Abstract
Propose
This study aims to systematically review the randomized controlled trials that address the effectiveness and safety of herbal medicine in patients with type 1 diabetes.
Methods
The Cochrane Library (latest issue); MEDLINE (until recent); EMBASE (until recent); AMED (Allied and Complementary Medicine Database) (until recent); and CINHAL (until recent) were searched electronically for the identification of trials until October 2019. Articles were initially screened based on title and abstract and then by full text by two independent authors. References of retrieved studies were hand-searched for further studies. Risk of bias was assessed according to the Cochrane handbook of systematic reviews of interventions. The results were summarized into GRADE (grading of recommendations, assessment, development and evaluation) tables. No meta-analysis was applicable as only one study was found for each intervention.
Results
Four RCTs were finally included in the systematic review with an overall moderate quality of conduct and low quality of reporting. The sample sizes were very small. The results of these RCTs show that cinnamon pills and Berberine/Silymarine compound capsules may decrease blood glucose indices from baseline, while fenugreek seeds and fig leaf decoction do not show any statistically significant effect.
Conclusions
The evidence is scarce and no recommendations can be made based on current evidence. Further trials with more rigorous methodology and stronger quality of reporting are needed to make conclusions.
Keywords: Medicinal plants, Herbal medicine, Type-1 diabetes, Systematic review
Introduction
Diabetes mellitus is a chronic metabolic disorder that is marked by hyperglycemia resulting from defective insulin secretion, insulin action or both. Short-term complications include diabetic ketoacidosis and the nonketotic hyperosmolar syndrome. These complications are commonly categorized as microvascular (retinopathy, nephropathy, neuropathy) and macrovascular (coronary artery disease, cerebrovascular disease, and peripheral vascular disease). Diabetes and its complications are a major cause of morbidity and mortality worldwide. Many of the long-term complications can be prevented by appropriate blood glucose control [1, 2].
Type 1 diabetes mellitus is a clinical syndrome in which the destruction of the pancreatic islet -cells leads to progressive insulin deficiency and hyperglycemia that in turn result in microvascular complications such as retinopathy, nephropathy, and neuropathy as well as macrovascular complications like coronary heart disease [3–5]. It comprises about 5%–10% of all people with diabetes [6]. In contrast, type 2 diabetes is mainly caused by peripheral insulin resistance and a relative insulin deficiency which can be associated with nutrition and certain dietary elements [7, 8].
Insulin is the first-line treatment in patients with type 1 diabetes. However, insulin does not address the main pathological mechanism of type-1 diabetes which is immune-mediated beta-cell damage and insulin secretion; instead, it is a replacement for inadequate endogenous insulin [9–12]. Medicinal plants have historically been used and have been shown to be popular among patients with diabetes especially in Asian countries. At least about one third of patients with diabetes use herbal preparations as part of their treatment [13–19]. Several articles have reviewed these herbal preparations assumed to have antidiabetic properties focusing on their proposed mechanisms of action rather than reviewing the experimental evidence on their clinical effectiveness [20–23]. Systematic reviews are available that assess the therapeutic effectiveness of herbal medicine in type 2 diabetes [24, 25]. Nevertheless, the difference in pathogenesis and nature between type1 and type 2 diabetes warrants different management approaches in these patients. The experimental evidence for the effectiveness and safety of herbal interventions in type 1 diabetes has not been studied systematically. This study aims to systematically review the randomized controlled trials that address the effectiveness and safety of herbal medicine in patients with type 1 diabetes.
Methods
This systematic review was constructed according to the PRISMA guideline [26].
Eligibility criteria
Types of studies: Only randomized controlled trials were included in this systematic review.
Types of participants: Children and adults with type 1 diabetes were included. The diagnosis had to be established based on the appropriate criteria at the time the study was conducted.
Types of interventions: Any type of herbal medicines including extract from herbs, single herb or a compound of herbs alone or along with Insulin. No limitation was applied for the mode of administration or the method of preparation of the herbal medicine. Studies on medicinal herbs plus other therapies such as a holistic treatment, for example, herbs plus cupping or acupuncture, were excluded. The control intervention included placebo that should have been a drug without an effect on blood glucose levels.
Types of outcome measures
Primary outcomes: glycemic control (as measured by glycated hemoglobin levels (HbA1c) and fasting blood glucose levels); adverse events (for example liver toxicity, kidney damage).
Secondary outcomes: diabetes complications (for example, neuropathy, retinopathy, nephropathy, sexual dysfunction); health-related quality-of-life; all-cause mortality; costs.
Search and information sources
The Cochrane Library (latest issue); MEDLINE (until recent); EMBASE (until recent); AMED (Allied and Complementary Medicine Database) (until recent); Google Scholar and CINHAL (until recent) were searched electronically for the identification of trials. The sample search terms are presented in Table 1. Hawaiian herbal medicine was a search term because Hawaiian plant flora contain some of the most distinctive plant species in the world [1–3].
Table 1.
