Abstract
Background
Neuropathic pain is a complicated phenomenon in patients with diabetes. These patients have many problems, especially depression and Sleep disturbance. This study aimed to assess the effectiveness of Acceptance and Commitment Therapy on depression and Sleep disturbance in patients with painful diabetic neuropathy.
Methods
The current paper was conducted according to the clinical trial method with 50 participants. Participants were separated into intervention and control groups randomly. Based on the diagnosis of neurologists, all participants received standard medications to regulate neuropathic pain. The intervention group received ACT for eight sessions. The results were evaluated in the pre-test, post-test, and follow-up. The tools used were the Pittsburgh sleep quality index (PSQI) and Beck’s depression inventory. Statistical analysis includes descriptive statistics, and repeated-measures (ANOVA) conducted by SPSS (version 26) software. Results: Results showed that in the intervention group, the treatment led to improved depressive symptoms (F = 6.81, P < 0.05). Besides, for sleep quality, treatment in all subscales, except for the Hypnotic medicine subscale, significantly improved the intervention group’s situations. It was also observed that the overall quality of sleep in the ACT group showed a more significant improvement (P < 0.05). All the above results remained the same until the end of the follow-up period (P < 0.05).
Conclusion
ACT, as a complementary treatment, can improve the psychiatric symptoms and problems in people with neuropathic pain. Therefore, it is necessary to include psychotherapy services along with medical treatment in outpatient and hospitalization units.
Trial registration number
IRCT201802050388630N4. Registered in 02/05/2018.
Keywords: Acceptance and commitment therapy, Depression, Diabetes mellitus , Pain, Neuropathy, Sleep
Introduction
The International Association for the Study of Pain (IASP) has defined neuropathic pain as pain due to a lesion or disease caused by the somatosensory nervous system, especially the surrounding fibres (Aβ, Aδ and C fibres). Nearly 10 % of adults report neuropathic pain, and in diseases such as Diabetes mellitus and AIDS, the prevalence of this type of pain is reported to be 25–35% [1]. This pain dramatically reduces people’s quality of life and creates a wide range of disabilities in various functional dimensions for patients and their caregivers [1, 2].
Diabetes mellitus (DM) as one of the most common diseases [3], has a comorbidity rate of more than one-fourth of neuropathic pain [1]. DM has received particular attention as the most common metabolic disorder. The problems related to DM are not limited to the disease itself, but also has a wide range of medical (such as retinopathy, neuropathy, and diabetic foot ulcers) and psychological (reduced quality of life, disability, and emotional disorders) complications [4]. Besides, one of the main reasons for people living with diabetes inability to control blood glucose is the correlation of this disease with comorbid psychiatric problems. These comorbidities, along with other medical conditions, such as genetic predisposition and stressors resulting from disease, can lead to the failure of costly treatments involving large numbers of treatment staff [2, 4]. Among these comorbidities, depression plays a vital role. In fact, in addition to other medical and behavioral problems created by depression, it reduces self-care behaviors (an essential component in the successful treatment of diabetes) in individuals. The prevalence rate of depression in people living with diabetes is about four times more than non-diabetics. Thus, no adequate treatment would be achieved without considering depression improvement during comprehensive diabetes treatment [5–7]. On the other hand, disturbances in the quality and quantity of sleep are among the most important causes of functional decline in neuropathic pain, especially diabetic neuropathy [8]. Various sleep disorders, including hypersomnia, sleep deprivation, and poor sleep quality, may increase the risk of developing DM and even its severity in older people with diabetes [8]. Specifically, in a study, it was found that sleep quality has a significant negative correlation with the increasing probability of diabetics, and diabetes itself can damage the quality and duration of sleep [8]. Besides, according to a meta-analysis in this regard, various sleep disorders are seen in a range of 27 to 69% in people with diabetes, while these disorders affect about 2 to 4% of the healthy population. Moreover, sleep problems in people living with diabetes lead to a decrease in treatment outcomes in these individuals [9].
Among people with diabetes, people with diabetic neuropathy experience these problems more severely. One of the most important causes of sleep problems in people with diabetes is neuropathy [8]. Besides, mood problems such as anxiety and depression in people with diabetic neuropathy have a higher rate and lead to a reduction in treatment outcomes [2]. Besides, depression can interfere with the perception of neuropathic pain and lead to pathological pain perception [2]. Also, neuropathic pain leads to reduced social and occupational performance and promotes widespread disability [2, 3].
