Abstract
Background and Objectives:
Gut microbiota such as Faecalibacterium prausnitzii play a major role in the regulation of gut barrier, inflammation and metabolic functions. Microbiota–derived extracellular vehicles (EVs) have been recently introduced as functional units mediating the eukaryotic and prokaryotic cell-microbiota interactions. In this paper, the effect of F. prausnitzii and its EVs on mRNA expression levels of tight junction genes (ZO1 and OCLN) as well as PPARs and ANGPTL4 genes in the human epithelial colorectal adenocarcinoma (Caco-2) cell line was evaluated.
Methods
F. prausnitzii was cultured on the Brain Heart Infusion (BHI) broth medium under anaerobic conditions, and its EVs were extracted by ultracentrifugation. This bacterium and its EVs were treated on the Caco-2 cells. After 24 h, the expression of the genes encoding TJ proteins such as ZO1 and OCLN, PPARs and ANGPTL4 was evaluated by quantitative real-time PCR.
Results
Unlike F. prausnitzii, its EVs significantly increased the expression of ZO1 and OCLN genes, and PPARα, PPARγ and PPARβ/δ genes (except at a concentration of 100 µg/ml) as well as ANGPTL4 gene.
Conclusions
The results of this study demonstrated that F. prausnitzii–derived EVs increased the intestinal barrier permeability via TJs (ZO1 and OCLN) as well as PPAR-α, PPAR-γ and PPAR β/δ genes and their targeted gene (ANGPTL4) in the Caco-2 cell line. Accordingly, it is suggested that F. prausnitzii–derived EVs can be considered as a new bacterial postbiotic to cure dysbiosis-associated diseases including obesity and its related metabolic dysfunctions, according to the leaky gut hypothesis.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00605-1) contains supplementary material, which is available to authorized users.
Keywords: F. prausnitzii, EVs, Intestinal epithelial cell permeability, PPARs, ANGPTL4
Introduction
The significance of microbiota-derived EVs
There has been a great interest in microbiota-derived EVs in recent years. These vesicles not only have the ability to carry a wide variety of digestive enzymes but also are able to modulate host immune responses and signalling pathways. Thus, the use of specific bacterial strains–derived EVs may modulate immune signalling pathways, host nourishment and generation of bacterial metabolites [1]. Under dysbiosis conditions, the host-gut microbiota interactions will be impaired leading to many diseases such as obesity and type 2 diabetes. Increased gut permeability causes different dysfunctions including a change in the composition or function of gut microbiota. Gut barrier alterations are responsible for metabolic endotoxemia leading to a low level of inflammation and metabolic dysfunctions [1].
Gastrointestinal microbiota plays a major role in body signalling so that any change in the pattern of microbiota (dysbiosis) may result in some diseases such as obesity and its related metabolic syndrome [2]. Among the firmicutes phylum, F. prausnitzii is an abundant microbiome species in the human intestine representing almost 8% of the total colonic microbiota with a key role in the intestinal health [3, 4]. Considering the key role of F. prausnitzii in the intestinal health and regulation of the immune system and inflammation pathways, this study aims at investigating the role of F. prausnitzii and its EVs in metabolic dysfunctions such as permeability of the intestinal epithelium. The aim of this study is to analyse F. prausnitzii-–derived EVs and to introduce new bacterial postbiotic based on the leaky gut hypothesis [5]. According to the leaky gut hypothesis, despite the anti-inflammatory effects of microbiota on the epithelial barrier, they can pass through the epithelial barrier and enter the bloodstream and may cause systemic consequences due to the high-fat diet (HFD) and obesity [6, 7]. Therefore, if only the F. prausnitzii is used as a probiotic, it may not have beneficial effects on the intestine health, immune system and inflammatory pathways, regulation during obesity and its related metabolic dysfunctions including inflammatory bowel disease (IBD) due to the leaky gut hypothesis. This study investigates the effect of F. prausnitzii and its EVs on the intestinal epithelial cell permeability through TJ proteins encoded mRNA expression such as zonula occludens (ZO) and occludin in the Caco-2 cell culture model. TJ proteins play an important role in the function of the intestinal barrier by connecting to the adjacent epithelial cells and blocking the paracellular space which in turn inhibits the entry of toxins and pathogens [8].
ANGPTL4 as a significant target gene of PPARs
Angiopoietin-like 4, as a fasting-induced adipose factor (FIAF), is known also as a PPARγ angiopoietin-related protein or hepatic fibrinogen/angiopoietin-related protein. It has been studied in many tissues as a multipurpose signalling protein. Despite its expression in liver, adipose tissue, intestine, brain and thyroid, it has also been observed in the heart, kidney, skeletal muscles, spleen, pituitary gland, hypothalamus and placenta [9].