PubMed search terms
| (((“Type 1 Diabetes Mellitus”[tiab] OR (Diabetes Mellitus[tiab] AND Type I[tiab]) OR “Type 1 Diabetes”[tiab] OR (Diabetes[tiab] AND Type 1[tiab]) OR (Diabetes Mellitus[tiab] AND Ketosis-Prone[tiab]) OR (Diabetes Mellitus[tiab] AND Ketosis Prone[tiab]) OR “Ketosis-Prone Diabetes Mellitus”[tiab] OR (Diabetes[tiab] AND Autoimmune[tiab]) OR “Autoimmune Diabetes”[tiab] OR (Diabetes Mellitus[tiab] AND Juvenile-Onset[tiab]) OR (Diabetes Mellitus[tiab] AND Juvenile Onset[tiab]) OR “Juvenile-Onset Diabetes Mellitus” OR “Juvenile-Onset Diabetes” OR (Diabetes[tiab] AND Juvenile-Onset[tiab]) OR “Juvenile Onset Diabetes”[tiab] OR (Diabetes Mellitus[tiab] AND Insulin-Dependent[tiab]) OR (Diabetes Mellitus[tiab] AND Insulin Dependent[tiab]) OR “Insulin-Dependent Diabetes Mellitus”[tiab] OR IDDM OR “Insulin Dependent Diabetes Mellitus 1”[tiab] OR (Diabetes Mellitus[tiab] AND Brittle[tiab]) OR “Brittle Diabetes Mellitus”[tiab] OR (Diabetes Mellitus[tiab] AND Sudden-Onset[tiab]) OR (Diabetes Mellitus[tiab] AND Sudden Onset[tiab]) OR “Sudden-Onset Diabetes Mellitus”[tiab]) AND (Medicine[tiab] AND Herbal[tiab]) OR “Hawaiian Herbal Medicine”[tiab] OR “Hawaiian Herbal Medicines”[tiab] OR (Herbal Medicine[tiab] AND Hawaiian[tiab]) OR (Herbal Medicines[tiab] AND Hawaiian[tiab]) OR (medicinal[tiab] AND herb[tiab]) OR (medicinal[tiab] AND plant[tiab]) OR (medicinal[tiab] AND herbs[tiab]) OR (medicinal[tiab] AND plants[tiab]) OR (Medicine[tiab] AND Hawaiian Herbal[tiab]) OR (Medicines[tiab] AND Herbal[tiab]) OR Herbalism[tiab]) AND (“glycemic control”[tiab] OR “adverse events”[tiab] OR “diabetic complication”[tiab] OR “Fasting plasma glucose”[tiab] OR “glycated hemoglobin”[tiab] OR “Diabetes-Related Complications”[tiab] OR “Complications of Diabetes Mellitus”[tiab] OR “Diabetes Mellitus Complication”[tiab] OR “Life Quality”[tiab] OR “Health-Related Quality Of Life”[tiab] OR “Health Related Quality Of Life”[tiab] OR HRQOL[tiab] OR (Cost [tiab] AND Health Care[tiab]) OR “Health Care Cost”[tiab] OR “Health Costs”[tiab] OR “Healthcare Costs”[tiab] OR “Medical Care Costs”[tiab] OR “Treatment Costs”[tiab])) |
All related components of lipid profile including [Ketosis-Prone Diabetes Mellitus, Autoimmune Diabetes, Juvenile-Onset Diabetes Mellitus, Insulin-Dependent Diabetes Mellitus, Brittle Diabetes Mellitus and IDDM] added to searched queries based on scientific MeSH terms, EMTREE or the keywords. The results were limited to human subjects and refined for patients with type 1 diabetes. Reference Manager bibliographic software was used to manage searched citations. Duplicate entries were searched by considering the title of the published papers, authors, the year of publication, and specifications of the sources types. Based on a 2003 systematic review of herbal preparations for diabetes, another search was performed with additional search terms including all of the proposed herbal preparations [24]. Citation tracking was performed for all of the retrieved studies. Authors of relevant identified studies and other experts (authors of reviews) were contacted in order to obtain additional references, unpublished trials, or ongoing trials. We attempted to identify additional studies by searching the reference lists of included trials. A librarian in health sciences was involved in all the stages of the search.
Study selection
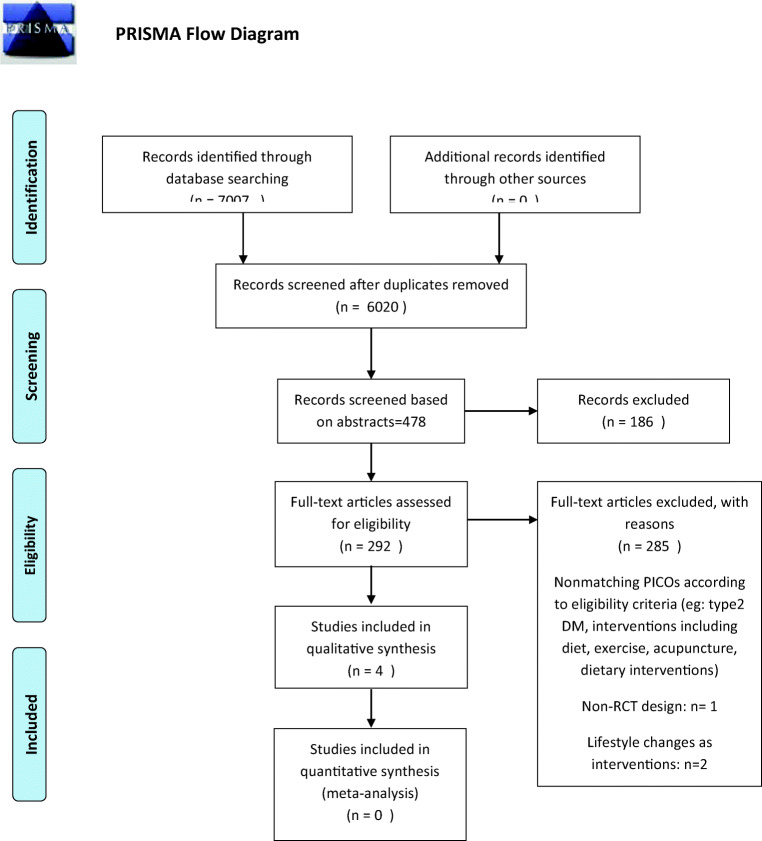
Two authors independently determined the studies for eligibility by scanning the title, abstract or both sections of all the retrieved records. All potentially relevant investigated as full text. Any differences in opinion were resolved by a third author. If resolving disagreement was not possible authors would be contacted for clarification (Fig. 1).
Fig. 1.
PRISMA Flow diagram
Data collection process
For studies that fulfilled our inclusion criteria, two authors independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies). Any disagreements were resolved by discussion or, if required, by a third party. Any relevant missing information on the trial were sought from the original author(s) of the article, if required.
