Abstract
Background
Endothelial dysfunction, which is a vascular response to oxidative stress and inflammation, involves a cascade of downstream events that lead to decreased synthesis of insulin-mediated vasodilator nitric oxide (NO) and increased production of vasoconstrictor protein endothelin-1 (ET-1). NO, and ET-1 production by endothelial cells is regulated by phosphatidylinositol 3-kinase (PI3K)-Akt-eNOS axis and mitogen-activated protein kinase (MAPK) axis of the insulin signaling pathway, respectively.
Methods
After treating the human umbilical vein endothelial cells (HUVECs) with either palmitate complexed with bovine serum albumin (BSA) (abbreviated as PA) or the aqueous Cichorium intybus L. (chicory) seed extract (chicory seed extract, abbreviated as CSE) alone, and simultaneously together (PA + CSE), for 3, 12, and 24 h, we evaluated the capacity of CSE to reestablish the PA-induced imbalance between PI3K/Akt/eNOS and MAPK signaling pathways. The level of oxidative stress was determined by fluorimeter. Insulin-induced levels of NO and ET-1 were measured by Griess and ELISA methods, respectively. Western blotting was used to determine the extent of Akt and eNOS phosphorylation.
Results
Contrary to PA that caused an increase in the reactive oxygen species (ROS) levels and attenuated NO production, CSE readjusted the NO/ROS ratio within 12 h. CSE improved the metabolic arm of the insulin signaling pathway by up-regulating the insulin-stimulated phospho-eNOS Ser1177/total eNOS and phospho-Akt Thr308/total Akt ratios and decreased ET-1 levels.
Conclusions
CSE ameliorated the PA-induced endothelial dysfunction not only by its anti-ROS property but also by selectively enhancing the protective arm and diminishing the injurious arm of insulin signaling pathways.
Keywords: Oxidative stress, Chicory seed extract (CSE), Nitric oxide, HUVEC cell, Palmitate (PA)
Endothelial dysfunction is a malfunction of the endothelium, which covers the inner surface of the entire vascular tree and regulates vascular wall homeostasis. It is the initial step of the undesirable events leading to pathological conditions such as atherosclerosis, cardiovascular disease, hypertension, and diabetes, and interconnected with vascular inflammation and oxidative stress [1, 2].
Endothelial dysfunction results from sterile inflammation induced by insulin resistance (IR), metabolic syndrome, hyperglycemia, and lipotoxicity [3]. Endothelial IR, also referred to as peripheral IR, is insulin-insensitivity in the vascular endothelium [4]. Lipotoxicity is defined as high circulating levels of non-esterified saturated free fatty acids (FFA), diacylglycerides, cholesterol, and ceramides; and causes low-grade chronic inflammation in the vital organs, including heart and vessels, as well as in the organs important for calorie sensing and energy metabolism, such as hypothalamus, adipose tissue, skeletal muscle, liver, pancreas, and gastrointestinal tract [5].
An imbalance between the PI3K and MAPK axes of the insulin signaling pathway provides a link between IR and endothelial dysfunction. The metabolic function of insulin is to maintain glucose homeostasis in the body via induction of glucose uptake in insulin-sensitive tissues such as skeletal muscle, fat and heart, inhibition of gluconeogenesis in the liver, and inhibition of lipolysis in adipose tissue. In microvasculature, insulin acts to regulate blood flow, vascular tone, and nutrient delivery to its target tissues by sustaining a steady balance between its more (anti-atherogenic or metabolic arm) and less (mitogenic/pro-atherogenic arm) favorable effects [6]. The anti-atherogenic actions of insulin depend on nitric oxide (NO) production via the activation of phosphatidylinositol 3-kinase (PI3K)-Akt-eNOS axis of the insulin signaling pathway. Nitric oxide is the major vasodilator synthesized by endothelial nitric oxide synthase (eNOS); the endothelial IR is equivalent to deterioration of IRS-1/PI3K/AKT/ eNOS signaling [4]. In a variation, the pro-atherogenic actions of insulin are mediated by endothelin-1 (ET-1), whose secretion from the endothelium is regulated by the mitogen-activated protein kinase (MAPK) axis of the insulin signaling pathway [7]. Both NO and ET-1 originate from the endothelium; NO is a vasodilator and counteracts platelet aggregation, VSMC proliferation, and leukocyte adhesion. ET-1, however, is a vasoconstrictor and contributes to the formation of plaque by stimulating smooth muscle cell proliferation and increasing expression of adhesion molecules. In normal conditions, NO counterbalances the contracting effects of ET-1. An uneven production of these dilator and constrictor substances leading to ET-1/NO imbalance rests at the base of endothelial dysfunction.