Despite these problems, neuropathy is still one of the most challenging areas in the treatment of people living with diabetes. Various pharmacies have so far failed to reduce neuropathic pain in these individuals [10] optimally, so special attention needs to be paid to this group of people. Since “pain” is a bio-psycho-social factor, it is important to consider psychological therapies in these people.
The role of psychotherapy and behavioral medicine in improving DM and its comorbid symptoms has been gaining increasing attention in the last decade. However, this area is in its early stages of development [11]. Despite differences in treatment style (intensive care and hospital care versus self-care besides medical supervision), to manage both the acute and chronic effects of diabetes on life, it is necessary to combine medical and psychological therapies [5, 11].
One of the treatments that have received particular attention in behavioral medicine in recent years is Acceptance and Commitment Therapy (ACT). According to this treatment, physical and mental pain is a natural part of life. While pain may be a part of the human condition, the human being is not the same as suffering and pain. The inevitable acceptance of pain and struggle, including physical illness and distress, is the first step toward psychological flexibility. Therefore, the main goal of ACT treatment is to increase patients’ psychological flexibility about thoughts, feelings, and behaviors. Psychological flexibility is the ability to accept one’s own complicated personal feelings and thoughts while at the same time trying to have a meaningful life according to one’s values. Even though this treatment is much newer than the other conventional psychotherapies, especially Cognitive Behavior Therapies (CBT), and it has advantages over them.
Most importantly, it is difficult for older people with diabetes to change their cognitions. Moreover, the existence of pain and psychological and physical limitations caused by the disease is so pervasive and severe that with these treatments, it is not possible to gradually begin to correct the cognitions and change the attitude of life in these people. Acceptance and commitment therapy, instead, seek to increase a person’s resilience and create a purposeful and value-based life in individuals. Finally, unlike CBT, where progress disorder by disorder and is not suitable for comorbid disorders, acceptance, and commitment therapy serves as a metacognitive therapy targeting the core of vulnerability [8, 12–14]. Therefore, the present study, as the first clinical trial, examines the effectiveness of acceptance and commitment therapy on depression and sleep quality in diabetic neuropathic pain patients.
Materials and methods
Trial design
The present study is a randomized controlled trial consisting of three pre-test-post-test and three-month follow-up periods. Interventions are offered simultaneously to both groups.
Participants
Participants included 149 people living with diabetes with pain admitted to the neurology department of Farabi Hospital in Kermanshah, whose symptoms were similar to the diagnostic criteria. Neurologist made the diagnosis of diabetic neuropathic pain. Preliminary assessments were performed by independent assessors to meet the inclusion and exclusion criteria. The inclusion criteria included (1) Diagnosis of diabetic neuropathy by a neurologist; (2) age of 35 to 65 years, (3) score of at least 13 in depression scales (4) having at least primary school education, (5) have not anxiety due to personal problems such as divorce and the death of a close relative, (6)lack of other psychiatric disorders based on DSM_5, (7)no history of psychiatric disorders, somatoform disorders,(8) and have not receive any type of psychotherapy in the past 6 months.
Exclusion criteria included (1) unwillingness to continue the sessions; (2) Absence of more than two sessions; (3) Initiation secondary psychotherapy separately, and (4) Detection abuse of drug use during the intervention and follow-up during the research.
Papulation
In order to determine the sample size, the following formula was used, and the previous studies were considered to estimate the number of to examine the number of participants. The samples in this study were 50 persons, interested and qualified individuals entered the study and were randomly assigned to intervention and control groups of 25 individuals. This randomization sequence was produced whit computer version of a random block size that divided participants to either the ACT group or the control group in a 1:1 ratio.