Quality assessment
Quality of reporting was assessed using CONSORT and its specific extension for herbal interventions. The Cochrane Risk of Bias tool was used to assess risk of bias within studies. Special issues regarding cross-over design in 2 of the trials were addressed according to the Cochrane handbook of systematic reviews. Two authors assessed risk of bias within each trial independently. Any disagreement was resolved by consensus and by consultation of a third party in case of persistent disagreement. The GRADE (Grading of recommendations, Assessment, Dissemination, and Evaluation) approach was used to assess quality of evidence for all study outcomes in summary of findings tables.
Summary measures
Results of each individual study were extracted based on predetermined characteristics and outcomes as defined by the study protocol. These templates included Study ID/year, Study Design, Target population, age range, Mean baseline BMI, Mean number of years since the diagnosis of DM1, Sample size, Number of Drop-outs, Baseline insulin regimen, Study duration, Description of Intervention, and Description of comparison. Mean differences were used to analyze the effect sizes of continuous outcomes. The effect sizes for dichotomous data were expressed in terms of relative risks or odds ratio.
How does the review differ from the protocol?
The search strategy was changed to include a secondary search that included the list of proposed medicinal plants used in the treatment of type-1 diabetes. The length of follow up was not limited to the predetermined protocol because of the scarcity of trials in the field and that this limitation would result in even fewer studies being included and reviewed. The outcome measures that are finally reported here were also extended according to the retrieved studies. The included studies did not have a sufficient description of their diagnostic criteria. No studies had long-term follow-up and thus our predetermined secondary outcomes were not assessable in any of the studies.
Results
Study selection
Among 7007 search results initially retrieved, 6006 remained after removing duplicates. 478 Studies were assessed for eligibility based on their titles and abstracts. Two-hundred-and-ninety-two studies that were assessed based on their full-texts, many studies were excluded because they included patients with type 2 diabetes. Two studies were excluded as they assessed the role of exercise and dietary interventions in patients with type1 diabetes rather than specific herbal medications [27, 28]. One clinical trial was excluded as it had used a metabolomics approach and did not assess our predetermined outcomes. Eventually, four studies were included to the systematic review.
Study characteristics
Only four studies met the criteria to be included to this systematic review; these included RCTs aimed to evaluate the effect of a) Cinnamomum zeylanicum(cinnamon) pills, b) Ficus carica (fig)leaf decoction, c) Berberis aristata/Sylibum marianum capsules, and d) Trigonella foenum-graecum(fenugreek) powder added to local bread, in glycemic control among patients with type 1 diabetes mellitus in comparison to control groups.
The studies included different patient age groups; The study on cinnamon included 72 adolescents (mean age: 14 ± 1.4 years) [29], the study on fenugreek included 10 adults(mean age: 22.7 ± 2.7 years) [30], the Berberis/Sylibum trial included 85 adults with type-1 diabetes(mean age: 29.8 ± 7.2) [31], and the Fig-leaf study included 10 patients with type-1 diabetes(mean age:29 ± 2.2) [29].The trial length and design varied among the studies. The study characteristics are presented in detail in Table 2.
Table 2.
Table of Study Characteristics
