Introduction
Reactive oxygen species (ROS) and lipid peroxidation (LPO) levels may increase in diabetic state and lead to oxidative stress, which plays a critical role in the progression of diabetes. There are various sources of ROS, including cytochrome P450 monooxygenases (CYP450s), which may be modulated in terms of their activities and expressions under diabetic conditions. This study is aimed to investigate the effects of streptozotocin-induced diabetes and insulin treatment on hepatic cytochrome P450 1A1 (CYP1A1) and cytochrome P450 2E1 (CYP2E1) activities and LPO levels. Methods: CYP1A1 and CYP2E1 activities were measured with ethoxyresorufin O-deethylase and p-nitrophenol hydroxylase activities, respectively. LPO levels were then corroborated via thiobarbituric acid reactive substances. Results: In diabetic rats, a marked 2.1- and 2.4-fold increase in hepatic CYP1A1 activity and 1.8- and 1.6-fold increase in hepatic CYP2E1 activity were observed compared to controls and insulin-treated diabetic rats, respectively. Hepatic LPO levels in diabetic rats did not significantly change compared to controls. However, in insulin-treated diabetic rats, LPO levels are 0.92- and 0.89-fold remarkably decrease compared to controls and diabetics, respectively. Conclusion: The present study suggests that insulin might have a useful role in the modulation of CYP1A1 and CYP2E1 activities as well as LPO levels in the liver of diabetic rats.
Keywords: CYP450, CYP1A1, CYP2E1, Diabetes, Insulin, Streptozotocin
Introduction
Diabetes mellitus is one of the most common metabolic diseases worldwide. Many studies have been carried out to clarify its mechanisms and develop more efficient therapeutic strategies. The increased oxidative stress in different tissues such as the liver, pancreas, kidney, and brain has been reported to associate with the development of diabetes and diabetic complications [1, 2]. As the liver is an essential organ in terms of its role in glucose homeostasis [3], it is crucial to understand elaborately how a diabetic state particularly affects hepatic functions.
Hyperglycaemia, or high blood glucose, is a serious symptom of diabetes. Oxidative stress can be occurred by the generation of reactive oxygen species (ROS) as a consequence of high blood glucose levels [4]. The increase in ROS production is vital due to their role in the inflammation, aging, atherosclerosis, and carcinogenesis [5]. ROS can contribute to the disruption of the membrane integrity and structural and functional alterations in biomolecules that regulate cell metabolism, including lipids, proteins, and nucleic acids. [6]. They can be generated from many sources such as oxidative phosphorylation, nicotinamide adenine dinucleotide phosphate oxidase, glucose autoxidation, and cytochrome P450 monooxygenases (CYP450) [4]. Over-expressions of CYP450s may lead to oxidative stress through the formation of ROS in diabetes [4], and lipid peroxidation can be one of the major outcomes induced by oxidative damage [7]. The increased ROS-induced lipid peroxidation can cause cellular damage, abnormality of blood coagulation (hypercoagulation), hypertension, and cardiovascular disease in diabetic patients [8].
CYP450s have a significant role in the oxidative metabolism of various lipophilic compounds such as fatty acids, endogenous steroids, carcinogens, as well as drugs [9]. Among CYP450s, cytochrome P450 1A1, 1A2, 1B1, 2E1, and 3A4 are the main isozymes contributing to the metabolism of xenobiotics [10–12]. Beyond their usual role in detoxification, some CYP450s could have potentially harmful effects in their increased activities since they may promote oxidative stress due to their ability of excessive ROS production. [4, 5, 13]. As the expressions and activities of CYP450s may be affected by several pathophysiological conditions such as diabetes, hypertension, cancer [14], one of the reasons behind the impaired function of the liver in diabetes could be alterations in hepatic CYP450s enzymes, including cytochrome P450 1A1 (CYP1A1), cytochrome P450 2E1 (CYP2E1) [15–17]. CYP1A1 and CYP2E1 are regarded as the oxidative stress-related enzymes [18, 19]. CYP2E1-dependent ROS production can be an important aspect of diabetes [16], and CYP1A1, known for its capacity to activate carcinogenic compounds [12, 20], may be associated with oxidative stress-induced diabetic complications [21]. However, there is hardly ever information on the relationship between CYP1A1 activity and oxidative stress in the diabetic state. Furthermore, insulin, the key regulatory hormone in diabetes, may also influence the expressions and activities of these enzymes [22, 23], but the effects of insulin on the activities of hepatic CYP1A1 and CYP2E1 and liver lipid peroxidation levels are not fully known. Accordingly, this study aimed to investigate the effects of insulin treatment on hepatic CYP1A1 and CYP2E1 activities as well as LPO levels in streptozotocin (STZ)-induced diabetic rats to determine the possible link between hepatic oxidative stress and insulin in diabetes through the activity of these enzymes and lipid peroxidation.