Vascular lipotoxicity, IR, and endothelial dysfunction may emerge due to oxidative stress, as well. Oxidative stress is defined as the disproportionate increase of oxidants beyond the buffering capacity of the preventive/repair mechanisms, and enzymatic/non-enzymatic antioxidant defenses [8–10]. As follows, a surge in ROS accompanied by depletion of NO is the earliest event in the endothelial dysfunction [6]. Insulin also has a redox-regulating role that becomes impaired during IR [10, 11]. According to the mechanisms mentioned above, a possible therapeutic approach in the treatment of endothelial dysfunction would be to restore the natural equilibria between PI3K and MAPK axes of the insulin signaling pathway, and between the levels of NO and ROS.
Herbal supplements have been useful in traditional medicine in a variety of clinical settings. Persian Medicine refers to Cichorium intybus L. (chicory) as a valuable medicinal plant [12]. The primary evidence regarding the effectiveness of chicory in the treatment of cardiovascular diseases was provided by experiments with isolated toad heart showing that chicory extracts could reduce cardiac rate like quinidine [13]. Besides, we have previously reported the insulin-sensitizing activity of CSE [14] and its ameliorating action on IR [15]. Most of the health benefits derived from plants are attributed to the detoxification and free radical scavenging activities of their constituent phytochemicals [16–28] and their alternative modes of action, such as their capacity to regulate gene expression, have remained largely unexplored.
The purpose of the present study was to induce endothelial dysfunction in HUVECs using palmitate (PA, which stands for palmitate-BSA). We preferred to use PA for this purpose because according to several studies not only does oleic acid not impair insulin signaling but it also prevents PA-induced H2O2 production and protects against cardiovascular insulin resistance [29–32]. As endothelial dysfunction is manifested by an upsurge in ROS and a depletion of NO [6], ROS and NO were measured in PA-treated cells. We measured the expression of the phosphorylated as well as the unphosphorylated Akt and eNOS proteins by Western blotting and determined the ET-1 levels by ELISA to evaluate the PA-induced impediments in the metabolic- and mitogenic- arms of the insulin signaling pathway. To investigate the capacity of CSE to invert these effects, we simultaneously co-treated the cells with PA + CSE to investigate CSE’s potentiality to prevent ROS/NO and ET-1/NO imbalance. For further comparison, other groups of HUVECs were exposed to chicory seed extract (CSE) but not to PA. We used water extract because it is closest to homemade preparations. Besides, as water comprises 80–90% of plants’ total weight, the plant components may better retain their natural shape and proportions in a watery extract.
Materials and methods
Cell culture
The HUVEC cell line was purchased from the Pasteur Institute of Iran. The cells were grown at 37 °C in a humidified atmosphere of 95% air, 5% CO2, in complete DMEM, i.e., low glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 mg/mL streptomycin, and 100 units/mL penicillin.
Preparation of CSE
The water extract of CSE (20% w/v solution) was prepared as previously explained [15]. A sample of the seeds, voucher number PMP-710, were deposited at the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences. Every 100 g of powdered seed yielded about 4.6 g of lyophilized substance. At the start of every experiment, a fresh stock CSE solution (10 mg/ml) was prepared in serum-free DMEM and filter sterilized before use [0.45-μm pore size disposable PES membrane filter; BioFACT, Korea].
Preparation of PA-BSA complex
The conjugation of PA to bovine serum albumin fatty acid-free (FFA-free BSA; Sigma-Aldrich, München, Germany) was achieved as described elsewhere [33]. The PA stock solution (200 mM) was prepared in ethanol and diluted with 10% FFA-free BSA-DMEM to a stock solution of 5 mM at 37 °C in a shaking water bath. After filter sterilization, this solution was stored at −20 °C and used within two weeks. Upon use, the stored stock solution was thawed at 37 °C and diluted to appropriate concentrations using serum-free DMEM. Throughout the manuscript, PA-BSA will be referred to as PA.