| Study ID/year | Study Design | Target population | Mean baseline BMI | Mean number of years since the diagnosis of DM1 | Sample size | Drop-out | Baseline insulin regimen | Study duration | Intervention | Comparison | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
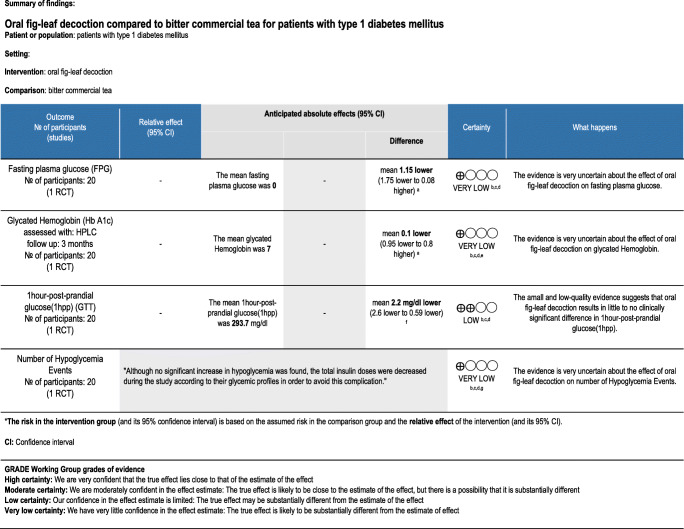
| Serraclara, {Serraclara, 1998 #79}1998 | Randomized controlled trial(RCT)-cross-over design | Adults, Mean age: 29 ± 2.2 years | Not reported | 9 ± 6.3 years | 10 | 2 | two daily doses of subcutaneous insulin | 1 month per intervention(total 2 months) | Fig-leaf decoction | Non-sweet commercial tea |
7 day/week glycemic profiles, Post-prandial glucose (2 h-pp) c-peptide Glycated hemoglobin(Hb A1c) Episodes of clinical hypogly-caemia Total daily insulin intake |
|
ALTSCHULER, {Altschuler, 2007 #80} 2007 |
Double blind-RCT | Adolescents, Mean age: 14.7± 1.4 | Not reported | 7.1 ± 4.6 years | 72 | 15 | Basal/bolus | 90 days | Cinnamon pills | Placebo (lactose) pills |
A1C, total daily insulin intake, adverse events |
| Sharma, {Sharma, 1990 #78}1990 | Randomized controlled trial- cross-over design |
Mean age: 22.7 ± 2.7 |
17 ± 0.97 | 7 years | 10 | 0 | Fixed dose of insulin not sufficient to control BS+ 40-50 g increase in daily carbohydrate intake | 20 days(total)/ two ten-day periods for each group | Fenugreek powder added to local bread | No Fenugreek powder |
Oral glucose tolerance test 24-h urinary glucose Areas under the glucose and insulin concentration curves |
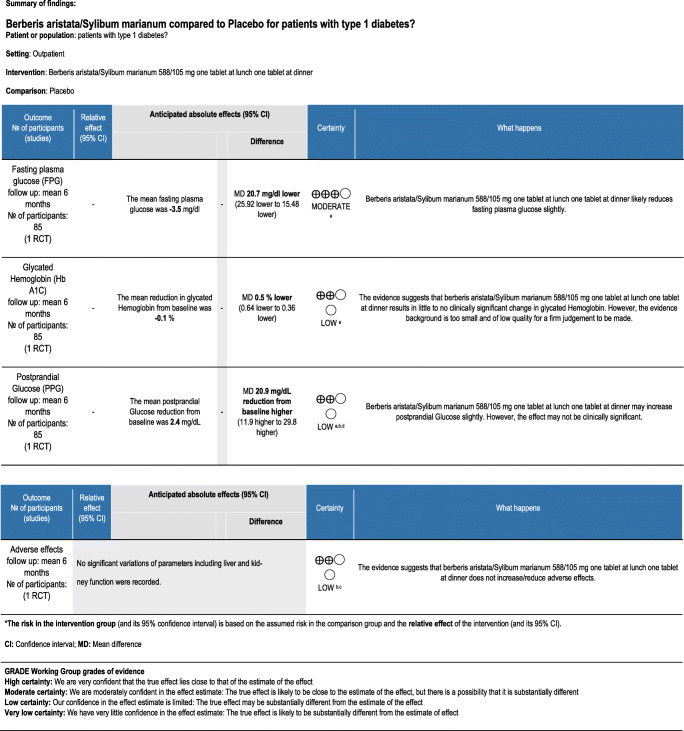
| Derosa, {Derosa, 2016 #57} | Double-blind Randomized placebo controlled trial |
Adults ≥18 years old Mean age: 29.8 ± 7.2 |
22.6 ± 1.8 | 15.1 ± 2.8 years | 85 | 4 | Meal-time rapid-acting insulin analogs with or without basal log-acting insulin analogs | 6-months | Berberis aristata/Sylibum marianum 588/105 mg, 1 tablet at lunch and 1 tablet at dinner, for six months | identical, opaque, white capsules(placebo) |
Reduction of insulin dose necessary to reach adequate glycemic control Glycated hemoglobin(Hb A1c) Fasting plasma glucose (FPG), Post-prandial glucose (PPG), |
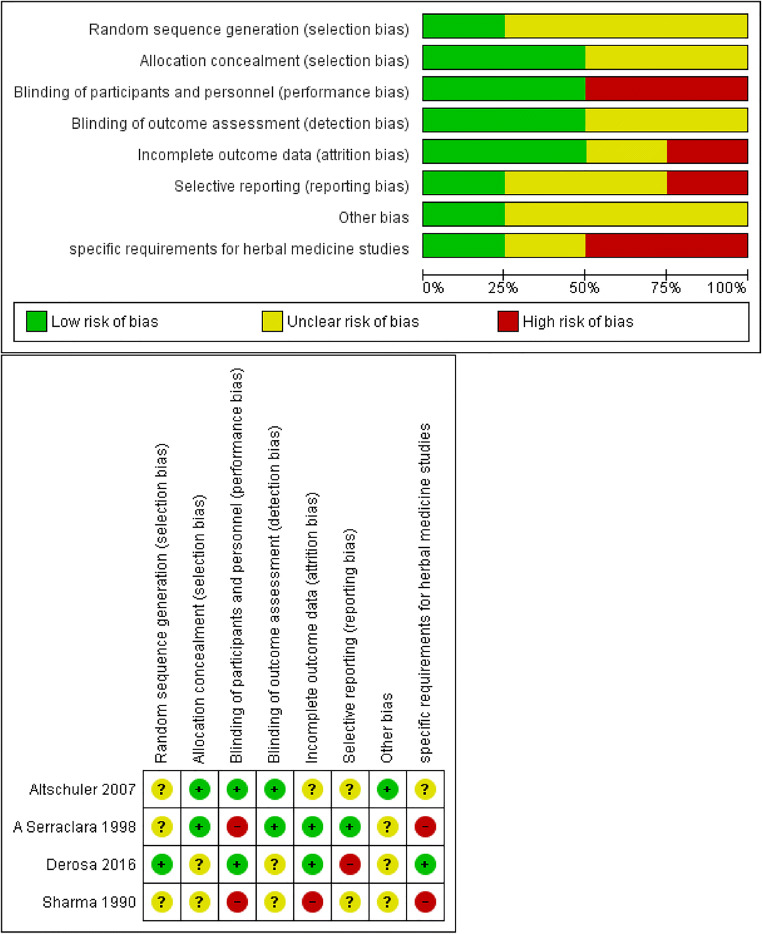
Risk of bias assessment
The risk of bias varied significantly from unclear, and low to high. The quality of reporting was suboptimal. This was especially a concern since they did not have sufficient description of their methods including their methods of randomization and sequence generation, as well as blinding which made it difficult for the researchers to draw clear judgements about risk of bias.
Three studies had unclear risk of bias in random sequence generation due to inadequate reporting of randomization methods [29, 30, 32]. Two studies had unclear risk of bias in allocation concealment [30, 31]. Two studies had high risk of bias in blinding of participants due to the distinguishable nature and appearance of their interventions from the controls. [30, 32] One study had a high rate of drop-outs, but this was unlikely to affect its results as the researchers had appropriately used an intention-to-treat analysis [29]. Outcome data were reported completely in all of the studies except one which had an inadequate reporting of adverse effects; Risk of selective reporting was unclear for two studies since protocols were not available [29, 30], and high for one study which was suspected of inadequate reporting of adverse effects. [31]
A high risk of bias was suspected in two of the studies regarding the special issues concerning use of herbal interventions, the plant accreditation and details of preparation of the intervention [30, 32].
One study used lactose pills (which contain glucose) as placebo which could potentially affect the results. Because of the opposite direction of the results of the study relative to the perceived bias, the risk of bias from this issue is perceived as low [30].