Materials and methods
Materials
Folin-ciocalteu’s phenol reagent, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, nicotinamide adenine dinucleotide phosphate (NADP+), sodium salt, streptozotocin 7-ethoxyresorufin, p-nitrophenol, thiobarbituric acid, albumin, ethyl alcohol, glycerol, potassium chloride, sodium potassium tartrate, and tris base were obtained from Sigma-Aldrich (USA). Copper sulphate and iron (II) sulphate heptahydrate were from the British Drug House (UK). Dipotassium hydrogen phosphate, hydrochloric acid, magnesium chloride, methyl alcohol and sodium hydroxide were purchased from Merck and perchloric acid was obtained from Riedel (Germany). All chemicals used were the highest purity available from commercial sources.
Animals and study design
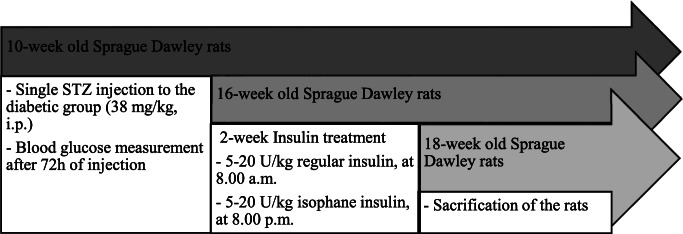
Adult 10-week old male Sprague-Dawley rats with 280–300 g body weight were used throughout the experiments. Twenty-seven rats provided by Bilkent University (Ankara, Turkey) were housed individually in standard single cages and maintained under the controlled conditions (12-h light-dark cycle; room temperature 22±1 ºC, %60 relative humidity). Animals had access to tap water and standard rat chow (Purina, Turkey) ad libitum. The animals were randomly divided into three groups: control (n = 05), diabetic rats (n = 11), and diabetic rats treated with insulin (n = 11). Experimental diabetes was induced by a single intraperitoneal injection of 38 mg/kg streptozotocin, diluted in citrate buffer (pH:4.5). Different moderate doses of streptozotocin ranging from 35 mg/kg to 45 mg/kg were tested and evaluated with preliminary experiments, 38 mg/kg was preferred, at which the most effective dose with minimum mortality. After 72 h of following the injection of streptozotocin, blood glucose levels were measured by glucose monitor Accuchek (Roche Diagnostics, Mannheim, Germany) for each group once a week. Rats were considered diabetic when the levels were ≥300 mg/dL. Weekly body weight changes of all animals were also assessed during the experimental period. 6 weeks after injection of streptozotocin, diabetic rats treated with insulin. The insulin dose was determined individually and daily regarding blood glucose levels of rats. Blood glucose levels were measured before injection, and the insulin dose was set thoroughly. As each rat had different blood glucose levels, the dose of insulin for each of them was different. Thus, we indicated a range of insulin doses between 5 and 20 U/kg regular insulin at 8:00 am (Actrapid, Novo Nordisk): 5–20 U/kg isophane insulin at 8:00 pm (Humulin NPH, Eli Lilly) for 2 weeks. The rats were not fasted, and their blood glucose levels were also measured again just before sacrificing. Animals were sacrificed under high dose anesthesia (ketamine/xylazine) at the end of the 2-week insulin-treatment period, and their liver tissues were carefully dissected and immediately stored at -80 ºC for further analysis. A diagram illustrating the experimental timeline is given in Fig. 1. All procedures used in this study were approved by the Ankara University Ethics Committee for Animal Experiments.
Fig. 1.
Schematic figure illustrating the timeline of the experimental protocol. The abbreviations of the figure include STZ (streptozotocin); U (Unit); i.p. (intraperitoneal)
Preparation of microsomes
The rat liver tissues were rapidly excised, weighed, and homogenized with 1.15% potassium chloride (KCI) (w/v) at 250 x g in an ice-cold bath. The resulting microsomal pellets were centrifuged at 11 000 x g for 25 min. The supernatant fractions were centrifuged again at 108 000 x g for 60 min. The pellets of ultracentrifugation were homogenized with 20% glycerol and were stored at -80 ºC until use.