ANGPTL4 expression can be regulated through different modulators depending on the targeted organs, which is accompanied by different activities. The main regulator belongs to PPARs (PPARα, PPARγ and PPARβ/δ). ANGPTL4 modulators, their target cells and organs as well as their associated physiological activities are currently known [9]. The expression of intestinal ANGPTL4 is strongly regulated through the microbial community of the intestine [9]. The secretion of ANGPTL4 in the bloodstream inhibits the function of blood lipase lipoproteins, an enzyme responsible for converting the lipoprotein triglycerides to monoglycerides and fatty acids in the bloodstream. Therefore, ANGPTL4 increases the plasma triglyceride while reducing the intake of free fatty acids and cholesterol into the tissues [10–16].
As previously mentioned, peroxisome proliferator-activated receptors (PPARα, PPARγ, and PPARβ/δ) are main ANGPTL4 modulators. The human superfamily of nuclear receptors includes 48 transcription factors which are activated by their specific ligands, and regulate different genetic, inflammatory and metabolic processes. PPARs are members of the superfamily of nuclear receptors. PPARs include three members of PPARα, PPARγ and PPARβ/δ which are also known as NR1C1, 2, 3, respectively [15, 17].
PPARs widely exist in different organs. Despite some spatial barriers between PPARs in some organs such as liver, adipose tissue and gut microbiota, there are still some interactions. Briefly, metabolites produced by microbiota are absorbed by intestinal epithelium and draw inflammatory cells. Metabolites are also transferred to the liver, adipose tissue, heart, blood vessels and other organs through the bloodstream. Metabolites act as a ligand for PPARs in these organs. PPARs activation first leads to intestinal modulation and also immune response throughout the body and then to modulation of carbohydrate and fat metabolism [18]. Studies have shown the key role of PPARs in microbial inhabitation and adaptability of the gastrointestinal tract. For migration and viability of a specific niche, microbiota modulate the expression of PPARs in the intestinal epithelium and immune regulator cells and also alter the host inflammatory responses [19–29]. It has been shown that gut microbiota and PPARs interact with each other in several diseases such as irritable bowel syndrome (IBS), IBD, obesity and metabolic syndromes such as dyslipidemia, insulin resistance, type 2 mellitus diabetes and liver diseases such as non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases such as atherosclerosis [18].
Given the important role of ANGPTL4 and PPARs in host-gut microbiota interactions and according to Kersten S. et al. who introduced ANGPTL4 as a novel targeted gene of PPARs [30], the other objective of this study is to investigate the effect of F. prausnitzii and its EVs on the expression of mRNA, ANGPTL4 and PPARs (PPARα, PPARγ, and PPARβ/δ) in the Caco-2 cell culture model.
Materials and methods
Bacterial strain and culture conditions
F. prausnitzii strain A2-165 was provided from the DSMZ standard bacterial collection (DSM NO. 17,677) and subsequently was cultured under anaerobic conditions (80% N2, 10% H2 and 10% CO2) at 37 °C [31].
Preparation and isolation of extracellular vesicles
Typically, F. prausnitzii EVs were extracted by ultracentrifugation at 200,000 g for 2 h at 4 °C as previously described [32, 33].
Physicochemical analysis of EVs
To confirm the physicochemical properties of the extracted EVs, physicochemical control was conducted by measuring the total protein concentration through OD measurement (Bradford protein assay), Scanning Electron Microscopy (SEM) and SDS-PAGE.
Bradford protein assay
To measure the concentration of the purified total protein, NanoDrop Lite Spectrophotometer (Thermo Scientific, USA) was used at the wavelength of 280 nm. Bradford assay was performed according to its standard protocol using 100 µl of protein and bovine serum albumin (BSA; 1 mg/ml) as standard, read at 595 nm [34].
SDS-PAGE
The protein contents of the EVs were separated using SDS-PAGE on 12% separating gel. To this end, 25 µl of each sample was loaded and stained by Coomassie brilliant blue G250 dye.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to confirm the integrity, stability and to determine the spherical shape and size of the vesicles. In this regard, filtered EVs in sucrose were coated on 400-mesh gold grids and stained with 2% uranyl acetate. The SEM micrographs were captured by a HITACHI S-4160 Microscope (Nano-electronic Laboratory, Tehran University).
Cell culture treatment
The established Caco-2 (ATCC® HTB-37) as a human epithelial colorectal adenocarcinoma cell line was cultured in DMEM (pH 7.0–7.5) with high glucose (Capricorn Scientific GmbH, Germany), supplemented with 10% FBS (Biochrom, Berlin, Germany) and 1% Penicillin- Streptomycin (Gibco BRL). The Caco-2 cells were seeded at 0.3 × 106 cells/cm2 and grown as confluent monolayers for 24 h (overnight) in six-well plates (Nunc) at 37 °C, under a 5% CO2/ 95% air atmosphere. Confluent monolayers were infected in 1.5 ml of the cell culture medium without antibiotics and with heat-inactivated FBS 1% after 2 h of incubation at 37 °C under anaerobic conditions (as described above) at a multiplicity of infection (MOI) of 10 F. prausnitzii per epithelial cell and with purified EVs at a concentration of 50 and 100 µg/ml respectively, and with sucrose and PBS as control and then incubated for 24 h at 37 °C under anaerobic conditions. Monolayers were washed once with phosphate-buffered saline (PBS, pH = 7.2).