Cell viability assays
We determined the viability of the HUVECs by MTT assay. Briefly, cells were seeded in 96-well plates at a density of 8 × 104 per well in 200 μl medium until they reached 60%–70% confluence. After starvation in serum-free medium for 2 h, the medium was changed to one containing the indicated final concentrations of PA (90, 180, 450 and 900 μM), CSE (0.562, 1.125, 2.25, 4.5, and 9.0 mg/ml), or mixtures of PA and CSE [PA (90 μM) + CSE (0.562, 1.125, 2.25, and 4.5 mg/ml)] (Table 1). Different recipes were prepared in separate Falcon tubes, using the PA and CSE stock solutions and serum-free DMEM; we replaced 10% of each solution with FBS before addition onto the cells (200 μl). After incubation for 24 h at 37 °C in a humidified chamber, we added the MTT reagent (20 μl, 5 mg/ml in phosphate-buffered saline, abbreviated PBS); and incubation continued for another 4 h. Then, we replaced the media with 150 μl isopropanol. The absorbance, which is proportional to the number of live cells, was recorded using a microplate reader (BioTek, USA) at 570 nm. In this experiment, the group called blank which consisted of complete DMEM containing10% FFA-free BSA, and 0.02% ethanol, was arbitrarily assigned 100% viability according to Ye et al. and Fratantonio et al. [34, 35]. We expressed the cell viability as the optical density ratio of the treatment to the blank.
Table 1.
Study groups
| Groups Procedure | Reagent | Blank | Con |
Con-Insulin (Con with 100 nM insulin) |
PA (90 μM) |
PA/CSE PA (90 μM)/CSE (mg/ml) |
CSE (mg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTT | PA | – | NA | NA | + | + | + | + | + | – | – | – | – |
| CSE | – | NA | NA | – | 0.562 | 1.125 | 2.25 | 4.5 | 0.562 | 1.125 | 2.25 | 4.5 | |
| ROS | PA | NA | – | NA | + | + | + | + | + | – | – | – | – |
| CSE | NA | – | NA | – | 0.562 | 1.125 | 2.25 | 4.5 | 0.562 | 1.125 | 2.25 | 4.5 | |
|
Nitric Oxide Western blot ET-1 Elisa |
PA | NA | – | – | + | + | + | + | – | – | – | ||
| CSE | NA | – | – | – | 1.125 | 2.25 | 4.5 | 1.125 | 2.25 | 4.5 | |||
| Insulin | NA | – | + | + | + | + | + | + | + | + | |||
NA, not applied. Blank consisted of complete DMEM containing10% FFA-free BSA, and 0.02% ethanol. The Con is the control group that consisted of complete DMEM. A Con-Insulin group, the same as control with added insulin, was not considered for MTT and ROS experiments. For measuring nitric oxide and ET-1 concentration, and for Western blotting, nine groups were prepared as described in the last 3 rows of the table
The groups treated with the lowest concentration of chicory seed extract (CSE) were not included in the final three experiments. PA stands for palmitate-BSA. The concentrations given for PA and CSE are the final concentrations, after adjusting for the replacement of 10% of each solution with FBS before addition onto the cells (refer to methods section). The actual concentrations were 100, 200, 500 and 1000 μM for PA and 0.625, 1.25, 2.5, 5, and 10 mg/ml for CSE
Study groups
After selecting the appropriate concentrations for PA and CSE according to MTT results, the study groups were assigned as control (Con, complete DMEM), PA (exposed to PA only), PA + CSE (treated with PA and three or four different concentrations of CSE), and CSE (treated with three or four different concentrations of CSE without exposure to PA). The groups receiving 0.562 mg/ml of CSE were later excluded from the study as they did not produce significant changes in MTT nor ROS assays. As the focus of the present study was to investigate the effect of PA and CSE on the insulin-induced outcomes of the metabolic and mitogenic arms of the insulin signaling pathway, an extra group was included called Con-insulin, when preparing different groups of cells for measuring NO, ET-1 levels and Western blotting. All groups, except Con, were treated with 100 nM insulin 30 min before measurements or cell collection (Table 1) according to Jeong et al. [33].