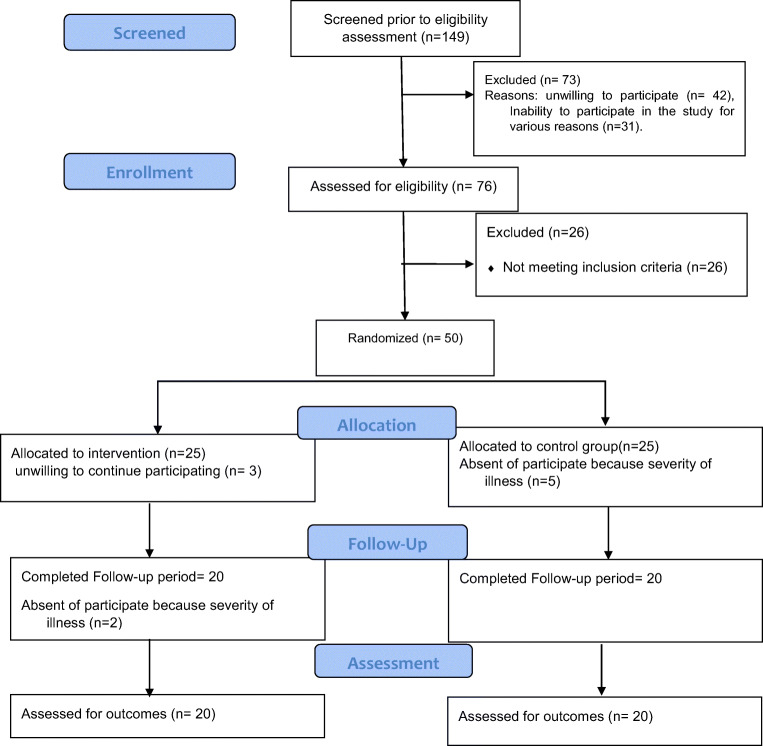
Finally, 50 patients were selected after screenings through diagnosis made by a specialist. After the screening stage, a consent form was provided to participants informing them about the research process, and a clinical interview session was conducted by a clinical psychologist to achieve their consent. During the study, in the intervention group, three people did not continue the study due to unwillingness to participate and two because of the severity of the disease, and the researchers’ calls and follow-ups were unanswered. In the control group, five participates unable to attend the sessions due to the severity of the disease, so the study was performed on 40 subjects. Since this study was a part of a more extensive country-wide study, the education and marital status variables are equal. Figure 1 shows the flowchart diagram of the study.
Fig. 1.
Participant flow diagram
Interventions
The intervention group received acceptance and commitment therapy [13], and the control group received common psycho-education intervention in medical centers under the treatment protocol established by the Ministry of Health of Iran for 8 weeks. At the beginning of the study, the research tools were examined using the mentioned scales. Acceptance and commitment therapy sessions (Table 1) and routine therapy in the form of multiple randomized blocks (1 session of 90 min per week) were held by two trained clinical psychologists (one expert in ACT for the intervention group and the other in behavioral cognition for the control group). Both treatments were the same in terms of the number of sessions and the number of individuals in each block. Of course, both groups continued their medical treatment under the supervision of a specialist.
Table 1.
A summary of contents of ACT sessions
| An introductory session: building a proper connection with the aim of making rapport and building trust. | |
| Session 1: Building a therapeutic relationship; an introduction to acceptance and commitment therapy and its purposes. Explaining the rules of sessions. Presenting data about chronic pain in neuropathy and the various types of it. | |
| Sessions 2 and 3: Introduction of creative helplessness and make a connection between this concept with escape and relieving the pain in a real-life situation. Discussion of ineffective control strategies and learning their uselessness. Introduce the concept of acceptance and its association with the resistance, denial and internal despair | |
| A booklet is given about the content of the session and planning related the exercises for people to practice at home. | |
| Sessions 4–5: Teaching the concept of mindfulness and three mental states (wise, reasonable, emotional) and their relations with pain. Training two bunches of mindful abilities. First, include viewing, participation, and description. Second, include a non-judgmental posture and general self-consciousness. Emphasis on living in the present moment by mindful living style. | |
| Session 6: Make an association between mindfulness, yoga and meditation with perception of the pain. Introducing distraction strategies with 5skills include contributing to using away, awareness activities, comparisons, thoughts, and emotional regulation. | |
| Session 7: Teaching ways for identifying values and keep behaviors in line with these values (committed action). Make a group discussion about cognitive defusion and sleep problems. They were learning about problem-solving techniques. | |
| Session 8: interpersonal effectiveness practice. Participants discovering assertiveness abilities about getting help from others. Other skills include Verbal and Non-verbal communication, Problem-solving and decision-making skills. |
Outcomes
Beck’s depression inventory (BDI)
The questionnaire, which includes 21 items, was designed to measure feedback and symptoms of depression. The reliability coefficient varied from 0.48 to 0.86. In studies related to Iranian culture, the reliability of the Beck inventory was reported as high in different values with the mean of 0.85 [12].