RNA extraction and cDNA synthesis
The total RNA was extracted from cells using RNX-Plus Solution (RN7713C, Sinacolon, Karaj, Iran) according to the manufacturer’s instructions. Spectrophotometry (260 and 280 nm) was used to assess the purity of the isolated RNA samples. Reverse transcription was carried out by a Thermo Scientific RevertAid™ First Strand cDNA Synthesis Kit (K1621; Fermentas, Waltham, MA).
Quantitative real-time PCR
The real-time PCR was performed by a LightCycler® 96 System (Roche, Mannheim, Germany) in a total volume of 20 µl containing Power SYBR Green master mix (2X) (Takara, Tokyo, Japan), primer (0.4 µM), cDNA (20 ng/µl) and nuclease-free water. The sequences of the primers used are listed in Table 1. GAPDH reference gene based on geNorm used in Piana et al.. study was selected as the most stable and appropriate gene for normalization of target genes data [35]. GAPDH (endogenous housekeeping gene) was done in duplicate for each sample. Forty thermal cycles were applied in the following order: 30 s at 95 °C (holding temperature), 40 cycles at 95 °C for 5 s, at 54 °C for 30 s and at 72 °C for 30. A melting point analysis was carried out by heating the amplicon from 55 to 95 °C and a characteristic melting point curve was obtained for each product. To calculate genes expression difference between the samples, first, the data from Ct were corrected based on the efficiency of the primers using Genex 6 software, and then 2−ΔΔCT method was used to calculate the fold change. PCR efficiency for each primer was obtained using LinReg PCR software between 0.8 and 0.94. Analysis of variance (ANOVA) and student’s t-test were used to compare the values obtained for the test and control samples. Statistical analyses were performed with the help of SPSS 21 and GraphPad Prism 7 (GraphPad, La Jolla, CA).
Table 1.
The primers used in real-time PCR
| Gene Name | Expected size (bp) | Gene Symbol | Primer pair sequence (5’ 3’) | Reference |
|---|---|---|---|---|
| glyceraldehyde-3-phosphate dehydrogenase | 166 | GAPDH |
Forward: CAAGATCATCACCAATGCCT Reverse: CCCATCACGCCACAGTTTCC |
[36] |
| zona occludens-1 | 184 | ZO1 |
Forward: CGGGACTGTTGGTATTGGCTAGA Reverse: GGCCAGGGCCATAGTAAAGTTTG |
[37] |
| occludin | 105 | OCLN |
Forward: TCCTATAAATCCACGCCGGTTC Reverse: CTCAAAGTTACCACCGCTGCTG |
[37] |
| angiopoietin like 4 | 64 | ANGPTL4 |
Forward: CGTACCCTTCTCCACTTGGG Reverse: GCTCTTGGCGCAGTTCTTG |
[38] |
| peroxisome proliferator activated receptor alpha | 186 | PPARα |
Forward: CTGGAAGCTTTGGCTTTACG Reverse: TGTCCCCGCAGATTCTACAT |
[38] |
| peroxisome proliferator activated receptor beta/delta | 117 | PPARβ/δ |
Forward: ACAGCATGCACTTCCTTCCA Reverse: TCACATGCATGAACACCGTA |
[38] |
| peroxisome proliferator activated receptor gamma | 159 | PPARγ |
Forward: GAGCCCAAGTTTGAGTTTGC Reverse: CAGGGCTTGTAGCAGGTTGT |
[38] |
Results
Morphological characterization of EVs
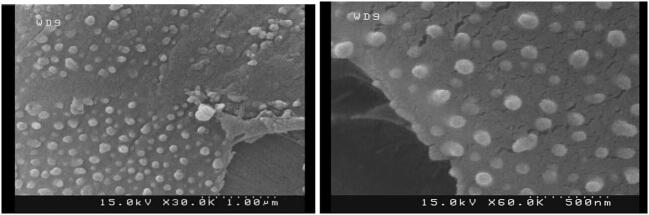
Following extraction and purification, morphological characteristics of F. prausnitzii A2-165 were studied by scanning electron microscopy. As seen in the SEM micrographs (Fig. 1), the size of vesicles ranges from 30 to 250 nm, maintaining their spatial and natural morphology. In comparison with the standard protein marker, the motion pattern of EVs in a 12% SDS-PAGE gel present different protein profiles with a molecular weight of 11 to 245 kDa (Fig. 2). The total protein concentrations of EVs were analysed by NanoDrop and Bradford assays. The concentration of the purified total protein obtained by Bradford assay was approximately 0.5 mg/ml which was also confirmed by NanoDrop method.
Fig. 1.