Measurement of intracellular ROS accumulation
ROS scavenging activity was measured using the cell-permeable dichloro-dihydro-fluorescein diacetate (DCFH-DA, Sigma Aldrich, St. Louis, USA). HUVECs were seeded in black 96-well as well as ordinary 6-well plates. After adherence, cells were treated with indicated concentrations of PA, PA + CSE, or CSE for 3, 12, and 24 h and stained with 25 μM DCFH-DA (30 min, 37 °C). These time points have been considered separately in other studies [33, 34, 36]. The fluorescence intensity was measured using a multi-detection plate reader (BioTek, USA) at an excitation and emission wavelength of 488 nm and 525 nm, respectively [37]. The 6-well plates were used to capture images by a fluorescence microscope (Zeiss, Germany).
Measurement of nitrite (NO2-) accumulation
Nitrite accumulation was used as an indicator of NO production in the medium. HUVECs were treated with PA, PA + CSE, or CSE (Table 1) for 3, 12, and 24 h, several hours after seeding (8 × 104) in a 96-well plate to allow them to adhere. All cells, except Con, were exposed to insulin (100 nM) 1 h before assay. The cell culture media were incubated with NaOH and ZnSO4 to precipitate proteins at room temperature. A 100 ml sample of each supernatant was mixed with 100 ml of Griess reagent (1% sulfanilamide, 0.1% N-(1-Naphthyl)ethylenediamine dihydrochloride (NEDA·2HCl), and 2.5% H3PO4; Sigma, St. Louis, USA) per well of 96-well plate. Absorbance was read in a microplate reader at 540 nm against a standard curve of NaNO2 (1.5–100 mM) in the culture medium [38].
Western blotting analysis
HUVECs grown in 25 cm2 flasks were treated with PA, CSE, PA + CSE for 12 h (Table 1). Cells were harvested, washed with PBS, and lysed in urea lysis buffer (7 M Urea, 2 M Thiourea, 65 mM 1,4-dithiothreitol (DTT), and 1% (v/v) protease inhibitor cocktail) on ice. After centrifugation (30 min, 10,000×g, 4 °C), proteins (40 μg) contained in the supernatants [39] were separated on 8% SDS-PAGE electrophoresis (BioRad, USA), and transferred to nitrocellulose membrane. After blocking with Tris-buffered saline containing 3% BSA (overnight, 4 °C), membranes were incubated overnight at 4 °C with 3% BSA containing the primary antibodies for Akt (1:1000 dilution), P-Akt (T308,1:1000), eNOS (1:1000) (Abcam, Cambridge, MA, USA), p-eNOS (S1177, 1:200) (Biorbyt, Cambridge, UK), and GAPDH (1:500) (Abnova, Taipei, Taiwan) (overnight at 4 °C). After 3 washes in Tris-buffered saline containing 0.2% Tween 20 (TBST), membranes were incubated with horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG,1:7000) for 2 h at room temperature and visualized by enhanced chemiluminescence (ECL; Amersham, Italy).
ELISA assay for endothelin-1
HUVECs, seeded in 96-well plate, were exposed to PA, CSE, or PA + CSE (Table 1), for 12 h. Supernatants were collected and centrifuged (10 min at 250×g) and used to determine the concentration of protein and ET-1 using BCA Protein Quantification kit (Pars-Tous, Iran) and the enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, MA, USA), respectively.
Statistical analysis
Statistical analysis was performed by SPSS software (version 20, Chicago, IL). The normal distribution of continuous variables was checked by the Kolmogorov–Smirnov test in each group. For continuous variables with normal distribution in groups, the comparison was carried out by one-way analysis of variance (ANOVA) followed by the Scheffe and Dunnett T3 post hoc tests according to the homogeneity of variances in subgroups observed or not, respectively. In groups without normal distribution, after the Kruskal-Wallis test, the post hoc nonparametric two-independent samples test/Mann Whitney u was used for analysis. All assays were conducted in duplicate and each experiment was performed at least three times or as indicated in the legends. A probability (P) value of less than 0.05 was considered significant in all statistical analyses. Data were presented as the mean ± SEM. The diagrams were drawn using Prism 8 software.