Pittsburgh sleep quality index (PSQI)
The questionnaire contains 18 items about variables related to sleep quality in the past month. The overall score ranges for sleep quality are from zero (Completely healthy sleep quality) to 21 (completely impaired sleep quality). An overall score above 5 means poor sleep quality. This questionnaire has a reliability of 0.83. Also in the Iranian community, this questionnaire had a Cronbach’s alpha of 0.77 and a sensitivity coefficient of 0.94 [15].
Blinding
Both groups were blinded to research. Patients were informed of participating in the study and the purpose of evaluating the efficacy of the treatment but were unaware of the other group. In the week following the end of treatment, post-test and 12 weeks after the end of treatment, follow-up was performed by the hospital’s psychologists with a master’s degree in clinical psychology. It should be noted that these investigators were not aware of the type of interventions received, the groups, the research goals, and hypotheses.
Statistical method
Analyzing the demographic information (frequency, mean, and standard deviation) and repeated measures ANOVA was performed by SPSS 26 software. P value was considered 0.05 and the results were interpreted with 95% probability considering the novelty of this field and possibility of unknown variables that will detected with more researches.
Therapeutic coherence
In each of the sessions, the voice was recorded with the patients’ agreement and the content of the sessions was matched with the protocol. The results showed that the about 83–95% of the protocols was performed in each session.
Ethical considerations
Before conducting the research, a meeting was held to justify all patients as a group. In this session, ethical issues and explanations about the research were given to the individuals. Then, a consent form was obtained for all people. Participants were assured that their results and identities would be kept confidential and under no circumstances, their medical information will be given to any of the people outside the medical staff. The present study was reviewed before the implementation by the Research Ethics Committee of Kermanshah University of Medical Sciences (Ethics Code (IR). KUMS.REC.1397.939) Besides, this study with the code (IRCT201802050388630N4) has been registered in Iranian Registry of Clinical Trials (IRCT).
Results
The demographic and research variables at all three periods were shown in Table 2. As it seen, there are no significant differences in these variables (at pretest) (p > 0.1). However, in the post-test and follow-up stages, the results showed the significant effectiveness of the intervention group compared to the control group (p < 0.05).
Table 2.
Mean and standard deviation of demographic variables in the groups at the test phases
| Variable | Intervention group Mean (SD) | Control Group Mean (SD) | p value | |
|---|---|---|---|---|
| Age | (9.32)58.6 | (9.7)56.03 | 0.86b | |
| Sex | Woman a | 9 (45%) | 12(60%) | 0.52 |
| Man a | 11(55%) | 8(40%) | ||
| Depression | Pre-test | 27.6(6.4) | 28.1(7.5) | 0.82 |
| Post-test | 20.4(5.6) | 25.1(7.2) | 0.025* | |
| Follow-up | 20.7(5.9) | 25.1(5.4) | 0.021* | |
To examine the research hypothesis, normality tests were used for each of the variables. Below, each variable was examined separately.
Depression
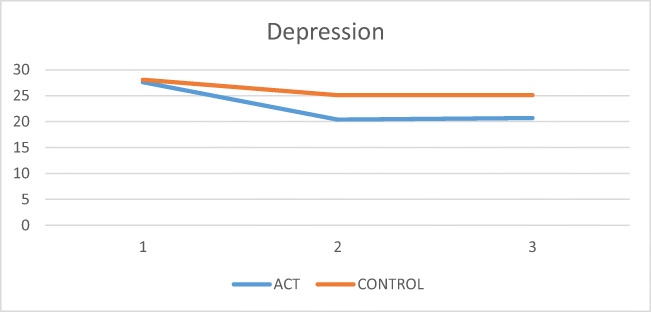
To compare the two groups in depression scores over time, repeated measures ANOVA test was used. First, the assumption of covariance matrices equality was investigated, and the results showed homogeneity of the variances (Box’s M = 6). P = 0.43). The Mauchly’s test also showed Mauchly’s test of sphericity of W = 0.9 and a significant level of 0.7. The results of intergroup test and intergroup interaction are shown in Table 3. The recovery indicator is also shown in Fig. 2. Based on Table 3, the level of effectiveness (F = 6.81) indicates the significant effectiveness in the intervention group compared to the control group (p < 0.05).