Determination of protein concentration
Total protein levels of the prepared liver microsomes were determined by the method of Lowry et al. [24]. Briefly, proteins reacted with copper ions and reduced with the folin-ciocalteu reagent in alkaline medium and the absorbance of the coloured product was then measured at 750 nm, using bovine serum albumin as a standard protein.
Determination of hepatic pNP-hydroxylase activity (CYP2E1)
Microsomal CYP2E1 (p-Nitrophenol-2-hydroxylase) activity was assayed, as described by Reinke and Moyer [25] with some modifications [26]. CYP2E1 activity was determined with CYP2E1 hydroxylates p-nitrophenol (pNP), its 2-hydroxylation to form of 4-Nitrocatechol was measured by spectrophotometrically. 1 mL of reaction medium contains microsomal protein, pNP as a substrate, 400 mM, Tris buffer (pH 6.8), 100 mM glucose-6-phosphate, and cofactor including 20 mM NADP+, 100 mM magnesium chloride (MgCI2), 400 mM potassium phosphate buffer (pH 7.8) and 500 U/0.15 mL (Units per millilitre) glucose-6-phosphate dehydrogenase. The reaction medium (Tris buffer, pNP, and microsomal protein) was added to test tubes placed in a shaking water bath at 37 ºC, and the reaction was then initiated by the addition of NADPH-generating system. The samples were allowed to incubate at 37 ºC for 30 min in a shaking water bath. At the end of the incubation, each reaction was terminated by the addition of 500 µl of 0.75 N (v/v) perchloric acid, then centrifuged at 7 300 x g for 45 min to remove denatured proteins in tubes. 250 µl of 2 m NaOH was added to each test tubes to complete the ionization of 4-nitrocatechol which formed in the reaction. Once the color change observed in the tubes, the reaction medium was centrifuged again at 7 300 x g for 15 min for the removal of NaOH precipitates. Following the last centrifugation, the absorbance was rapidly read at a wavelength of 546 nm by spectrophotometrically.
Determination of hepatic EROD activity (CYP1A1)
CYP1A1 activity (7-ethoxyresorufin O-deethylase, EROD) in microsomes was determined as previously described by Burke et al. [27]. 7-Ethoxyresorufin is a substrate in which CYP1A1 transforms into resorufin measured fluorometrically. 1 mL of reaction medium contains microsomal protein, 7-ethoxyresorufin as a substrate, 10 mM albumin, 50 mM Tris.HCl buffer (pH: 7.8), 100 mM glucose-6-phosphate, 400 mM Tris buffer (pH: 6.8), and cofactor including 20 mM NADP+, 100 mM MgCI2, 400 mM potassium phosphate buffer (pH 7.8) and 500 U/ 0.15 mL glucose-6-phosphate dehydrogenase. The reaction medium (Tris buffer, 7-ethoxyresorufin, and microsomal protein) was added to test tubes taken into a shaking water bath at 37 ºC, and the reaction was then initiated by the addition of NADPH-generating system to each test tube. The samples were allowed to incubate at 37 ºC for 5 min in a shaking water bath. At the end of the incubation, each reaction was terminated by the addition of 3 mL ice-cold methanol, then centrifuged at 7 000 x g for 20 min to remove denatured proteins in tubes. Following the centrifugation, 3 mL of supernatant was taken into another tube, and the absorbance was measured at the excitation wavelength of 538 nm and an emission wavelength of 587 nm by fluorometrically.
Determination of liver lipid peroxidation levels
Microsomal lipid peroxidation (LPO) levels were determined with thiobarbituric acid reactive substances (TBARS) spectrophotometrically as described by Wills and Bishayee [28–30]. 1 mL of reaction medium contains microsomal protein, 100 mM potassium phosphate buffer (pH: 7.4), 0.02 mM Fe++, 9 mM KCl, 400 mM Tris buffer (pH 6.8), 100 mM glucose-6-phosphate, and cofactor including 20 mM NADP+, 100 mM MgCI2, 400 mM potassium phosphate buffer (pH 7.8) and 500 U/0.15 mL glucose-6-phosphate dehydrogenase. The reaction medium was added to test tubes placed in a shaking water bath at 37 ºC, and the reaction was then initiated by the addition of NADPH-generating system. The samples were allowed to incubate at 37 ºC for 30 min in a shaking water bath. At the end of the incubation, each reaction was stopped by the addition of 500 µl of 25% trichloroacetic acid (TCA), then centrifuged at 7 000 x g for 20 min to remove denatured proteins in tubes. After centrifugation, 1 mL of supernatant was taken into another tube. 0.5 mL of thiobarbituric acid (TBA) was added to each test tube, boiled for 20 min in a hot water bath. Following the boiling, the absorbance was read at a wavelength of 530 nm spectrophotometrically.