There was a concern regarding one of the studies’ choice of HbA1C as an outcome in the study with a cross-over design and a one-month intervention. This study did not have a wash-out period. This concern was based on the fact that HbA1C is describe to assess the state of glycemic control in the past three months. Thus, it is difficult to ascertain whether the results at the end of the 2-month period are due to the second intervention alone or the combination of the first and the second intervention.
Risk of bias within each study is presented in Fig. 2.
Fig. 2.
Risk of bias within the studies
Results of individual studies
The four included studies evaluated the effects of four different herbal interventions. Three of the four studies showed statistically significant effects for the interventions. The results of individual studies are presented in Table 3.
Table 3.
GRADE summary of findings tables
Summary of findings and effectiveness of treatments
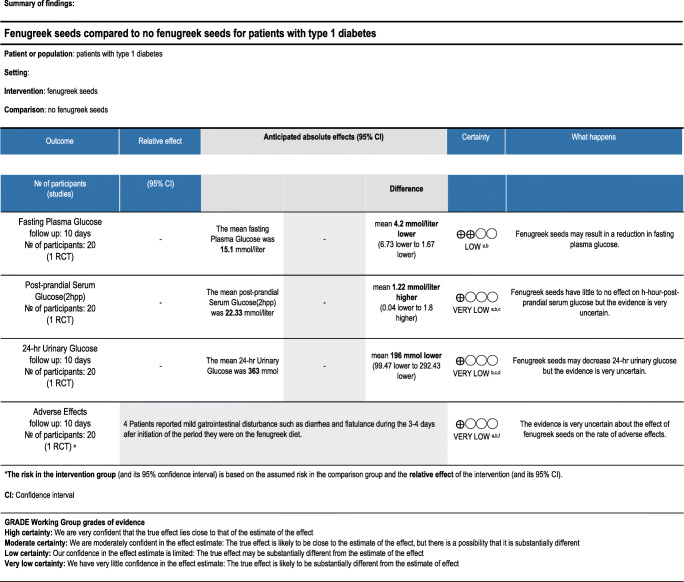
The quality of the evidence regarding the use of fenugreek in patients with type 1 diabetes was low to very low for all of the outcomes. Only one trial was retrieved which assessed 20 patients. Patients in the fenugreek group had a significantly increased mean urinary glucose and fasting plasma glucose after 10 days. However, Indices showing glucose tolerance were slightly improved in the group of patients taking fenugreek. There were six patients in the fenugreek group experiencing minor gastrointestinal adverse effects compared to no people in the placebo group. More details are presented in Table 3. [30]
One trial compared the effects of oral fig leaf decoction with placebo in patients with type 1 diabetes. The quality of evidence differed between outcomes and is presented in Table 3. There was no statistically significant difference in Hb A1C and fasting plasma glucose between the group taking oral fig leaf decoction and the placebo group. Glucose tolerance improved significantly in the group of patients taking oral fig leaf in comparison to placebo. Although the rate of hypoglycemia did not differ between groups, the insulin dosage had been reduced to prevent such an event [32].
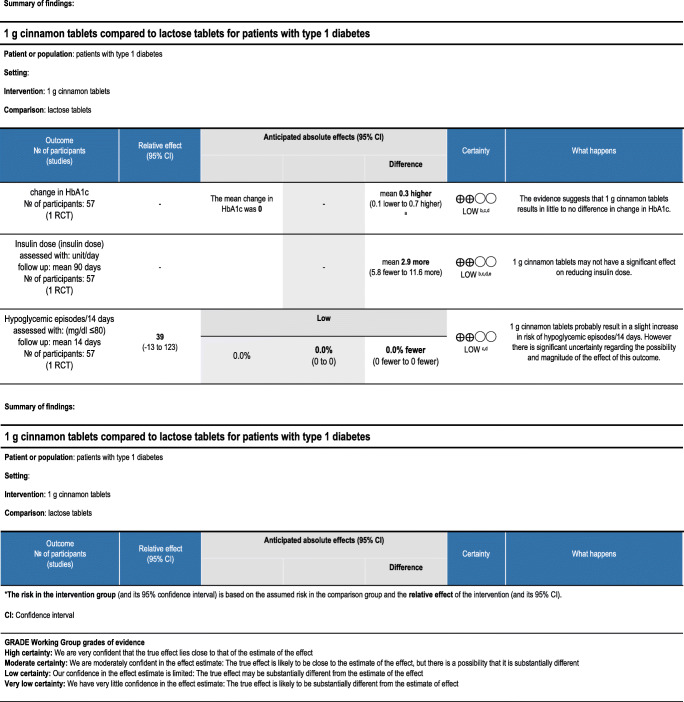
The overall quality of evidence for the use of cinnamon in type 1 diabetes is low based on a single RCT including 57 patients. The effect of cinnamon on HbA1c was not statistically significant. The change in insulin dosage varied among participants and was not statistically different either. Neither was the number of hypoglycemic episodes [29].
One randomized trial examined the effect of B. aristata/S. marianum combination on glycemic control and adverse events in type-1 diabetes. There is moderate certainty that B. aristata/S. marianum pills may slightly improve fasting plasma glucose. The degree of certainty is low for evidence regarding the effect of this herbal preparation on glycated hemoglobin, postprandial glycemia, and adverse effects. In addition, although these outcomes were statistically improved from baseline, the difference in improvement from baseline was not significantly different between groups [31].
The findings were summarized into GRADE tables as presented below (Tables 3).
Discussion
This systematic review aimed to evaluate the efficacy of herbal medicine as a complementary treatment for type 1 diabetes. We found only four randomized studies which used fenugreek, cinnamon, oral fig leaf decoction, and Berberine-Silymarine compound.