Table 3.
Repeated measures ANOVA for variables between ACT group and control group in the pre-test, post-test and follow up
| Variable | Source | Type III Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squared | Observed Power a | |
|---|---|---|---|---|---|---|---|---|---|
| Depression | Tests of Within-Subjects Effects | factor1 | 691.217 | 2 | 345.608 | 8.72 | 0.00 | 0.188 | 0.96 |
| factor1 * group | 111.717 | 2 | 55.85 | 1.41 | 0.24 | .036 | 0.296 | ||
| Error(factor1) | 2991.067 | 76 | 39.356 | ||||||
| Tests of Between-Subjects Effects | group | 310.4 | 1 | 310.4 | 6.812 | .013 | .152 | .720 | |
| Error | 1731.583 | 38 | 45.568 |
Fig. 2.
Comparison mean score of depression between ACT group and control group in the pre-test, post-test and follow up
Sleep quality
To evaluate the quality of sleep and related sub-scales subscales, each subscale and the overall score were examined at first using the independent group t-test scores in three periods: pretest, post-test, and follow-up. The results showed that there was no significant difference in pretest in any of the subscales, but different results were obtained in the post-test and follow-up in different subscales (Table 4).
Table 4.
Mean and standard deviation of sub-variables in the intervention and control groups
| Variable | Intervention group Mean (SD) | Control Group Mean (SD) | p value | |
|---|---|---|---|---|
| Sleep quality | Pre-test | 1.55 ± 1.14 | 1.35 ± 1.13 | .583 |
| Post-test | 0.75 ± 0.78 | 1.7 ± 1.21 | .006* | |
| Follow-up | 0.65 ± 0.74 | 1.6 ± 0.99 | .002* | |
| Sleep latency | Pre-test | 2 ± 0.56 | 1.8 ± 0.83 | .379 |
| Post-test | 1.3 ± 0.47 | 1.95 ± 0.75 | .002* | |
| Follow-up | 1 ± 0.56 | 1.6 ± 0.82 | .01* | |
| Sleep duration | Pre-test | 0.44 ± 2.25 | 2.35 ± 0.48 | .503 |
| Post-test | 2.05 ± 0.22 | 2.2 ± 0.41 | .159 | |
| Follow-up | 2 ± 0.001 | 2.5 ± 0.512 | .000* | |
| Sleep efficiency | Pre-test | 1.95 ± 1.27 | 2.05 ± 1.23 | .802 |
| Post-test | 1.55 ± 1.31 | 2.30 ± 0.97 | .048* | |
| Follow-up | 1.45 ± 1.09 | 2.45 ± 0.88 | .003* | |
| Sleep disturbance | Pre-test | 1.9 ± 0.55 | 1.8 ± 0.52 | .560 |
| Post-test | 1.2 ± 0.41 | 1.8 ± 0.61 | .001* | |
| Follow-up | 1.2 ± 0.4 | 1.9 ± 0.51 | .000* | |
| Hypnotic medicine | Pre-test | 0.5 ± 0.51 | 0.7 ± 0.47 | .206 |
| Post-test | 0.95 ± 0.88 | 1 ± 0.91 | .862 | |
| Follow-up | 0.4 ± 0.50 | 0.65 ± 0.81 | .249 | |
| Daytime dysfunction | Pre-test | 2.15 ± 0.74 | 1.95 ± 0.82 | .426 |
| Post-test | 1 ± 0.45 | 1.75 ± 0.63 | .000* | |
| Follow-up | 1.15 ± 0.58 | 2 ± 0.72 | .000* | |
| Total | Pre-test | 12.3 ± 2.14 | 12 ± 2.10 | .658 |
| Post-test | 8.8 ± 1.98 | 12.7 ± 2.2 | .000* | |
| Follow-up | 7.8 ± 1.53 | 12.75 ± 1.68 | .000* | |
As shown in Table 4, except for the Hypnotic medicine subscale, in the rest of the subscales, the intervention group showed a higher ability to reduce sleep-related symptoms after the follow-up phase than the control group.