Data Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) version 23. The data were expressed as the means ± SD (standard deviation). Three groups of data were compared with one-way ANOVA followed by the non-parametric Mann-Whitney U test. After Mann-Whitney U, the non-parametric Kruskal-Wallis H test was used for the multicomparison. p < 0.05 was considered statistically significant.
Results
Physical and physiological parameters in diabetic state
The blood glucose levels of control and insulin-treated diabetic rats were about 116.8 mg/dL and 93.72 mg/dL, respectively. However, in diabetic rats, the blood glucose levels were approximately 445 mg/dL, corresponding 3.8- and 4.7-fold statistically significant (p < 0.05) increase compared to controls and insulin-treated diabetic rats, respectively. As a characteristic of diabetes, we observed remarkable (p < 0.05) weight loss in diabetic rats compared to insulin-treated diabetic rats. However, no significant change in body weights was noted between diabetic rats and controls. The data of the changes in body weight and blood glucose levels before sacrifice are shown in Table 1.
Table 1.
The body weights and blood glucose levels of the control, diabetic and insulin-treated diabetic rats
| Control | Diabetic | Insulin-treated Diabetic | |
|---|---|---|---|
| Blood glucose levels (mg/dL) | 116.8 ± 9.1 | 445.0 ± 32.9* | 93.72 ± 12.2 o |
| Body weight (g) | 335.4 ± 48.5 | 295.7 ± 30.7 | 344.0 ± 24.5 o |
All the values are represented as means ± SD; n = 05 for the control group, n = 11 for the diabetic rats, and n = 11 for the diabetic rats treated with insulin. Significant changes are expressed as *(p < 0.05) in comparison with control group; O (p < 0.05) in comparison with the diabetic group
Cytochrome P450 isoforms activity in liver microsomes of sprague dawley rats
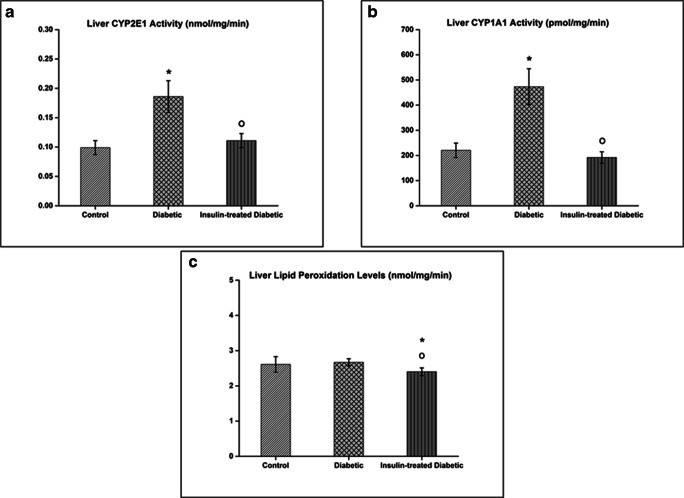
In diabetic rats, CYP1A1 activity showed 2.14- and 2.45-fold marked increase compared to control and insulin-treated diabetic rats, respectively (p < 0.05). The optimized method for measuring CYP2E1 activity was also previously reported in our group [26]. The present results indicated the statistically significant 1.88- and 1.68-fold induced liver CYP2E1 activity in diabetic rats compared to controls and insulin-treated diabetic rats, respectively (p < 0.05). However, hepatic CYP1A1 and CYP2E1 activities in diabetic rats were normalized by the treatment of insulin. The data are shown in Fig. 2.
Fig. 2.
Hepatic CYP2E1 (a) and CYP1A1 (b) activities and lipid peroxidation (c) levels in the controls and treatment groups of Sprague Dawley rats. Results are presented as means ± SD; n = 05 for the control group, n = 11 for the diabetic rats, and n = 11 for the diabetic rats treated with insulin. Significant changes are expressed as *(p < 0.05) in comparison with the control group; O (p < 0.05) in comparison with the diabetic group
Lipid peroxidation levels in liver microsomes of sprague dawley rats
In insulin-treated diabetic rats, LPO levels are statistically significant 0.92- and 0.89-fold decrease compared to control and diabetic group, respectively (p < 0.05). Interestingly, no marked difference was noted between diabetic rats and controls. These results demonstrated that insulin was able to decrease the levels of LPO (Fig. 2).
