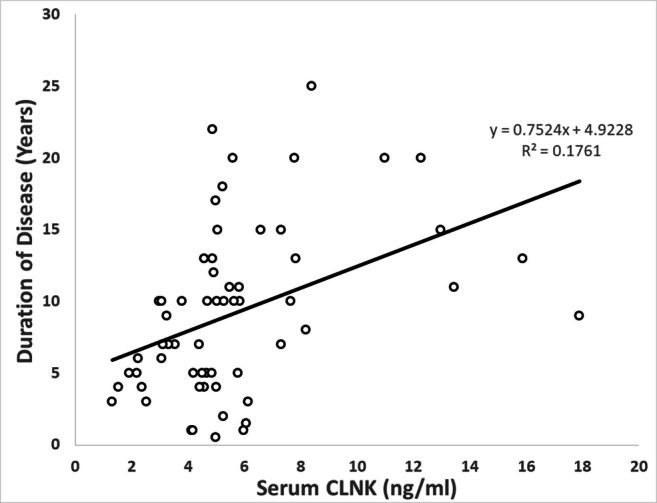
The result of the correlation study showed a highly significant positive correlation between CLNK and the duration of disease in the patients’ group (rho = 0.420, p < 0.001) as presented in Fig. 1. The results showed that R2 = 0.1761, which represents that 17.61% of the variance in the duration of illness is explained by serum CLNK level which is statistically significant (p < 0.001). To obtain a better view of the dependence of CLNK level on the and duration of the illness, the duration of the disease is distributed into 5 years periods and the average of CLNK for each period was calculated and plotted in Fig. 2. The figure clearly presents the increase in the average serum CLNK with increasing the duration of disease. There is no significant correlation between serum CLNK and other parameters.
Fig. 1.
The correlation between CLNK and duration of disease in the T2DM patients’ group
Fig. 2.
Distribution of mean CLNK according to the periods of duration of T2DM disease
Discussion
The major finding of the present study is the increase in serum CLNK level in T2DM patients in comparison with the control group as presented in Table 1. Because of the lack of literature about the level of CLNK in the serum of T2DM patients, there is no simple explanation for this result. In the literature, only one research measured the serum CLNK in humans, namely in thalassemia patients [32]. The suggested explanation for the increase of CLNK in thalassemia patients in comparison with healthy controls depends on the reciprocal effects between immune system signaling and immature erythrocytes that release soluble receptors and signaling molecules including CLNK in the blood [32]. In our T2DM patients, the factor that may underpin the explanation of increased CLNK is via the LGI associated with T2DM disease [33, 34]. The disease is started with chronic LGI associated with obesity, which is involved in the development of IR that increases the risk of T2DM [35]. When LGI is associated with lipid toxicity, together they appear to be major assaults on insulin sensitivity in insulin-responding tissues [36]. Hyperinsulinemia also has a bidirectional relationship with chronic LGI, which may develop into systemic inflammation and systemic inflammation causing IR and eventually compensatory hyperinsulinemia, which increases oxidative stress and inflammation processes [37]. Therefore, it is confirmed that T2DM associated with LGI, IR, and obesity that interact with each other in a multidirectional mechanism [38, 39]. For example, there is strong evidence that activation of inflammatory pathways interferes with normal metabolism and disrupts proper insulin signaling resulting in increased expression of pro-inflammatory cytokines [40]. These cytokines can attach to their receptors on the cell membrane and produce an inflammatory reaction and exacerbating IR [41]. Chronic activation of immune-inflammatory pathways lead to IR, thus rising risk of T2DM [42]. IR development is generally correlated with pro-inflammatory cytokines-dependent LGI responses in various tissues [43]. These cytokines eventually prevent the activation of receptors of the insulin signaling in β-cells islets [25] by activating kinases that phosphorylate serine residues of insulin receptor substrate leading to suppression of insulin signaling [44]. IR could be produced by altered recruitment of downstream adaptor proteins that CLNK belongs to [45]. The CLNK homology adaptor protein SLP-76 interacts physically with a signaling molecule, HPK1 (homeodomain-interacting protein kinase-1) [17], which may cause IR by modifying the insulin receptors [46]. Therefore, the connection between CLNK and the T2DM may be due to the mutual interaction between CLNK and various molecules and receptors involved in the inflammation and the insulin signaling.