Repeated measures ANOVA was used to evaluate the scores of sleep quality subscales and the overall score in three phases. All sub-scales, except Hypnotic medicine, followed assumption of Mauchly’s test of sphericity. Table 5 shows the scales that used the ANOVA test. For the Hypnotic medicine subscale, according to the Kruskal–Wallis test as a nonparametric test, the significance level of p = 0.2 for the pre-test, p = 0.9 for the post-test, and p = 0.38 for follow-up were obtained, respectively. The results indicated that there was no significant difference between acceptance and commitment therapy group and control group did not differ in the use of sleeping medicines, and both groups did not show an improvement in Hypnotic medicine.
Table 5.
Repeated measure variance analysis (ANOVA) in sleep subscales
| Variable | mauchly | Sig mauchly | Sig mauchly | F | Observed Power | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Sleep quality | 0.99 | 0.96 | 0.96 | 9.19 | 0.84 | 0.195 |
| Sleep latency | 0.98 | 0.81 | 0.81 | 8.54 | 0.815 | 0.184 |
| Sleep duration | 0.857 | 0.58 | 0.58 | 11.16 | 0.902 | 0.227 |
| Sleep efficiency | 0.955 | 0.42 | 0.42 | 8.54 | 0.813 | 0.184 |
| Sleep disturbance | 0.996 | 0.92 | 0.92 | 20.36 | 0.993 | 0.349 |
| Daytime dysfunction | 0.97 | 0.64 | 0.64 | 15.38 | 0.96 | 0.288 |
| Total | 0.93 | 0.26 | 0.26 | 55.36 | 0.9 | 0.593 |
Discussion
In the present study, the results indicated that ACT could significantly improve depression severity and overall sleep quality. The present study was one of the first clinical trials on the effectiveness of acceptance and commitment therapy on sleep quality and depression in patients with diabetic neuropathy, so there is little research history in this area. However, the results of previous studies are consistent with the present study.
Regarding sleep quality, the overall score indicates the effectiveness of acceptance and commitment therapy on sleep quality in diabetic neuropathy patients. However, no significant difference was found between the two groups in the Hypnotic medicine subscale. Of course, as observed in the table, the dose of sleeping medicines taken in both groups was shallow from the beginning, and the lack of change could not be due to the treatment inability; instead, there is no problem in this area that requires treatment in these people. Since Iranian society uses fewer medicines in its life than in Western societies, this lack of use to improve sleep is somewhat predictable [16].
One study investigated the effectiveness of Acceptance-based behavioral therapy in the insomnia of people with chronic pain. The results showed that the treatment improved sleep quality. These results persisted until the follow-up period [17]. In one study on insomnolent patients with chronic pain, the results showed that by improving the components of “value-based life” and “flexibility,” sleep quality increased significantly [18]. In addition to sleep studies in people with chronic pain, studies in people with DM with these problems have also shown the effectiveness of acceptance and commitment therapy. For example, in a randomized clinical trial study acceptance and commitment therapy were significantly improved quality of life and treated primary insomnia in people who did not respond to cognitive-behavioral therapy, which was consistent with the findings. The present study [19]. Also conducted that acceptance and commitment therapy, when added to cognitive-behavioral therapy, significantly improved sleep-related problems [20]. Regarding depression, results of similar studies suggest the effectiveness of acceptance and commitment therapy on depression. For example, in a study investigating the effectiveness of this treatment in diabetic children aged 7 to 15 years, the results showed that this treatment significantly reduced depression and guilt and increased the quality of life in these people [21].