SEM micrographs of EVs extracted from F. prausnitzii, (A) low (scale bar: 1 µm) and (B) high (scale bar: 500 nm) resolution
Fig. 2.
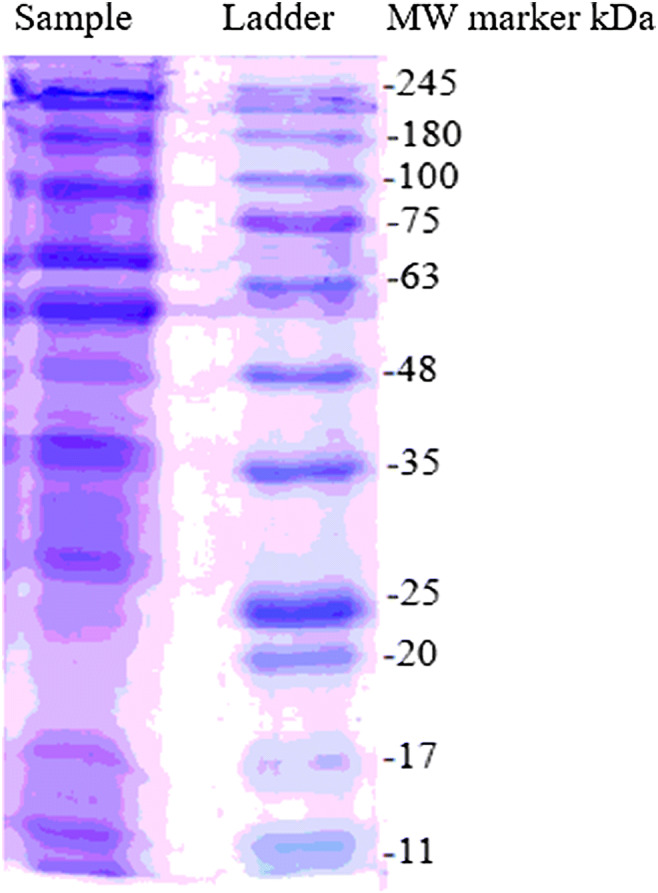
Protein electrophoretic mobility view in 12% SDS-PAGE. Sample: EVs extracted by ultracentrifugation; MW: molecular-weight size marker (CinnaGen, Cat. No. PR901641-tris-glycine 4–20%)
The effects of F. prausnitzii and its EVs on TJs
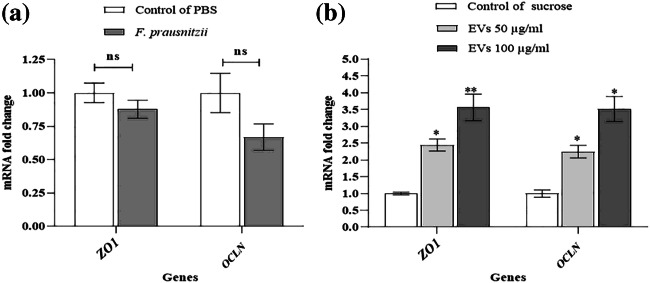
F. prausnitzii in the Caco- 2 cell line insignificantly decreased the expression of ZO1 and OCLN genes at the mRNA level (Fig. 3a). Interestingly, the expression of ZO1 and OCLN genes was significantly increased in response to 50 and 100 µg/ml concentrations of EVs at the mRNA level (Fig. 3b).
Fig. 3.
The effects of F. prausnitzii and its EVs on TJs: (a) the Caco-2 cells were treated with F. prausnitzii at MOI 10 and (b) different concentrations of F. prausnitzii–derived EVs (50 µg/ml and 100 µg/ml); *, ** p < 0.05 and p < 0.01 were considered statistically significant, respectively. ns represents no significance. GAPDH was used as an internal control
The effects of F. prausnitzii and its EVs on PPARS and ANGPTL4
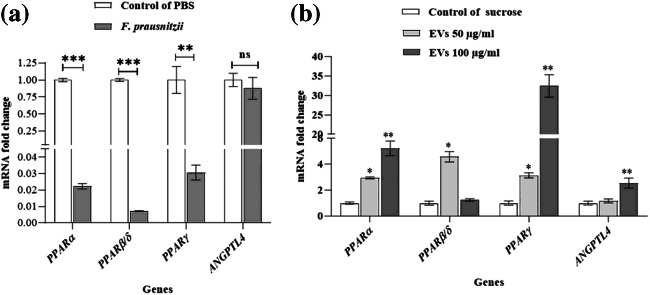
F. prausnitzii significantly decreases the expression of all PPARs genes (PPARα, PPARβ/δ, and PPARγ) at the mRNA level, while the expression of mRNA the ANGPTL4 decreased insignificantly (Fig. 4a). EVs (50 µg /ml) increased the expression of the PPARα, PPARβ/δ, and PPARγ at the mRNA level, but no significant difference was observed for the ANGPTL4 gene at the same concentration. Moreover, EVs (100 µg/ml) increased the expression of the PPARα, PPARγ, and ANGPTL4 at the mRNA level. However, no significant difference was found for PPARβ/δ at this concentration.