Results
Effect of PA and CSE on HUVECs viability
Cell viability was decreased by increasing concentrations of PA such that a 30% decrease in cell proliferation was achieved by PA at 90 μM (Fig. 1). Cell viability was around 80% or higher with CSE doses less than 4.5 mg/ml; a higher dosage of CSE (9 mg/ml) led to ~40% reduction in cell viability (Fig. 1); based on these results, one dose of PA (90 μM) and three doses of CSE (1.125, 2.25 and 4.5 mg/ml) were selected for use in subsequent experiments. To assess the effect of co-treatment on cell viability, cells were co-treated, simultaneously with PA and the selected doses of CSE. The results suggested that CSE (at 1.125 mg/ml) overcame the inhibitory effect of PA and allowed the number of viable cells to reach 94% compared to 72% with PA (90 mM, p < .05) (Fig. 1).
Fig. 1.
Effect of CSE, PA, and their combination, PA + CSE, on HUVECs viability. HUVECs were separately treated with different doses of PA-BSA (a), 0.562, 1.125, 2.25, 4.5 and 9.0 mg/ml of CSE (b), or simultaneously with PA-BSA (90 mM) and CSE (0.562, 1.125, 2.25, and 4.5 mg/ml) (c) for 24 h. Cell viability was measured by MTT assay. Data are shown as mean ± SEM of at least three separate duplicate experiments; *p < .05, **p < .01, ***p < .001, and ****p < .0001. PA, an abbreviation for palmitate-BSA; CSE, chicory seed extract; and Con, control cells without any treatment
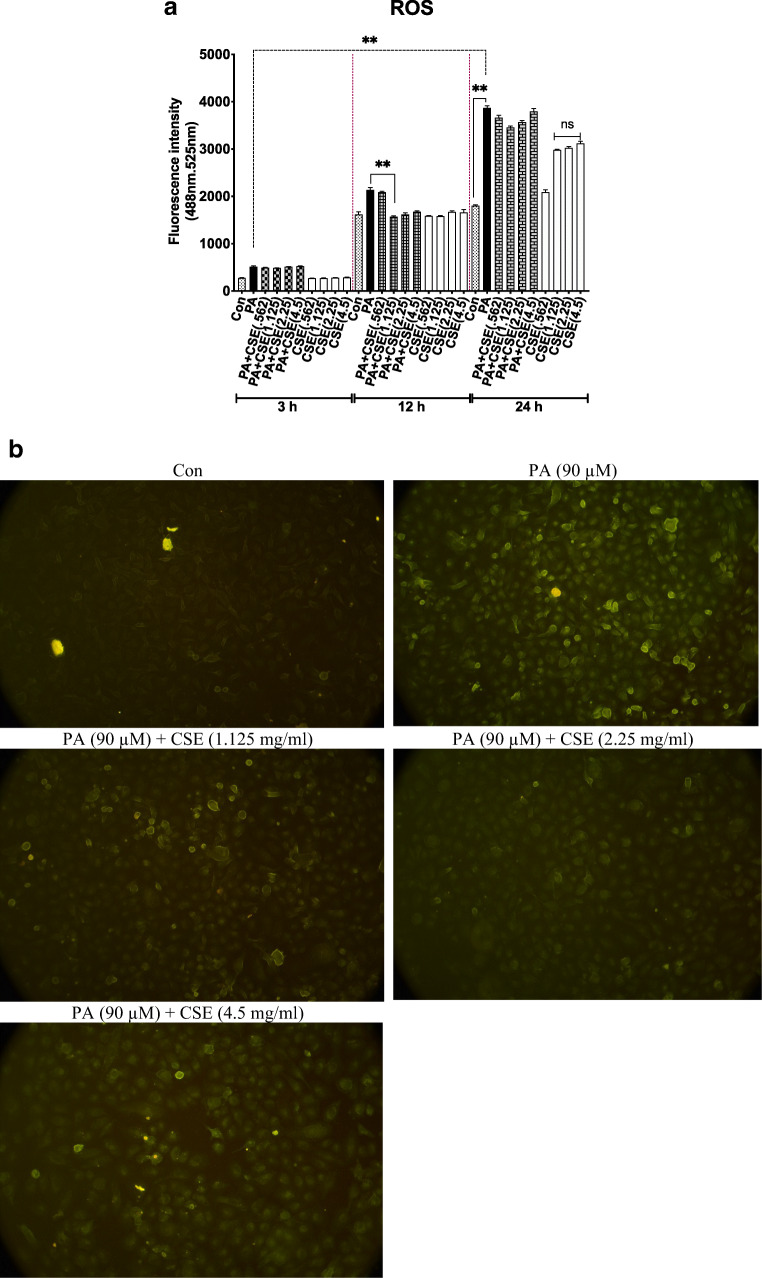
Effect of PA and CSE on ROS production in HUVECs
HUVECs were exposed to PA (90 μM) in an attempt to mimic the natural condition of endothelial cell exposure to hyperlipidemia. The increase in ROS production was rapid and time-dependent during 3, 12, and 24 h (Fig. 2a, black bars) in line with Fratantonio et al. during 3 h [40], Zhang et al. during 12 h [41], Kim et al. during 18 h [42] and Mahmoud et al. during 3 and 24 h [43].