The main point in improving depression and sleep quality in these people are related to pain. Because these people experience severe pain, pain becomes the central axis of their lives. They repeatedly try to avoid and control pain. Since this pain has a firm neurological basis, it is not controlled by avoiding thinking about it. The person perceives this inability to control as a personal disability, and therefore he feels low self-esteem and self-efficacy. These defects, along with other problems of the disease, exacerbate depression in them. Since the majority of the intellectual capacity is to deal with pain, the time and ability to manage the other aspects of individual-social life is severely reduced, and as a result, functioning in these areas is associated with defects and conflicts that make these patients more depressed. Another problem with these people is trying to control or suppress unwanted thoughts that can lead to a prolonged delay in the onset of sleep. In fact, by avoiding anxious behaviors, anxiety increases, and this anxiety triggers impulsive responses (the opposite point for getting ready for bed).
In this treatment, the person does not see the need to control pain, stimuli, and distressing thoughts related to the course of the disease. He also learns that these experiences are permanent and daily for him, so their mere presence does not mean atonement for unforgivable sin or loss of life. This concept, which is taught to a person in the form of metaphors and mindfulness practices, leads to a reduction in his distress. In fact, with this experience, he becomes empowered [13, 22, 23]. On the other hand, by changing one’s lifestyle and acting following one’s inner values, one becomes closer to his goals, has a higher function, and naturally feels more satisfied. These, in turn, reduce distressing symptoms of depression and anxiety [24].
On the other hand, people who experience chronic insomnia as a result of trying to avoid and control pain may lose some of the values of their lives because they are mostly focused on controlling insomnia. By reducing the centrality of pain in life, a person with a commitment to critical personal values can provide a path to lead life. People with sleep disorders have two precious values called “sleep improvement” and “pain relief.” However, instead of helping to improve sleep, these values lead to sleep being at the core of a person’s main problem. For example, one plans to achieve other value-based goals of his life, and to achieve that, adjusts his or her sleep schedule with more interest. In acceptance and commitment therapy, the person reduces this anxiety and experiences a more relaxed sleep with mindfulness and acceptance techniques [24, 25]. Overall, acceptance and commitment therapy, by improving value-oriented life, mindfulness skills, and increasing flexibility in individuals, reduces the severity of depression and improves sleep. Besides, since there is an interaction between the severity of depression and the quality of sleep, by breaking this vicious cycle, a change in each of them causes an improvement in the other. Hence, by repeating these practices, one becomes more capable in dealing with pain, and as is clear from the results of the present study, these people stabilize the achieved improvements in the follow-up period by being more equipped with these capabilities [12, 13, 23, 26].
Despite these findings, the present study had some limitations. First, to study the most effective treatment components, there was no group to receive other third-wave therapies. Second, the study had only a three-month follow-up period, and due to the treatment and hardware facilities of the city where the research was conducted, it was not possible to run a long-term investigation. It is suggested that in future research, by examining the mediating and confounding variables, the results of similar research be examined. Second, other constructs affecting the improvement of the quality of life in these individuals should be examined.
Acknowledgements
We would like to express our gratitude to the staff of the hospitals affiliated with Kermanshah University of Medical Sciences who have assisted in the implementation of this study. The Vice Chancellor for Research and Technology of Kermanshah University of Medical Sciences is also highly appreciated for providing the financial resources for the research.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest in this project.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pain IAftSo . Epidemiology of neuropathic pain: how common is neuropathic pain, and what is its impact? Washington: IASP; 2014. [Google Scholar]
- 2.Torta R, Ieraci V, Zizzi F. A review of the emotional aspects of neuropathic pain: from comorbidity to co-pathogenesis. Pain and therapy. 2017;6(1):11–17. doi: 10.1007/s40122-017-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC) 2017. [PubMed]