Discussion
Diabetes mellitus is an endocrine-metabolic disorder characterized by hyperglycaemia resulting from defects in insulin secretion or impairment in the body’s ability to use insulin efficiently. Its mechanisms have long been studied to develop more efficient therapeutic strategies, but effective approaches to preventing and treating diabetes are still rather limited. Oxidative stress may be one of the primary mechanisms by which its increased level in different tissues such as the liver, pancreas, kidney, and brain can trigger the development of diabetes and diabetic complications [31]. Furthermore, there also might be a correlation between diabetes and lipid peroxidation that is a major harmful consequence of increased oxidative stress [32]. On the other hand, the liver, which has a unique role in the glucose homeostasis, can be severely affected by diabetic state, and the impairment of liver functions such as fatty liver disease, hepatocellular carcinoma, cirrhosis, and hepatic enzymes alterations may occur [15]. Hepatic CYP450s enzymes could be regulated by diabetes due to its pathological conditions, such as hyperglycaemia or other molecules associated with the disease [1]. Among CYP450s, CYP1A1 and CYP2E1 are two of the most potent CYP isozymes to generate ROS [4, 21, 33]. In this regard, the induction of CYP1A1 and CYP2E1 activities may have severe consequences related to oxidative damage [34].
In the present study, after streptozotocin treatment, a marked increase was shown in diabetic rats’ blood glucose levels versus controls. The average blood glucose levels of insulin-treated diabetic rats were not significantly (p < 0.05) different from control rats and there was a statistically (p < 0.05) remarkable increase in the average body weight of insulin-treated diabetic rats in comparison to diabetic rats, indicating that insulin therapy is effective to normalize blood glucose levels and triggers the weight gain [35].
In our work, a significant increase in hepatic CYP1A1 and CYP2E1 activities in diabetic rats was noted. These findings are in accordance with Shankar et al. [36] and Haider Raza et al.’s [1] works that showed an increment in CYP1A1 and CYP2E1 enzyme activities compared to controls in STZ-induced diabetic male Sprague-Dawley and Wistar rats, respectively. However, Shankar and co-workers also reported that there is no significant change in CYP2E1 activity and also a decrease in CYP1A1 activity in STZ-induced diabetic male Swiss Webster mice compared to non-diabetic mice, suggesting highlights the importance of the inter-species in terms of alterations in these enzyme activities under diabetic condition. Besides the diversity within species, an increase in CYP1A1 and CYP2E1 activity could be attributed to the increased ketone bodies in diabetic rats [36]. As an alternative energy source, ketone bodies are produced by the oxidation of fatty acids in the liver, and insulin deficiency can lead to hyperlipidaemia as well as hyperketonaemia. Since ketone bodies are one of the substrates of the CYP2E1, their augmented production may trigger an increase in CYP2E1 activity [22, 37–39]. Nevertheless, the changes in the level of ketone bodies cannot be sufficient to explain the alterations in the activities of CYP450s. In diabetic conditions, lipids, carbohydrates, and hormones such as insulin, glucagon, leptin, and growth hormone and testosterone may also attribute to changes in CYP450s activity [40].
The insulin treatment normalized hepatic CYP1A1 and CYP2E1 activities in diabetic rats, suggesting that insulin has a regulatory effect on CYP450s. However, Borbás and his colleagues showed that with insulin therapy, increased CYP1A1 activity in diabetic rats was restored to the level of control rats, while CYP2E1 activity did not change either in STZ-induced diabetic rats or insulin-treated STZ-induced diabetic rats [23]. Although at first glance our findings seem to contradict with their results in terms of CYP2E1 activity, they observed a major standard deviation that could be attributed to the inter-individual variation of sensitivity to insulin, and also a significant decrease in CYP2E1 activity had been shown in insulin-treated non-diabetic rats. This may be due to the suppression effect of insulin on CYP2E1 transcription to prevent oxidative stress [23, 41]. Indeed, several potential mechanisms could be offered about the normalizing effect of insulin on hepatic CYP450s. In diabetes, growth hormone deficiency may occur, leading to alterations in CYP450s [42], and insulin-related normalization of growth hormone-mediated procedures could be one of the possible explanations for changes in CYP450s activity [43].