The other part that needs to be confirmed is the correlation between CLNK and inflammation. It is noticed previously that CLNK, as an adaptor protein, plays a crucial role in the T-cell signaling pathway mediated by the T-cell receptor, which is necessary for the adaptive immune response and important for differentiation, proliferation, and cytokine secretion [47]. It is expressed exclusively in cytokine-stimulated hemopoietic cells, including IL-2-induced T cells and NK cells, and IL-3-induced myeloid cells and mast cells [48].
Another important finding of the present study is the strong association between CLNK and the duration of T2DM illness. The strongest association came from the finding that CLNK regulates the IFN-γ production by different immune cells [49]. The immune activation, that associated with increased IFN-γ production, has a harmful effect on the β-cells and may even kill the cells [50, 51]. Another important factor is the CLNK expression inside NK cells is needed to improve the subsequent acquired immune responses. At the advanced stage of immune responses, CLNK can restrict excessive NK-cell activation, where abundant cytokines upregulate CLNK expression in NK cells [23]. Since CLNK is needed for complete activation of NK T-cells at such an advanced stage of immune responses, activated NK T-cells (expressing CLNK) produce high amounts of cytokines including IL-4 and IFN-γ which can ultimately decide the nature of the subsequent acquired immune responses [49]. All these immune sequences of CLNK may be associated with the progression or developing the complications of diabetes with time.
Figure 1 showed the dependence of the duration of illness on the serum CLNK level. The figure showed that 17.61% of the variation change in the duration of the disease is dependent on the serum CLNK level. The duration of diabetes reflects the course of the disease and patients with longer illness duration have more complications [52, 53]. Previous research found an association between the duration of diseases and the development of immune system concentrations [52]. In glycemic controlled patients without complications, the duration of the disease is negatively correlated with the monocyte RANTES and positively correlated with various types of neutrophil Toll-like receptors. The nature of correlation (negative or positive) depends on the type of Toll-like receptor, degree of glycemic control, and the presence of complications [52]. The duration of diabetes mellitus also affects the concentration of IL-1β in both types of diabetes [54] and showed a negative correlation with serum level of brain-derived neurotrophic factor (BDNF) [55]. These changes in the immune biomarkers with time indicating continuous deterioration of immune system defense with the duration of illness.
Figure 2 shows the distribution of mean CLNK according to the duration periods of T2DM. The long duration of the disease leads to more complications [56]. Many diabetes complications appear with the progress of disease and correlated with the duration of disease including coronary artery diseases, nephropathy, retinopathy, and neuropathy [57]. As the duration of disease prolonged, the serum level of many biomarkers including uric acid and microalbuminuria increased [58] indicating the progression of nephropathy with time. Therefore, this is a good point to consider CLNK as a predictor for diabetes complications that needs more investigation.
Conclusion
The level of serum CLNK is strongly associated with the duration of illness and not associated with the IR parameters. As the prolonged duration of the disease leads to the development of diabetes complications and deterioration of the immune system, the elevation of the CLNK level may be used as a predictor for diabetes complications.
Limitations of the Study
The first limitation is the relatively small sample size. The second limitation is the strict criteria for choosing the subjects of the study. All subjects were nonsmokers, and the patients were free of obvious complications and on one type of treatment (glibenclamide). These criteria are useful in removing the cofounders affecting the CLNK level but they are ideal and do not represent the real-life state.
Author contribution
All the contributing authors have participated in the preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Contributor Information
Suhaer Zeki Al-Fadhel, Email: suhaeralfadel@yahoo.com.
Nibras H. Abdulsada Al-Ghuraibawi, Email: nibrash.abdalsada@uokufa.edu.iq.