- 4.Fonseca VA. Clinical diabetes: translating research into practice. Philadelphia: Elsevier; 2006. [Google Scholar]
- 5.Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin. 2013;42(3):529–544. doi: 10.1016/j.ecl.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Hasan SS, Mamun AA, Clavarino AM, Kairuz T. Incidence and risk of depression associated with diabetes in adults: evidence from longitudinal studies. Community Ment Health J. 2015;51(2):204–210. doi: 10.1007/s10597-014-9744-5. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd C, Roy T, Begum S, Mughal S, Barnett A. Measuring psychological well-being in south Asians with diabetes; a qualitative investigation of the PHQ-9 and the WHO-5 as potential screening tools for measuring symptoms of depression. Diabet Med. 2012;29(1):140–147. doi: 10.1111/j.1464-5491.2011.03481.x. [DOI] [PubMed] [Google Scholar]
- 8.Brzecka A, Madetko N, Nikolenko VN, Ashraf GM, Ejma M, Leszek J, et al. Sleep disturbances and cognitive impairment in the course of type 2 diabetes - possible link. Curr Neuropharmacol. 2020;18. [DOI] [PMC free article] [PubMed]
- 9.Khalil M, Power N, Graham E, Deschênes SS, Schmitz N. The association between sleep and diabetes outcomes–a systematic review. Diabetes Res Clin Pract. 2020;161:108035. doi: 10.1016/j.diabres.2020.108035. [DOI] [PubMed] [Google Scholar]
- 10.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindholm-Olinder A, Fischier J, Fries J, Alfonsson S, Elvingson V, Eriksson JW, Leksell J. A randomised wait-list controlled clinical trial of the effects of acceptance and commitment therapy in patients with type 1 diabetes: a study protocol. BMC Nurs. 2015;14(1):61. doi: 10.1186/s12912-015-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davoudi M, Omidi A, Sehat M, Sepehrmanesh Z. The effects of acceptance and commitment therapy on man smokers’ comorbid depression and anxiety symptoms and smoking cessation: a randomized controlled trial. Addict Health. 2017;9(3):129–138. [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- 14.Gregg JA, Callaghan GM, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. J Consult Clin Psychol. 2007;75(2):336–343. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- 15.Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh sleep quality index (PSQI-P) Sleep Breath. 2012;16(1):79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 16.Ershadpour R, Zare Marzouni H, KALANI N. Review survey of the reasons of the prevalence of self-medication among the people of Iran. Navid No. 2015;18(60):16–23. [Google Scholar]
- 17.Zetterqvist V, Grudin R, Rickardsson J, Wicksell RK, Holmström L. Acceptance-based behavioural treatment for insomnia in chronic pain: a clinical pilot study. J Contextual Behav Sci. 2018;9:72–79. doi: 10.1016/j.jcbs.2018.07.003. [DOI] [Google Scholar]
- 18.McCracken LM, Williams JL, Tang NK. Psychological flexibility may reduce insomnia in persons with chronic pain: a preliminary retrospective study. Pain Med. 2011;12(6):904–912. doi: 10.1111/j.1526-4637.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Manual by Hayes A Quality of life improvements after acceptance and commitment therapy in nonresponders to cognitive behavioral therapy for primary insomnia. Psychother Psychosom. 2014;83:371–373. doi: 10.1159/000365173. [DOI] [PubMed] [Google Scholar]
- 20.Dalrymple KL, Fiorentino L, Politi MC, Posner D. Incorporating principles from acceptance and commitment therapy into cognitive-behavioral therapy for insomnia: a case example. J Contemp Psychother. 2010;40(4):209–217. doi: 10.1007/s10879-010-9145-1. [DOI] [Google Scholar]
- 21.Moghanloo VA, Moghanloo RA, Moazezi M. Effectiveness of acceptance and commitment therapy for depression, psychological well-being and feeling of guilt in 7–15 years old diabetic children. Iran J Pediatr. 2015;25(4):e2436. doi: 10.5812/ijp.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsberg S, Wijk I, Livheim F, Toft E, Johansson U-B, Anderbro T. Acceptance and commitment therapy (ACT) for adult type 1 diabetes management: study protocol for a randomised controlled trial. BMJ Open. 2018;8(11). [DOI] [PMC free article] [PubMed]
- 23.Coto-Lesmes R, Fernandez-Rodriguez C, Gonzalez-Fernandez S. Acceptance and commitment therapy in group format for anxiety and depression. A systematic review. J Affect Disord. 2020;263:107–120. doi: 10.1016/j.jad.2019.11.154. [DOI] [PubMed] [Google Scholar]
- 24.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York: Guilford Press; 2011. [Google Scholar]
- 25.Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: a metacognitive model of insomnia. Behav Res Ther. 2012;50(11):651–660. doi: 10.1016/j.brat.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momtaz YA, Hamid TA, Bagat MF, Hazrati M. The association between diabetes and cognitive function in later life. Curr Aging Sci. 2019;12(1):62–66. doi: 10.2174/1874609812666190614104328. [DOI] [PMC free article] [PubMed] [Google Scholar]