Although the mechanism of fenugreek (Trigonella. f) action in humans is not understood, animal studies have revealed the ability of dietary fiber including fenugreek seeds to delay gastric emptying, and suppress release of gastric inhibitory peptides and insulinotropic hormones. In another study, fenugreek oil was shown to restore a normal architecture of pancreas and kidney in comparison with the control group in alloxan-induced diabetic rats [33]. Although several studies showed a hypoglycemic effect for Fenugreek, the majority of these studies were carried out in animals and in people with type 2 diabetes and cannot be applied clinically for patients with type 1 diabetes. The only published randomized study for the efficacy of fenugreek in humans, only showed a significant effect on fasting plasma glucose levels; however, the mechanism of potential effect in humans with type one diabetes cannot be inferred yet and the only human trial on patients with type one diabetes did not show significant positive effects on glycemic control.
Cinnamon (Cinnamomum. z) is one of the oldest herbal plants, a widely used spice that is postulated to improve glucose control by promoting phosphorylation of insulin receptor tyrosine kinase which increases insulin sensitivity. In addition, cinnamon extracts have been implicated in the production of transcription factors that modify insulin resistance through possible modulation of GLUT4 transporters which mobilize glucose into the cell. In animal models of type 1 diabetes, a hot water extract of cinnamon has been shown to exert insulin independent catabolic effects on serum glucose via upregulation of mitochondrial uncoupling protein-1(UCP-1) in brown adipose tissue in streptozocin-induced type-1 diabetes in rats [34]. In another study, it showed insulinotropic activity comparable to glibenclamide in rat models of type 1 diabetes and cultured pancreatic beta cell lines [35]. Several studies in people with type 2 diabetes show that cinnamon reduces fasting blood glucose levels but has no effect on other glycemic parameters or markers of insulin resistance; the studies have significant heterogeneity which limits their direct clinical application [36]. The only randomized study in humans which was included in this review had low quality and did not show a significant effect for cinnamon on glycemic control in patients with type 1 diabetes [29]. This may be due to the small sample size, different pathogenic mechanisms between type 1 and type 2 diabetes, and the inadequate duration of the only available study to detect any possible effect. Another possible reason may be the relatively lower doses-1 g/day used in this study in comparison with studies in type 2 diabetes that have used up to 6 g/day of cinnamon [37–39]; Nevertheless, the direction of the results of this trial-although not significant- is towards a harmful effect of cinnamon and higher doses could probably increase this difference toward a significant level rather than show a benefit for cinnamon [29].
Ficus. c belongs to the Moraceae family which is popularly called “figs” and has been used for diabetes among other ailments since ancient times [40]. The Ficus species contain bioactive metabolites such as phenolic acids, flavonoids, coumarins, vitamin E, sterols, glycosides, tannins, alkaloids, and triterpenoids [41–43]. Several in-vivo animal studies have shown the beneficial effects of these compounds on insulin secretion and blood glucose [41, 43–47]. One study showed that fig leaf extract may inhibit beta cell apoptosis in mice via inhibition of the AMPK/JNK/caspase-3 signaling pathway and its anti-oxidant properties [48]. Other proposed mechanisms of effect based on experimental evidence include decreased intestinal glucose absorption, affecting glucose homeostasis, and enhancement of glucose uptake by the skeletal muscle [40]. A recent RCT showed beneficial effects of two fig fruit extracts on glycemic and insulinemic indices in healthy adults [49]. The only randomized trial in the literature did not show significant effects of oral fig leaf decoction on blood glucose in patients with type-1 diabetes. However, the results were statistically significant for its effect on insulin resistance [32].
Berberine is an alkaloid isolated from the roots, rhizomes, and stem barks of many plants including B. aristata. In traditional Eastern medicine, it has been used to treat diabetes, inflammation, and infections for more than 1000 years. It has two distinct modes of action acting paradoxically on different mechanisms involved in diabetes pathophysiology. It has been shown that it increases insulin sensitivity and consequently reduces blood insulin levels in patients with type 2 diabetes. On the other hand, in type 1 diabetes and more advanced stages of type 2 diabetes where poor beta cell function is involved, it is able to increase insulin secretion via promoting beta cell regeneration, insulin expression, antioxidant enzyme activity, and decreasing lipid peroxidation [31, 50–55]. These effects have been studied in a few trials on patients with type 2 diabetes the results of which are consistent with the findings from animal studies [52–55]. Silymarine is the powder extract from seeds from a plant called S. marianum which is from the Asteracea(sunflower) family [56]. It has been proposed to have independent antidiabetic effects and has also been shown to increase the bioavailability and effectiveness of B. aristata extract when used in combination [51–57]. However, these effects have only partially been addressed in the only randomized study on patients with type 1 diabetes which showed a small but statistically significant reduction in daily insulin dose from baseline and in comparison to controls for patients receiving a combination of Berbesris asristata/Silybum marianum.
Limitations
The studies in this systematic review were of short duration and thus many patient-important outcomes such as the rate of diabetes complications were not addressed. Safety of these interventions cannot be assessed because adverse outcomes have not been reported adequately in the included studies. In addition, most of the studies evaluated patients with well-controlled diabetes and without complications which may not be representative of all patients with type-1 diabetes. There is special concern for use of these herbs in pregnant women with diabetes due to potential risk of toxicity and scarcity of evidence.
Although every attempt was made to include the names of all the commonly proposed plants in the search terms, the diversity of the medicinal herbs traditionally proposed for use in diabetes makes it difficult to ensure a comprehensive literature search that will retrieve all the possible RCTs.
There are too few randomized clinical trials addressing the use of medicinal plants in the management of type-1 diabetes. A meta-analysis could not be done because the herbal preparations(interventions) varied among the few included studies and no series of studies evaluated the effect of the same plant on the study outcomes.
Conclusions
There is insufficient evidence to draw conclusions about the efficacy of fenugreek, Berberine/Silymarine compound capsule, oral fig leaf decoction and cinnamon for glycemic control in type 1 diabetes. In addition, the evidence is inconclusive regarding the optimal doses and methods of preparations of these herbs and their safety in these patients.