In contrast to the results of the present study, Bukan et al. and Mukherjee et al. have reported elevated levels of hepatic lipid peroxidation in diabetic male albino rats compared to the control group [44, 45]. In Bukan et al.’s study, hepatic malondialdehyde (MDA) levels, which is one of the LPO products used to determine oxidative stress, in 24-week old diabetic rats were significantly higher than 8-week old diabetic and control rats, suggesting the possible effect of aging on the formation of LPO [44]. However, in our work, hepatic LPO levels did not significantly change in diabetic rats compared to the control group. The possible reasons for our findings may be that reactive oxygen species cannot have reached the level of causing lipid peroxidation, and the liver is less susceptible to oxidative stress [1]. On the other hand, it is worth noting that after insulin treatment, lipid peroxidation levels in insulin-treated diabetic rats were statistically remarkable (p < 0.05) decrease compared to those of diabetic rats and controls, indicating that insulin has a crucial role in reducing hepatic lipid peroxidation levels. Nonetheless, in addition to its harmful effects, LPO could be beneficial to perform some physiologically basic functions as a regulator of gene expression and mediator of cellular signalling [46]. Therefore, very low lipid peroxidation levels below those of physiological levels may cause undesirable consequences in the normal functioning of the body. In the current study, although hepatic LPO level in insulin-treated diabetic rats was lower than that of controls, it was not a level far below the physiological limit, which may cause the disruption of cell metabolism.
In conclusion, to the best of our knowledge, here we report for the first time the effect of insulin treatment on hepatic CYP1A1 and CYP2E1 activities by comparing them with hepatic lipid peroxidation levels in diabetic conditions. Increased hepatic CYP1A1 and CYP2E1 activities in STZ-induced diabetic rats suggest that oxidative stress is one of the potential mechanisms in the development of diabetes and its complications. However, CYP1A1- and CYP2E1-dependent ROS production in diabetes should be at a certain level for the formation of lipid peroxidation. Another important conclusion is that the regulatory hormone insulin can reduce the harmful effects of oxidative stress in diabetes by restoring the increased hepatic CYP450s activity and decreasing the lipid peroxidation levels. These findings may be useful for understanding the effects of diabetes on hepatic functions in terms of CYP450s activity related to lipid peroxidation levels. Yet, further studies are needed to elucidate the role of oxidative stress in diabetes.
Limitations
When compared to humans, differences in rodent physiology is the primary limitation of this study. Another limitation is that the present research was conducted only on the liver. Therefore, these parameters should also be studied in other tissues related to diabetes and its complications. On the other hand, the increase in oxidative stress in diabetes can be established a more explicit cause-effect relationship by measuring antioxidant enzymes such as glutathione peroxidase, glutathione reductase, and superoxide dismutase in the liver as well as other tissues. The final limitation is that the experimental duration of diabetes and insulin treatment can be extended to evaluate the long-term effects of the disease in terms of oxidative stress.
Abbreviations
- CYP1A1
cytochrome P450 1A1
- CYP2E1
cytochrome P450 2E1
- CYP450
cytochrome P450 monooxygenase
- EROD
7-ethoxyresorufinO-deethylase
- Fe++
Iron(II)
- KCI
potassiumchloride
- LPO
lipid peroxidation
- M
molarity
- MDA
malondialdehyde
- MgCI2
magnesiumchloride
- mins
minutes
- mM
millimolar
- N
normality
- NADP+
nicotinamideadenine dinucleotide phosphate
- NaOH
sodiumhydroxide
- nm
nanometer
- ºC
degreesCelsius
- pNP
p-nitrophenol
- ROS
reactive oxygen species
- TBARS
thiobarbituricacid reactive substances
- Tris.HCl
trishydrochloride
- U/mL
unitspermillilitre
- v/v
volume/volume
- w/v
weight/volume
- µL
microliter
Authors’ contributions
G.K., R.B., and B.C.E. contributed to the concept, design, and analysis of data. E.A.I. has carried out all injections. G.K. and R.B. performed the experimental works. G.K., R.B., and B.C.E. participated in drafting the article.