Fig. 4.
The effect of F. prausnitzii and its EVs on PPARs and ANGPTL4: (a) the Caco-2 cells treated with F. prausnitzii (MOI = 10) and (b) the Caco-2 cells treated at different concentrations of F. prausnitzii–derived EVs (50 µg/ml and 100 µg/ml); *, **, *** p < 0.05, p < 0.01 and p < 0.001 were considered statistically significant, respectively. ns represents no significance. GAPDH was used as an internal control
Discussion
F. prausnitzii, a species with proven anti-inflammatory properties, is able to produce butyrate and many SCFAs, which were found to be reduced in Crohn’s disease, obesity, asthma and major depressive disorders. Today, it can be considered the next generation probiotics (NGPs). Since the introduction of the leaky gut hypothesis in recent years [7], there are doubts regarding the use of live bacteria as probiotics, and the safety and health effects of probiotics are no longer acceptable. Hence, any component of probiotics such as EVs (known as postbiotics) and/or prebiotics have attracted the attention of many research centers around the world [5, 39]. For this reason, the effect of F. prausnitzii and its EVs on mRNA expression levels of tight junction genes (ZO1 and OCLN) as well as PPARs and ANGPTL4 genes in the human Caco-2 cell line was evaluated in this study.
According to the recent literature, EVs are functional units secreted by all bacteria and can significantly affect the permeability of the intestine. EVs contain several microbe-associated molecular patterns (MAMPs) capable of interacting with immune and epithelial cells. The benefits or side effects of EVs on the host are dependent on the strain-specific microbe. It has been demonstrated that probiotics and the host may directly interact through probiotics–derived EVs [18, 40, 41]. Ahmadi Badi et al.. found the role of Bacteroides fragilis and its OMVs in host-gut microbiota interactions, particularly in immunomodulation in the Caco-2 cell line [40]. According to our results, unlike the F. prausnitzii, its EVs increased the permeability of the intestinal barrier at the mRNA level through TJs (ZO1 and OCLN) expression. Alvarez et al. showed that the OMVs secreted by Escherichia coli Nissle 1917 probiotics and ECOR63 commensal increased the intestinal barrier activity by regulating the expression of intestinal epithelial TJ proteins [42]. This is comparable with that found in our study. According to Rabiei et al., the Caco-2 cells treated with F. prausnitzii and its EVs caused a significant increase in the expression of TNF-α, IL-4, IL-8 and IL-10 and a significant decrease in the expression of IL-1, IL-2, IL-6, IL-12, IL-17a and IFN-γ compared to the control group (P < 0.05). However, the EVs derived from F. prausnitzii showed greater efficacy in decreasing the inflammatory cytokines and increasing the anti-inflammatory cytokines [43].
Jafari et al. show that the F. prausnitzii supernatant and derived EVs are able to dysregulate the expression of some specific cytokines. However, the response of bacterium-secreted EVs was more significant than the bacterial supernatant for some key cytokines [32, 34]. A recent study by Chelakkot et al. revealed the intestinal regulatory effects of A. muciniphila–derived EVs (AmEV). The results of this study showed that an increase in FITC-dextran induced permeability and a decrease in occludin protein expression were improved by AmEVs in the Caco-2 cells [44]. Ashrafian et al. also reported that A. muciniphila and its EVs have a key role in the integrity of the intestinal barrier and reduction of inflammation [36]. These results are in good agreement with our results.
In this study, proteins banding patterns of F. prausnitzii–derived EVs were observed by the means of SDS-PAGE, but they are not only proteins that can alter physiochemical conditions of the cells. Thus, proteomic or lipidimic experiments are suggested to detect F. prausnitzii–derived EVs, which are involved in the integrity of the intestinal barrier. EVs are considered an alternative postbiotic in cases where viable bacteria can be harmful to human health (e.g. in the case of patient immunodeficiency or when the intestinal barrier is impaired or in the case of leaky gut hypothesis). EVs can diffuse through the mucosal layer and interact with the host preventing the danger of sepsis [1, 36]. Thus, due to the positive effects of F. prausnitzii–derived EVs on the expression of TJ proteins, they could be a good candidate as new bacterial postbiotics for curing obesity and associated metabolic disorders according to the leaky gut hypothesis.