Fig. 2.
Elimination of PA-induced ROS by CSE. ROS levels were measured by DCFH-DA fluorimetric assay in cells incubated with PA (90 μM), CSE (0.562, 1.125, 2.25, 4.5 mg/ml), or both for 3 h, 12 h and 24 h (a). After 12 h of treatment, higher fluorescence intensity was observed in the PA(90 μM)-treated group compared to control using a microscope (b) which seemed to decrease by low dose (1.125 mg/ml and 2.25 mg/ml) better than high (4.5 mg/ml) dose of CSE. Magnification is ×10. Data are shown as mean ± SEM of at least three separate duplicate experiments; **p < .01. The increase in ROS levels in the groups treated with CSE alone for 24 h was not significant, compared to Con. PA, an abbreviation for palmitate-BSA; CSE, chicory seed extract; and Con, control cells without any treatment
Although the exposure of cells to PA and CSE was simultaneous in the co-treatment (PA + CSE) groups, no ROS scavenging activity was observed in the first 3 h (Fig. 2A). At 12 h, however, all doses of CSE tended to decrease ROS levels (~32.5%, 29.3% and 26.6% reduction of ROS for CSE doses 1.125, 2.25, and 4.5 mg/ml, p = 0.033, p = 0.54, p > 0.99, compared to PA, respectively), but only one dose of CSE (1.125 mg/ml) had significant effect. An absence of anti-ROS activity by 24 h time (Fig. 2a) could be due to the consumption of the entire CSE components for buffering ROS. This and the observed increase in ROS levels (relative to Con) in CSE-only-treated groups (1.125, 2.25, and 4.5 mg/ml, for 24 h without PA) could be due to the decay or oxidation of CSE components. The concentration of ROS in different groups, visualized by fluorescence staining at 12 h of treatment, is shown in Fig. 2b.
Effect of PA and CSE on insulin-induced NO production
PA tended to decrease the NO production by HUVECs during 12 and 24 h (42.9% and 35.6%, p < .01, and p < .05, respectively compared to Con-insulin) (Fig. 3) in accordance with Jeong et al. [33] who reported reduced NO levels during 12 h. Unlike Mahmoud et al. [43], nitric oxide levels did not decrease following 3 h treatment with PA. In PA + CSE groups, the NO production was triggered by CSE (all doses) during both 12 and 24 h (CSE doses 1.125, 2.25 and 4.5 mg/ml led to ~25.6%, 27.9% and 51.2% increase during 12 h; and 35.4%, 35.3% and 44.2% increase during 24 h; respectively, compared to PA) (Fig. 3); however, the effect of only one dose of CSE was significant during 12 h (4.5 mg, p = 0.017). Treatment of cells with CSE alone tended to significantly increase NO production relative to Con-insulin (4.5 mg/ml, for 12 h without PA, p = 0.047). The overall effect of CSE on lowering ROS and boosting NO levels was a significantly reduced ROS/ NO ratio (or increased NO/ROS ratio) during 12 h treatment (Fig. 4). For this reason, 12 h treatment was chosen for the rest of the study (Western blotting and ET-1 activity).
Fig. 3.