Data svailability
Data are accessible upon request from the authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures used in this study was approved by the Ankara University Local Ethics Committee for Animal Experiments (2010-56-283).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raza H, Ahmed I, John A, Sharma AK. Modulation of xenobiotic metabolism and oxidative stress in chronic streptozotocin-induced diabetic rats fed with Momordica charantia fruit extract. J Biochem Mol Toxicol. 2000;14(3):131–9. doi: 10.1002/(SICI)1099-0461(2000)14:3<131::AID-JBT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53(1):185–94. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 3.König M, Bulik S, Holzhütter H-G Quantifying the contribution of the liver to glucose homeostasis: a detailed kinetic model of human hepatic glucose metabolism. PLoS Comput Biol. 2012;8(6):e1002577. doi: 10.1371/journal.pcbi.1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niedowicz DM, Daleke DL The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43(2):289–330. doi: 10.1385/cbb:43:2:289. [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum AI. Role of Cytochrome P450 and oxidative stress in alcohol-induced liver injury. React Oxygen Species. 2017;4(11):303–19. [Google Scholar]
- 6.Memişoğulları R. Diyabette serbest radikallerin rolü ve antioksidanların etkisi düzce. Tıp Fakültesi Dergisi. 2005;3:30–9. [Google Scholar]
- 7.Dib M, Garrel C, Favier A, Robin V, Desnuelle C. Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol. 2002;249(4):367–74. doi: 10.1007/s004150200025. [DOI] [PubMed] [Google Scholar]
- 8.Hall ED, Wang JA, Bosken JM, Singh IN. Lipid peroxidation in brain or spinal cord mitochondria after injury. J Bioenerg Biomembr. 2016;48(2):169–74. doi: 10.1007/s10863-015-9600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manikandan P, Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug Targets. 2018;19(1):38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich FP. The 1992 Bernard B. Brodie Award Lecture. Bioactivation and detoxication of toxic and carcinogenic chemicals. Drug Metab Dispos Biol Fate Chem. 1993;21(1):1–6. [PubMed] [Google Scholar]
- 11.Puga A, Nebert DW, McKinnon RA, Menon AG. Genetic polymorphisms in human drug-metabolizing enzymes: potential uses of reverse genetics to identify genes of toxicological relevance. Crit Rev Toxicol. 1997;27(2):199–222. doi: 10.3109/10408449709021619. [DOI] [PubMed] [Google Scholar]
- 12.Stiborova M, Martinek V, Rydlova H, Koblas T, Hodek P. Expression of cytochrome P450 1A1 and its contribution to oxidation of a potential human carcinogen 1-phenylazo-2-naphthol (Sudan I) in human livers. Cancer Lett. 2005;220(2):145–54. doi: 10.1016/j.canlet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–38. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Curr Drug Metab. 2012;13(9):1327–44. doi: 10.2174/138920012803341302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55(1):77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113(1):88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Park HS, Park MH, Suh SH, Pang MG. Oxidative stress-related gene polymorphism and the risk of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;119(1):42–6. doi: 10.1016/j.ejogrb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 20.Walsh AA, Szklarz GD, Scott EE. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J Biol Chem. 2013;288(18):12932–43. doi: 10.1074/jbc.M113.452953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Chen M, Yan YE, Xiao FQ, Pan XL, Wang H. Growth retardation of fetal rats exposed to nicotine in utero: possible involvement of CYP1A1, CYP2E1, and P-glycoprotein. Environ Toxicol. 2009;24(1):33–42. doi: 10.1002/tox.20391. [DOI] [PubMed] [Google Scholar]
- 22.Oh SJ, Choi JM, Yun KU, Oh JM, Kwak HC, Oh JG, Lee KS, Kim BH, Heo TH, Kim SK. Hepatic expression of cytochrome P450 in type 2 diabetic Goto-Kakizaki rats. Chemico-Biol Interact. 2012;195(3):173–9. doi: 10.1016/j.cbi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Borbas T, Benko B, Dalmadi B, Szabo I, Tihanyi K. Insulin in flavin-containing monooxygenase regulation. Flavin-containing monooxygenase and cytochrome P450 activities in experimental diabetes. Eur J Pharm Sci. 2006;28(1–2):51–8. doi: 10.1016/j.ejps.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 25.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos Biol Fate Chem. 1985;13(5):548–52. [PubMed] [Google Scholar]