Since the discovery of PPARs in the early 1990 s, it has been specified that PPARs are vital for the genetic regulation of mammalian complex metabolic pathways such as fatty acids oxidation and lipogenesis [45]. There are different interactions between the host PPARs and gut microbiota. For instance, it has been shown that gut microbiota and PPARs interact in some diseases such as gastrointestinal disorders [46–54], obesity and metabolic syndrome [26, 52–61], cardiovascular system and liver disease. The expression of PPARs along with their targeted genes is affected by microbial changes. Various studies showed that PPARs facilitate the intestinal homeostasis for colonization and adaptation of the microbes. Despite contradictory results, these mechanisms include (1) production of inflammatory cytokines, (2) maintenance of mucosal haemostasis and integrity of the intestine and (3) modulation of the immune cells [18]. According to the recent studies, dysbiosis-induced alterations in intestinal permeability that lead to leaky gut can affect the expression of the genes encoding PPARs and their target genes. It was identified in this study that unlike F. prausnitzii, its EVs have a positive effect on mRNA expression of the genes encoding PPARs proteins (PPARα, PPARγ, and PPARβ/δ) in the Caco-2 cells [62, 63].
As the mostly found isoform of PPARs in intestinal epithelium, the isoform PPARγ plays a key role in fatty acid metabolism regulation through beta-oxidation and cell proliferation [64] as well as intestinal homeostasis [65, 66]. It was found in this study that the highest levels of gene expression belong to PPARγ, PPARα, PPARβ/δ in the Caco-2 cells, respectively. Many studies have been indicated that ANGPTL4 (FIAF) is the PPARs (PPARα, PPARγ, and PPARβ/δ) targeted gene in different tissues. In was shown in this study that unlike F. prausnitzii, its EVs positively affect the mRNA expression level of the gene encoding the ANGPTL4 in the Caco-2 cells. F. prausnitzii insignificantly decreased the mRNA expression of the gene encoding the ANGPTL4. This result is in good agreement with that found by Couvigny et al. who observed reduced expression of PPARγ and its targeted gene (ANGPTL4) by S. salivarius at the Caco-2 cell line [28].
Since angiopoietin-like proteins such as ANGPTL4 has been introduced as a new target to cure obesity and related metabolic diseases [67], modification of the ANGPTL4 via the manipulation of gut microbiota can be helpful for curing obesity. Due to the complexity of the gut microbiota and their interactions with the host, studying the immune systems and microbiota structures is vital owing to their regulatory effects [68]. Immune system dysfunction leads to malfunction of the intestinal barrier in some diseases which in turn results in the abnormal transfer of the gut microbiota from the intestinal epithelium [69]. It has been pointed out in a study that leaky gut induction in animal models made the intestinal mucosal layer become very thin and permeable, enabling the bacteria to penetrate the intestinal barrier [70]. Kang et al. showed that Akkermansia muciniphila, as a harmful microorganism, was not able to induce a protective effect at the leaky gut condition. In contrast, A. muciniphila–derived EVs could disrupt disease progression [71]. Therefore, it has been suggested that in the leaky gut syndrome, the probable harmful effect of gut microbiota could be replaced and inversed by gut microbiota-derived EVs treatment [1].
Conclusions
According to the results of this study, F. prausnitzii EVs showed a positive effect on the mRNA expression of TJ proteins (ZO-1 and Occludin) and the genes encoding PPARs and also their targeted gene (ANGPTL4). These results suggested that F. prausnitzii–derived EVs could be introduced as a new bacterial postbiotic effective on the host-gut microbiota interactions through modulating the PPARs signalling pathways and expression of their targeted genes such as ANGPTL4 and also the regulation of the intestine permeability (by the expression of TJ proteins). According to the results, it is proposed that F. prausnitzii–derived EVs can be introduced as a ligand or new agonist of PPARs. Therefore, obesity and related metabolic dysfunctions can be cured by targeting the PPARs via F. prausnitzii–derived EVs followed by modulation of the expression of genes encoding the ANGPTL4 protein. More research works should be conducted in this area.
Electronic supplementary material
(XLSX 9.34 KB)
(PDF 1.41 MB)
Acknowledgements
We thank all the personnel of Mycobacteriology and Pulmonary Research Department and Microbiology Research Center (MRC), Pasteur Institute of Iran for their assistance in this project.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abbas Akhavan Sepahi, Email: akhavansepahy@gmail.com.
Seyed Davar Siadat, Email: d.siadat@gmail.com.