Dunia M. Mohammed Ali, Email: duniam.mohammedali@uokufa.edu.iq
Hussein Kadhem Al-Hakeim, Email: headm2010@yahoo.com.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Hussain AM, Lafta RK. Burden of non-communicable diseases in Iraq after the 2003 war. Saudi Med J. 2019;40(1):72–8. doi: 10.15537/smj.2019.1.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, , et al. Mortality attributable to diabetes in 20–79 years old adults estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edn. Diabetes Res Clin Pract. 2019;2020:108086. [DOI] [PubMed]
- 5.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–73. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee M, Saxena M. Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes. 2014;5(4):493. doi: 10.4239/wjd.v5.i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94(2):146–50. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 9.Naidoo V, Naidoo M, Ghai M. Cell- and tissue-specific epigenetic changes associated with chronic inflammation in insulin resistance and type 2 diabetes mellitus. Scand J Immunol. 2018;88(6):e12723. doi: 10.1111/sji.12723. [DOI] [PubMed] [Google Scholar]
- 10.Eftekharian MM, Karimi J, Safe M, Sadeghian A, Borzooei S, Siahpoushi E. Investigation of the correlation between some immune system and biochemical indicators in patients with type 2 diabetes. Hum Antib. 2016;24(1–2):25–31. doi: 10.3233/HAB-150290. [DOI] [PubMed] [Google Scholar]
- 11.van Diepen JA, Robben JH, Hooiveld GJ, Carmone C, Alsady M, Boutens L, et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia. 2017;60(7):1304–13. doi: 10.1007/s00125-017-4261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phosat C, Panprathip P, Chumpathat N, Prangthip P, Chantratita N, Soonthornworasiri N, et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. BMC Endocr Disord. 2017;17(1):44. doi: 10.1186/s12902-017-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lainampetch J, Panprathip P, Phosat C, Chumpathat N, Prangthip P, Soonthornworasiri N, et al. Association of tumor necrosis factor alpha, Interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: A retrospective cohort study of Rural Thais. J Diabetes Res. 2019;2019:9051929. doi: 10.1155/2019/9051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin Chim Acta. 2012;413(15–16):1163–70. doi: 10.1016/j.cca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Verma NK, Tran T, Kelleher D. Adaptor protein regulation in immune signalling. Front Immunol. 2020;11:441. doi: 10.3389/fimmu.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan MS, Koretzky GA. Coordination of receptor signaling in multiple hematopoietic cell lineages by the adaptor protein SLP-76. Cold Spring Harb Perspect Biol. 2010;2(4):a002501-a. doi: 10.1101/cshperspect.a002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4(2):110–6. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Cruz-Munoz M-E, Zhong M-C, Chen R, Latour S, Veillette A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10(9):973. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Devine S, Caligiuri MA, He S. Methods for mobilizing hematopoietic stem cells. Google Patents; 2018.
- 20.Goitsuka R, Kanazashi H, Sasanuma H, Fujimura Y-i, Hidaka Y, Tatsuno A, et al. A BASH/SLP-76-related adaptor protein MIST/Clnk involved in IgE receptor-mediated mast cell degranulation. Int Immunol. 2000;12(4):573–80. doi: 10.1093/intimm/12.4.573. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara K, Hirano T. Molecular basis of the cell specificity of cytokine action. Biochim Biophys Acta (BBA) Mol Cell Res. 2002;1592(3):281–96. [DOI] [PubMed]
- 22.Cao MY, Davidson D, Yu J, Latour S, Veillette A. Clnk, a novel SLP-76–related adaptor molecule expressed in cytokine-stimulated hemopoietic cells. J Exp Med. 1999;190(10):1527–34. doi: 10.1084/jem.190.10.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidano S, Sasanuma H, Ohshima K, Seino K-i, Kumar L, Hayashi K, et al. Distinct regulatory functions of SLP-76 and MIST in NK cell cytotoxicity and IFN-γ production. Int Immunol. 2008;20(3):345–52. doi: 10.1093/intimm/dxm150. [DOI] [PubMed] [Google Scholar]
- 24.Tard C, Rouxel O, Lehuen A. Regulatory role of natural killer T cells in diabetes. Biomed J. 2015;38(6):484–95. doi: 10.1016/j.bj.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23(1):87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167(1):228–56. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. doi: 10.4239/wjd.v6.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006.