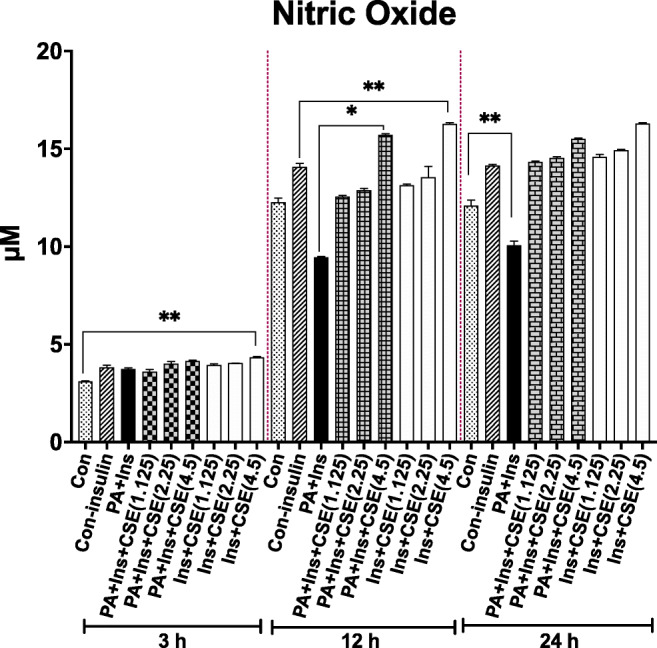
CSE modulation of NO release in PA-treated HUVECs. Production of NO did not change in PA, PA + CSE, or CSE groups, in a 3 h period. Stimulation of HUVECs with PA for 12 and 24 h caused a remarkable decrease in NO production. Simultaneous treatment with CSE (4.5 mg/ml) and treatment with CSE alone (4.5 mg/ml) led to significant increases in NO release during 12 h. Data are shown as mean ± SEM of at least three separate duplicate experiments; *p < .05, **p < .01. PA, an abbreviation for palmitate-BSA; CSE, chicory seed extract; NO, nitric oxide; and Con, control cells without any treatment
Fig. 4.

Adjustment of ROS: NO ratio by CSE. PA caused a rise in ROS: NO ratio (or a reduction in NO: ROS ratio, not shown) relative to control. In the PA-treated group, at all periods, ROS: NO was highest. After co-treatment, CSE readjusted the ratios to near Control values during 12 h and 24 h treatment time, although only one dose of CSE (4.5 mg/ml) during 12 h treatment time caused a statistically significant reduction. In groups that were treated with CSE alone, ROS: NO ratio was also reduced. Data are mean ± SEM; *p < .01, **p < .01. PA, the abbreviation for palmitate-BSA; CSE, chicory seed extract; ROS, reactive oxygen species, and Con, control cells without any treatment
Effect of PA and CSE on the Akt/eNOS signaling pathway at 12 h treatment
The PI3K/Akt/eNOS pathway links insulin to its metabolic (anti-atherogenic) actions. Stimulation of insulin receptor (INSR) leads to downstream activation of eNOS by its phosphorylation at Ser1177; whereas the enzyme responsible for this phosphorylation, Akt kinase (protein kinase B), is in turn phosphorylated at Thr308 by phosphoinositide-dependent kinases (PDKs) which are stimulated by lipid produced by PI-3 kinase (phosphatidylinositol 3-kinase). As decreased NO release by PA-treated HUVECs may be related to a decline in this pathway, eNOS and Akt protein expression and phosphorylation were analyzed by Western blotting. Contrary to some reports [44] but in line with others [45, 46], GAPDH did not change under different treatments and so was used as a loading control.
Effect of PA and PA + CSE on insulin-stimulated Akt phosphorylation
The Akt total protein expression displayed no change in different treatment groups, after adjusting against GAPDH (Fig. 5). Insulin-induced Akt (Thr308) phosphorylation was inhibited by PA (PA group compared with Con-Insulin, p < .05). In the PA + CSE groups, CSE led to a promotion of Akt phosphorylation (2.25 mg/ml p < .01 and 4.5 mg/ml, p < .001, compared to PA) suggesting amelioration of PA induced-IR in the endothelial cell by CSE. All doses of CSE alone could also increase Akt phosphorylation relative to Con-Insulin (Fig. 5).
Fig. 5.