References
- 1.Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-derived extracellular vesicles as new systemic regulators. Front Microbiol. 2017;8:1610. doi: 10.3389/fmicb.2017.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microbial ecology in health disease. 2015;26(1):26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinken A, Khan MT, Paglia G, Rodionov DA, Harmsen HJ, Thiele I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J Bacteriol. 2014;196(18):3289–302. doi: 10.1128/JB.01780-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira-Halder CV, de Sousa Faria AV, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):643–8. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Tsilingiri K, Rescigno M. Postbiotics: what else? Benef Microbes. 2013;4:101–7. doi: 10.3920/BM2012.0046. [DOI] [PubMed] [Google Scholar]
- 6.Maguire M, Maguire G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev Neurosci. 2019;30(2):179–201. doi: 10.1515/revneuro-2018-0024. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–26. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141(5):769–76. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 9.Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: health effects, modulating agents and structure–function relationships. Expert Rev Proteomics. 2012;9(2):181–99. doi: 10.1586/epr.12.12. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. Journal of lipid research. 2002;43(11):1770–2. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, et al. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc Natl Acad Sci. 2005;102(5):1767–72. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Müller M, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281(2):934–44. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 13.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci. 2006;103(46):17450–5. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai U, Lee E-C, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci. 2007;104(28):11766–71. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan L, Yu X-C, Liu Z, Hu Y, Sturgis LT, Miranda ML, et al. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J Biol Chem. 2009;284(3):1419–24. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee E-C, Desai U, Gololobov G, Hong S, Feng X, Yu X-C, et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J Biol Chem. 2009;284(20):13735–45. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int J Mol Sci. 2018;19(7):2124. doi: 10.3390/ijms19072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan AU, Rahman A, Kobori H. Interactions between host PPARs and gut microbiota in health and disease. Int J Mol Sci. 2019;20(2):387. doi: 10.3390/ijms20020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Are A, Aronsson L, Wang S, Greicius G, Lee YK, Gustafsson J-Å, et al. Enterococcus faecalis from newborn babies regulate endogenous PPARγ activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci. 2008;105(6):1943–8. doi: 10.1073/pnas.0711734105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64(5):982–92. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12(10):941. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 23.Shaw MH, Kamada N, Kim Y-G, Núñez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209(2):251–8. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol. 2013;190(10):5306–12. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d en Bogert Bv. Erkus O, Boekhorst J, Goffau Md, Smid EJ, Zoetendal EG, et al. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol. 2013;85(2):376–88. [DOI] [PubMed]
- 26.Kundu P, Ling TW, Korecka A, Li Y, D’Arienzo R, Bunte RM, et al. Absence of intestinal PPARγ aggravates acute infectious colitis in mice through a Lipocalin-2–dependent pathway. PLoS Pathog. 2014;10(1):e1003887. doi: 10.1371/journal.ppat.1003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, et al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio. 2014;5(4):e01438-14. doi: 10.1128/mBio.01438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couvigny B, de Wouters T, Kaci G, Jacouton E, Delorme C, Dore J, et al. Commensal Streptococcus salivarius modulates PPARγ transcriptional activity in human intestinal epithelial cells. PLoS One. 2015;10(5):e0125371. doi: 10.1371/journal.pone.0125371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275(37):28488–93. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson AH, Yakymenko O, Olivier I, Håkansson F, Postma E, Keita ÅV, et al. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48(10):1136–44. doi: 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- 32.Jafari B, Nejad RAK, Vaziri F, Siadat SD. Evaluation of the effects of extracellular vesicles derived from Faecalibacterium prausnitzii on lung cancer cell line. Biologia. 2019:1–10.
- 33.Badi SA, Moshiri A, Marvasti FE, Mojtahedzadeh M, Kazemi V, Siadat SD. Extraction and Evaluation of Outer Membrane Vesicles from Two Important Gut Microbiota Members, Bacteroides fragilis and Bacteroides thetaiotaomicron. Cell J(Yakhteh). 2020;22(3). [DOI] [PMC free article] [PubMed]
- 34.Jafari B, Khavari Nejad R, Vaziri F, Siadat S. Isolation and characterization of Faecalibacterium prausnitzii extracellular vesicles. Vaccine Res. 2017;4(3):51–4. [Google Scholar]
- 35.Piana C, Wirth M, Gerbes S, Viernstein H, Gabor F, Toegel S. Validation of reference genes for qPCR studies on Caco-2 cell differentiation. Eur J Pharm Biopharm. 2008;69(3):1187–92. doi: 10.1016/j.ejpb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafian F, Behrouzi A. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol Hepatol Bed Bench. 2019;12(2):163. [PMC free article] [PubMed] [Google Scholar]
- 37.Park H-Y, Kunitake Y, Hirasaki N, Tanaka M, Matsui T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci Biotechnol Biochem. 2015;79(1):130–7. doi: 10.1080/09168451.2014.951027. [DOI] [PubMed] [Google Scholar]
- 38.Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279(33):34411–20. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 39.Ashrafian F, Shahryari A, Behrouzi A, Moradi HR, Lari A, Hadifar S, Yaghobfar R, Ahmadi Badi S, Vaziri F, Siadat SD. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina-Tijeras JA, Gálvez J, Rodríguez-Cabezas ME. The immunomodulatory properties of extracellular vesicles derived from probiotics: A novel approach for the management of gastrointestinal diseases. Nutrients. 2019;11(5):1038. doi: 10.3390/nu11051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson MM, Søreide K. The gut microbiota influence on human epigenetics, health, and disease. Handbook of Epigenetics. Amsterdam: Elsevier; 2017. pp. 495–510.