There is insufficient evidence to support the use of medicinal plants in patients with type 1 diabetes. We suggest that more robust randomized studies be conducted in this field that measure more clinically-important outcomes such as real-time continuous glucose monitoring and long-term diabetes-related adverse effects, cover longer follow-ups and address outcome measures that better clarify the pharmacodynamics and the pharmacokinetics of commonly used medicinal herbs.
Acknowledgements
Authors would like to thank clinical librarians to retrieve information from literature.
Abbreviation
- MEDLINE
Medical Literature Analysis and Retrieval System Online,
- AMED
Allied and Complementary Medicine Database,
- Embase
Excerpta Medica Database,
- CONSORT
Consolidated Standards of Reporting Trials,
- CINAHL
Cumulative Index of Nursing and Allied Health Literature,
- BMI
Body Mass Index,
- DM
Diabetes Mellitus.
Authors’ contributions
HRB, MEK and JK Conceived and designed this study. FB, YM and RVA searched and retrieved all information. FB, YM and HRB conducted the risk of bias assessment and evaluation of study results. All authors contributed to writing the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by the Iran University of Medical Sciences (IUMS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farzaneh Barzkar, Email: barzkarfarzane@gmail.com.
Hamid Reza Baradaran, Email: baradaran.hr@iums.ac.ir.
Mohammad Ebrahim Khamseh, Email: khamseh.m@iums.ac.ir.
Roya Vesal Azad, Email: royavesal@yahoo.com.
Jalil Koohpayehzadeh, Email: jkoohpayezadeh@yahoo.com.
Yousef Moradi, Email: Yousefmoradi211@yahoo.com.
References
- 1.American DA. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 2.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1(suppl 1):S5–20. [DOI] [PubMed]
- 3.Ardalani H, Jandaghi P, Meraji A, Moghadam MH. The effect of Cynara scolymus on blood pressure and BMI in hypertensive patients: a randomized, double-blind, placebo-controlled, clinical trial. Complementary medicine research. 2020;27(1):40–46. doi: 10.1159/000502280. [DOI] [PubMed] [Google Scholar]
- 4.Ardalani H, Moghadam MH, Rahimi R, Soltani J, Mozayanimonfared A, Moradi M, Azizi A. Sumac as a novel adjunctive treatment in hypertension: a randomized, double-blind, placebo-controlled clinical trial. RSC Adv. 2016;6(14):11507–11512. [Google Scholar]
- 5.Jandaghi P, Noroozi M, Ardalani H, Alipour M. Lemon balm: a promising herbal therapy for patients with borderline hyperlipidemia—a randomized double-blind placebo-controlled clinical trial. Complementary therapies in medicine. 2016;26:136–140. doi: 10.1016/j.ctim.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Arykan D, Sivrikaya SK, Olgun N. Complementary alternative medicine use in children with type 1 diabetes mellitus in Erzurum, Turkey. J Clin Nurs. 2009;18(15):2136–2144. doi: 10.1111/j.1365-2702.2008.02464.x. [DOI] [PubMed] [Google Scholar]
- 7.Akhoundan M, Shadman Z, Jandaghi P, Aboeerad M, Larijani B, Jamshidi Z, Ardalani H, Khoshniat Nikoo M. The Association of Bread and Rice with metabolic factors in type 2 diabetic patients. PLoS One. 2016;11(12):e0167921. doi: 10.1371/journal.pone.0167921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and [beta]-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 9.Zalinska M, Szmigiero-Kawko M, Brandt A, Trzonkowski P, Mysliwiec M. [Insulin secretion in the early phase of type 1 diabetes mellitus (T1DM) and new hopes for maintaining it through therapy]. Pediatric endocrinology, diabetes, and metabolism. 2016;22(3). [DOI] [PubMed]
- 10.Vanikar AV, Trivedi HL, Thakkar UG. Stem cell therapy emerging as the key player in treating type 1 diabetes mellitus. Cytotherapy. 2016;18(9):1077–1086. doi: 10.1016/j.jcyt.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig B, Barthel A, Reichel A, Block NL, Ludwig S, Schally AV, et al. Modulation of the pancreatic islet-stress axis as a novel potential therapeutic target in diabetes mellitus. Vitam Horm. 2014;95:195–222. doi: 10.1016/B978-0-12-800174-5.00008-9. [DOI] [PubMed] [Google Scholar]
- 12.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial--type 1. Diabetes Care. 2005;28(5):1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 13.Kalra S, Kumar S, Kalra B, Agrawal N, Sharma A, Prusty V. Knowledge, attitudes and practices of type 1 diabetes patients regarding complementary and alternative medicine (CAM). 2010.