- 26.Başaran R, Özdamar ER, Eke BC. A study on the optimization of CYP2E1 enzyme activity in C57Bl/6 mouse brain and liver. FABAD J Pharm Sci. 2012;37(4):191–6. [Google Scholar]
- 27.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985;34(18):3337–45. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 28.Wills ED. Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J. 1969;113(2):333–41. doi: 10.1042/bj1130333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishayee S, Balasubramanian AS Lipid peroxide formation in rat brain. J Neurochem. 1971;18(6):909–20. doi: 10.1111/j.1471-4159.1971.tb12020.x. [DOI] [PubMed] [Google Scholar]
- 30.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99(3):667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadi G, Baloglu MC, Pektas MB. Differential gene expression in liver tissues of streptozotocin-induced diabetic rats in response to resveratrol treatment. PLoS One. 2015;10(4):e0124968. doi: 10.1371/journal.pone.0124968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza Bastos A, Graves DT, de Melo Loureiro AP, Junior CR, Corbi SC, Frizzera F, Scarel-Caminaga RM, Camara NO, Andriankaja OM, Hiyane MI, Orrico SR Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J Diabetes Complicat. 2016;30(8):1593–9. doi: 10.1016/j.jdiacomp.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Q, Li JK, Li F, Li RG, Zhan GQ, Li G, Du WX, Tan HB. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2015;21(7):2058–66. doi: 10.3748/wjg.v21.i7.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morel Y, de Waziers I, Barouki R. A repressive cross-regulation between catalytic and promoter activities of the CYP1A1 and CYP2E1 genes: role of H(2)O(2) Mol Pharmacol. 2000;57(6):1158–64. [PubMed] [Google Scholar]
- 35.Skovso S, Damgaard J, Fels JJ, Olsen GS, Wolf XA, Rolin B, Holst JJ. (2015) Effects of insulin therapy on weight gain and fat distribution in the HF/HS-STZ rat model of type 2 diabetes. Int J Obes. 2005;39(10):1531–1538. doi: 10.1038/ijo.2015.92. [DOI] [PubMed] [Google Scholar]
- 36.Shankar K, Vaidya VS, Apte UM, Manautou JE, Ronis MJ, Bucci TJ, Mehendale HM. Type 1 diabetic mice are protected from acetaminophen hepatotoxicity. Toxicol Sci. 2003;73(2):220–34. doi: 10.1093/toxsci/kfg059. [DOI] [PubMed] [Google Scholar]
- 37.Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016;95:268–77. doi: 10.1016/j.freeradbiomed.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnett C, Petrides L, Wilson J, Flatt P, Ioannides C. Induction of rat hepatic mixed-function oxidases by acetone and other physiological ketones: their role in diabetes-induced changes in cytochrome P450 proteins. Xenobiotica. 1992;22(12):1441–50. doi: 10.3109/00498259209056694. [DOI] [PubMed] [Google Scholar]
- 39.Lieber CS. Cyp2e1: from ash to nash. Hepatol Res. 2004;28(1):1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Kim CH, Lee JY, Jeon JS, Kim MJ, Chae SH, Kim HC, Oh SJ, Kim SK. Hepatic expression of cytochrome P450 in Zucker diabetic fatty rats. Food Chem Toxicol. 2016;96:244–53. doi: 10.1016/j.fct.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35(2):263–73. doi: 10.1053/jhep.2002.30691. [DOI] [PubMed] [Google Scholar]
- 42.Shimojo N, Ishizaki T, Imaoka S, Funae Y, Fujii S, Okuda K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol. 1993;46(4):621–7. doi: 10.1016/0006-2952(93)90547-A. [DOI] [PubMed] [Google Scholar]
- 43.Yamazoe Y, Murayama N, Shimada M, Yamauchi K, Kato R. Cytochrome P450 in livers of diabetic rats: regulation by growth hormone and insulin. Arch Biochem Biophys. 1989;268(2):567–75. doi: 10.1016/0003-9861(89)90324-X. [DOI] [PubMed] [Google Scholar]
- 44.Bukan N, Sancak B, Yavuz Ö, Koca C, Tutkun F, Özçelikay AT, Altan N. Lipid peroxidation and scavenging enzyme levels in the liver of streptozotocin-induced diabetic rats. Indian J Biochem Biophys. 2003;40:447–50. [PubMed] [Google Scholar]
- 45.Mukherjee B, Mukherjee JR, Chatterjee M. Lipid peroxidation, glutathione levels and changes in glutathione-related enzyme activities in streptozotocin-induced diabetic rats. Immunol Cell Biol. 1994;72(2):109–14. doi: 10.1038/icb.1994.17. [DOI] [PubMed] [Google Scholar]
- 46.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47(5):469–84. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are accessible upon request from the authors.