- 42.Alvarez C-S, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. doi: 10.3389/fmicb.2016.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabiei N, Badi SA, Marvasti FE, Sattari TN, Vaziri F, Siadat SD. Induction effects of Faecalibacterium prausnitzii and its extracellular vesicles on toll-like receptor signaling pathway gene expression and cytokine level in human intestinal epithelial cells. Cytokine. 2019;121:154718. doi: 10.1016/j.cyto.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2):e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 46.Bomba A, Nemcova R, Gancarcikova S, Herich R, Guba P, Mudronova D. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructo-oligosaccharides and polyunsaturated fatty acids. Br J Nutr. 2002;88(S1):95-S9. doi: 10.1079/BJN2002634. [DOI] [PubMed] [Google Scholar]
- 47.Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55(3):348–55. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyrin-Biroulet L, Beisner J, Wang G, Nuding S, Oommen ST, Kelly D, et al. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc Natl Acad Sci. 2010;107(19):8772–7. doi: 10.1073/pnas.0905745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zenhom M, Hyder A, de Vrese M, Heller KJ, Roeder T, Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J Nutr. 2011;141(5):971–7. doi: 10.3945/jn.110.136176. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Zhang M, Chen CC, Gillilland M, III, Sun X, El–Zaatari M, et al. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144(7):1478–87. doi: 10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mir SA, Nagy-Szakal D, Dowd SE, Szigeti RG, Smith CW, Kellermayer R. Prenatal methyl-donor supplementation augments colitis in young adult mice. PLoS One. 2013;8(8):e73162. doi: 10.1371/journal.pone.0073162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mardini HE, Grigorian AY. Probiotic mix VSL# 3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20(9):1562–7. doi: 10.1097/MIB.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 53.Selwyn FP, Cheng SL, Klaassen CD, Cui JY. Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab Dispos. 2016;44(2):262–74. doi: 10.1124/dmd.115.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Q, Ren Y, Lu J, Bartlett M, Chen L, Zhang Y, et al. A novel prebiotic blend product prevents irritable bowel syndrome in mice by improving gut microbiota and modulating immune response. Nutrients. 2017;9(12):1341. doi: 10.3390/nu9121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghose C, Perez-Perez G, Van Doorn L, Dominguez-Bello M, Blaser M. High frequency of gastric colonization with multiple Helicobacter pylori strains in Venezuelan subjects. J Clin Microbiol. 2005;43(6):2635–41. doi: 10.1128/JCM.43.6.2635-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu P, Hong F, Wang J, Wang J, Zhao X, Wang S, et al. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim Biophys Acta (BBA) Gen Subj. 2017;1861(11):2690–701. doi: 10.1016/j.bbagen.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Lu P, Sodhi CP, Yamaguchi Y, Jia H, Prindle T, Jr, Fulton WB, et al. Intestinal epithelial Toll-like receptor 4 prevents metabolic syndrome by regulating interactions between microbes and intestinal epithelial cells in mice. Mucosal Immunol. 2018;11(3):727. doi: 10.1038/mi.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bassaganya-Riera J, Dominguez-Bello MG, Kronsteiner B, Carbo A, Lu P, Viladomiu M, et al. Helicobacter pylori colonization ameliorates glucose homeostasis in mice through a PPAR γ-dependent mechanism. PLoS One. 2012;7(11):e50069. doi: 10.1371/journal.pone.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12(3):277–88. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 61.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Gu D, Xu N, Lei F, Du L, Zhang Y, et al. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benefic Microbes. 2014;5(3):335–44. doi: 10.3920/BM2013.0071. [DOI] [PubMed] [Google Scholar]
- 63.Badi SA, Khatami S, Irani S, Siadat SD. Induction effects of Bacteroides fragilis derived outer membrane vesicles on toll like receptor 2, toll like receptor 4 genes expression and cytokines concentration in human intestinal epithelial cells. Cell J (Yakhteh). 2019;12(1). [DOI] [PMC free article] [PubMed]
- 64.Wahli W. A gut feeling of the PXR, PPAR and NF-κB connection. J Intern Med. 2008;263(6):613–9. doi: 10.1111/j.1365-2796.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 65.Hasan AU, Ohmori K, Konishi K, Igarashi J, Hashimoto T, Kamitori K, et al. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARγ mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2015;406:10–8. doi: 10.1016/j.mce.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Ishaq S, Nunn L. Helicobacter pylori and gastric cancer: a state of the art review. Gastroenterol Hepatol Bed Bench. 2015;8(Suppl1):6. [PMC free article] [PubMed] [Google Scholar]
- 67.Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS J. 2011;278(4):559–64. doi: 10.1111/j.1742-4658.2010.07979.x. [DOI] [PubMed] [Google Scholar]
- 68.Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, et al. Peroxisome proliferator activated receptor γ in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55(8):1104–13. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, et al. Immunoregulatory actions of epithelial cell PPAR γ at the colonic mucosa of mice with experimental inflammatory bowel disease. PLoS One. 2010;5(4):e10215. doi: 10.1371/journal.pone.0010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang C-s, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 9.34 KB)
(PDF 1.41 MB)