- 14.Hasan SS, Ahmed SI, Bukhari NI, Loon WC. Use of complementary and alternative medicine among patients with chronic diseases at outpatient clinics. Complement Ther Clin Pract. 2009;15(3):152–157. doi: 10.1016/j.ctcp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Kumar D, Bajaj S, Mehrotra R. Knowledge, attitude and practice of complementary and alternative medicines for diabetes. Public Health. 2006;120(8):705–711. doi: 10.1016/j.puhe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Moolasarn S, Sripa S, Kuessirikiet V, Sutawee K, Huasary J, Chaisila C, Chechom N, Sankan S. Usage of and cost of complementary/alternative medicine in diabetic patients. J Med Assoc Thail. 2005;88(11):1630–1637. [PubMed] [Google Scholar]
- 17.Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br J Clin Psychol. 1996;35(1):37–48. doi: 10.1111/j.2044-8260.1996.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 18.Manya K, Champion B, Dunning T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement Altern Med. 2012;12:2. doi: 10.1186/1472-6882-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabian E, Toscher S, Elmadfa I, Pieber TR. Use of complementary and alternative medicine supplements in patients with diabetes mellitus. Annals of nutrition & metabolism. 2011;58(2):101–108. doi: 10.1159/000326765. [DOI] [PubMed] [Google Scholar]
- 20.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 21.Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of momordica charantia: active constituents and modes of actions. The open medicinal chemistry journal. 2011;5(Suppl 2):70–77. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29(5):580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 23.El-Tantawy WH, Temraz A. Management of diabetes using herbal extracts: review. Arch Physiol Biochem. 2018;124(5):383–389. doi: 10.1080/13813455.2017.1419493. [DOI] [PubMed] [Google Scholar]
- 24.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26(4):1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 25.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. The Annals of Family Medicine. 2013;11(5):452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumer JH, Drakeford JA, Wadsworth J, Savage DC. Effects of dietary fibre and exercise on mid-morning diabetic control--a controlled trial. Arch Dis Child. 1982;57(12):905–909. doi: 10.1136/adc.57.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori TA, Vandongen R, Masarei JR, Rouse IL, Dunbar D. Comparison of diets supplemented with fish oil or olive oil on plasma lipoproteins in insulin-dependent diabetics. Metab Clin Exp. 1991;40(3):241–246. doi: 10.1016/0026-0495(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 29.Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30(4):813–816. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]
- 30.Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990;44(4):301–306. [PubMed] [Google Scholar]
- 31.Derosa G, D'Angelo A, Maffioli P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clinical nutrition (Edinburgh, Scotland). 2016;35(5):1091–5. [DOI] [PubMed]
- 32.Serraclara A, Hawkins F, Pérez C. Domínguez E, Campillo JE, Torres MaD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39(1):19–22. doi: 10.1016/s0168-8227(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 33.Hamden K, Masmoudi H, Carreau S, Elfeki A. Immunomodulatory, β-cell, and neuroprotective actions of fenugreek oil from alloxan-induced diabetes. Immunopharmacol Immunotoxicol. 2010;32(3):437–445. doi: 10.3109/08923970903490486. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T, et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74(12):2418–2425. doi: 10.1271/bbb.100453. [DOI] [PubMed] [Google Scholar]
- 35.Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact. 2010;186(1):72–81. doi: 10.1016/j.cbi.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Namazi N, Khodamoradi K, Khamechi SP, Heshmati J, Ayati MH, Larijani B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Complementary therapies in medicine. 2019;43:92–101. doi: 10.1016/j.ctim.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 38.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136(4):977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 39.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Investig. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 40.Deepa P, Sowndhararajan K, Kim S, Park SJ. A role of Ficus species in the management of diabetes mellitus: a review. J Ethnopharmacol. 2018;215:210–232. doi: 10.1016/j.jep.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 41.Perez C, Canal JR, Torres MD. Experimental diabetes treated with ficus carica extract: effect on oxidative stress parameters. Acta Diabetol. 2003;40(1):3–8. doi: 10.1007/s005920300001. [DOI] [PubMed] [Google Scholar]
- 42.Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol. 2014;52(11):1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 43.Belguith-Hadriche O, Ammar S, Contreras MDM, Fetoui H, Segura-Carretero A, El Feki A, et al. HPLC-DAD-QTOF-MS profiling of phenolics from leaf extracts of two Tunisian fig cultivars: Potential as a functional food. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;89:185–93. [DOI] [PubMed]
- 44.Canal JR, Torres MD, Romero A, Perez C. A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiol Hung. 2000;87(1):71–76. doi: 10.1556/APhysiol.87.2000.1.8. [DOI] [PubMed] [Google Scholar]
- 45.Perez C, Dominguez E, Canal JR, Campillo JE, Torres MD. Hypoglycaemic activity of an aqueous extract from ficus carica (fig tree) leaves in streptozotocin diabetic rats. Pharm Biol. 2000;38(3):181–186. doi: 10.1076/1388-0209(200007)3831-SFT181. [DOI] [PubMed] [Google Scholar]
- 46.Irudayaraj SS, Stalin A, Sunil C, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARgamma expression in type 2 diabetic rats. Chem Biol Interact. 2016;256:85–93. doi: 10.1016/j.cbi.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Stephen Irudayaraj S, Christudas S, Antony S, Duraipandiyan V, Naif Abdullah AD, Ignacimuthu S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and beta-cells in type 2 diabetic rats. Pharm Biol. 2017;55(1):1074–1081. doi: 10.1080/13880209.2017.1279671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Li Y, Ma P, Chen J, Xie W. Ficus carica leaves extract inhibited pancreatic beta-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;122:109689. [DOI] [PubMed]
- 49.Atkinson FS, Villar A, Mula A, Zangara A, Risco E, Smidt CR, et al. Abscisic Acid Standardized Fig (Ficus carica) Extracts Ameliorate Postprandial Glycemic and Insulinemic Responses in Healthy Adults. Nutrients. 2019;11(8). [DOI] [PMC free article] [PubMed]
- 50.Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606(1–3):262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 51.Oh YS. Plant-derived compounds targeting pancreatic Beta cells for the treatment of diabetes. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:629863. doi: 10.1155/2015/629863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes, metabolic syndrome and obesity: targets and therapy. 2012;5:213. [DOI] [PMC free article] [PubMed]
- 53.Di Pierro F, Putignano P, Villanova N, Montesi L, Moscatiello S, Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clinical pharmacology: advances and applications. 2013;5:167. doi: 10.2147/CPAA.S54308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Pierro F, Bellone I, Rapacioli G, Putignano P. Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes, metabolic syndrome and obesity: targets and therapy. 2015;8:89. [DOI] [PMC free article] [PubMed]
- 55.Derosa G, Bonaventura A, Bianchi L, Romano D, D'angelo A, Fogari E, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther. 2013;13(11):1495–1506. doi: 10.1517/14712598.2013.832751. [DOI] [PubMed] [Google Scholar]
- 56.Stolf AM, Cardoso CC, Acco A. Effects of Silymarin on diabetes mellitus complications: a review. Phytother Res. 2017;31(3):366–374. doi: 10.1002/ptr.5768. [DOI] [PubMed] [Google Scholar]
- 57.Soto C, Raya L, Perez J, Gonzalez I, Perez S. Silymarin induces expression of pancreatic Nkx6.1 transcription factor and beta-cells neogenesis in a pancreatectomy model. Molecules. 2014;19(4):4654–4668. doi: 10.3390/molecules19044654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.