- 29.Al-Hakeim HK, Abdulzahra MS. Correlation between glycated hemoglobin and homa indices in type 2 diabetes mellitus: Prediction of beta-cell function from glycated hemoglobin. J Med Biochem. 2015;34(2):191–9. doi: 10.2478/jomb-2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangeeta S. Metformin and pioglitazone in polycystic ovarian syndrome: a comparative study. J Obstet Gynecol India. 2012;62(5):551–6. doi: 10.1007/s13224-012-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hakeim HK, Al-Mayali HH, Maes M. Cytokine dependent hematopoietic cell linker (CLNK) is highly elevated in blood transfusion dependent beta-thalassemia major patients. Available at SSRN 3369783. 2019. [DOI] [PubMed]
- 33.Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2018;9(1):1–58. doi: 10.1002/cphy.c170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 35.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilan PJ, Samokhvalov V, Koshkina A, Schertzer JD, Samaan MC, Klip A. Direct and macrophage-mediated actions of fatty acids causing insulin resistance in muscle cells. Arch Physiol Biochem. 2009;115(4):176–90. doi: 10.1080/13813450903079314. [DOI] [PubMed] [Google Scholar]
- 37.van den Oever IAM, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, et al. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–86. doi: 10.2147/dmsott.s9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11(4):e0154003-e. doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 41.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metabol. 2007;6(5):386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36(2):480–9. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fève B, Bastard J-P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 44.Aroor AR, McKarns S, DeMarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–52. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siragusa M, Fisslthaler B. Insulin Keeps PYK-ing on eNOS: Enhanced Insulin Receptor Signaling Induces Endothelial Dysfunction. Am Heart Assoc; 2017. [DOI] [PubMed]
- 46.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji Q, Ding Y, Salomon AR. SRC homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) N-terminal tyrosine residues regulate a dynamic signaling equilibrium involving feedback of proximal T-cell receptor (TCR) signaling. Mol Cell Proteomics. 2015;14(1):30–40. doi: 10.1074/mcp.M114.037861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boomer JS, Tan TH. Functional interactions of HPK1 with adaptor proteins. J Cell Biochem. 2005;95(1):34–44. doi: 10.1002/jcb.20401. [DOI] [PubMed] [Google Scholar]
- 49.Sasanuma H, Tatsuno A, Hidano S, Ohshima K, Matsuzaki Y, Hayashi K, et al. Dual function for the adaptor MIST in IFN-γ production by NK and CD4 + NKT cells regulated by the Src kinase Fgr. Blood. 2006;107(9):3647–55. doi: 10.1182/blood-2005-10-4102. [DOI] [PubMed] [Google Scholar]
- 50.Eizirik DL, Mandrup-Poulsen T. A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44(12):2115–33. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 51.Hostens K, Pavlovic D, Zambre Y, Ling Z, Van Schravendijk C, Eizirik DL, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Investig. 1999;104(1):67–72. doi: 10.1172/JCI6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta S, Maratha A, Siednienko J, Natarajan A, Gajanayake T, Hoashi S, et al. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Sci Rep. 2017;7(1):7633. doi: 10.1038/s41598-017-07230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akujuru EE, Aprioku JS, Okerengwo AA. Circulatory levels of pro-inflammatory cytokines (IL-6 and IL-1β) and neutrophil-lymphocyte ratio (NLR) in diabetic patients in Nigerian population. Comp Clin Pathol. 2020;29(2):539–45. [Google Scholar]
- 54.Amin K, Qadr SH, Hussein RH, Ali KM, Rahman HS. Levels of cytokines and GADA in type I and II diabetic patients. Prim Care Diabetes. 2020;14(1):61–7. doi: 10.1016/j.pcd.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Li B, Lang N, Cheng Z-F. Serum levels of brain-derived neurotrophic factor are associated with diabetes risk, complications, and obesity: A cohort study from Chinese patients with type 2 diabetes. Mol Neurobiol. 2016;53(8):5492–9. doi: 10.1007/s12035-015-9461-2. [DOI] [PubMed] [Google Scholar]
- 56.Hsu HY, Chiu HY, Lin HT, Su FC, Lu CH, Kuo LC. Impacts of elevated glycaemic haemoglobin and disease duration on the sensorimotor control of hands in diabetes patients. Diab/Metab Res Rev. 2015;31(4):385–94. doi: 10.1002/dmrr.2623. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi Y, Suzuki R, Yasukawa K, Oba K, Yamauchi T, Yatomi Y, et al. Oxidized albumin in blood reflects the severity of multiple vascular complications in diabetes mellitus. Metab Open. 2020:100032. [DOI] [PMC free article] [PubMed]
- 58.Latif H, Iqbal A, Rathore R, Butt NF. Correlation between Serum Uric Acid Level and Microalbuminuria in Type-2 Diabetic Nephropathy. Pak J Med Sci. 2017;33(6):1371–5. doi: 10.12669/pjms.336.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]