Amelioration of PA-induced decline in Akt and eNOS phosphorylation in HUVECs by CSE. Levels of phospho-Akt and phospho-eNOS as determined by immunoblot analysis (a) and densitometry analysis of p-Akt relative to total Akt and p-eNOS to total eNOS (b). Cells were incubated with PA (90 μM), CSE (1.125, 2.25, 4.5 mg/ml), or both for a total of 12 h followed by the addition of 100 nM insulin for 30 min before collection of the cells. A correlation seemed to exist between the dose of CSE and Akt phosphorylation but not between CSE dosage and eNOS phosphorylation even though eNOS phosphorylation is regulated by p-Akt, which may imply that at a high dose of CSE (4.5 mg/ml) eNOS phosphorylation may be regulated by other means such as other protein kinases (AMPK, PKA). Data are mean ± SEM of 3 separate experiments; *p < .05, **p < .01. PA, an abbreviation for palmitate-BSA; CSE, chicory seed extract; and Con, control cells without any treatment
Effect of PA and PA + CSE on insulin-stimulated eNOS phosphorylation
Exposure of HUVECs to PA for 12 h led to significantly lower eNOS phosphorylation without any change in the expression of total eNOS protein (Ser1177) (p < .01). Co-treatment with CSE increased the phosphorylation of eNOS compared to the PA group. When treated with CSE alone, all dosages increased eNOS levels but only the highest dose (4.5 mg/ml; p < .01) led to significantly higher production of p-eNOS relative to Con-Insulin.
Effect of PA and CSE on ET-1 production
According to our results, stimulation of HUVECs with PA, in the presence of insulin, increased ET-1 concentrations in supernatant cell cultures. This event was reverted by CSE which led to a decrease in the insulin-induced production of ET-1 in a dose-dependent manner (Fig. 6). These results were in line with those of Storniolo et al. who observed that the increased synthesis of ET-1 in ECV304 cells, upon their treatment with high glucose together with FFAs, was reversed by hydroxytyrosol and polyphenol fraction of extra virgin olive oil [47].
Fig. 6.

Inhibition by CSE of PA-induced ET-1 production in HUVECs. Cells were incubated with PA (90 μM), CSE (1.125, 2.25, 4.5 mg/ml), or both for 12 h, followed by the addition of 100 nM insulin for 30 min. ET-1 production was increased significantly in the PA group. Higher doses of CSE in the PA + CSE and CSE groups decreased the ET-1 levels. Data are mean ± SEM of three readings in each group; *p < .05, **p < .01. PA, an abbreviation for palmitate-BSA; CSE, chicory seed extract; and Con, control cells without any treatment
Discussion
Treatment with PA led to the expected outcomes concerning the induction of ROS production. Induction of ROS production by PA has been reported to have occurred in many cell types, including myocytes [48], hepatocytes [49], adipocytes [50], placenta [51], keratinocytes [52], peripheral blood mononuclear cell (PBMCs) [53] and endothelial cells [42]. The mechanism(s) for the induction of ROS production by PA may include one or any combination of the following: disruption of the electron transport chain (ETC) [54], activation of NADPH-oxidases (Nox family) [3, 55–57], uncoupling of eNOS that changes the enzyme’s activity from a producer of NO to a producer of ROS (superoxide) [58], enzyme activities such as xanthine oxidase, xanthine dehydrogenase, and cyclooxygenases (COX), and finally a decline in antioxidant defense systems such as glutathione peroxidase (GPx) [59].
Although in some situations, PA has led to the augmentation of NO production [53, 60], in the present study, treatment with PA led to the prevention of NO production by HUVECs, in line with many reports [47, 61, 62]. The reasons for decreased NO production by HUVECs after exposure to PA (Fig. 3) may include the uncoupling of eNOS and its degradation due to the enhanced formation of oxygen radical (•O-2, superoxide anion) [41, 63]. Other means of increased NO degradation include scavenging by oxyhemoglobin to yield methemoglobin and inorganic nitrate or reacting with thiol groups in proteins to yield S-nitrosothiols [58, 59].
As a plant rich in antioxidant phytochemicals, CSE was expected to be quick and strong against ROS. According to the results, CSE was able to lower ROS levels in PA + CSE(1.125 mg/ml) group during 12 h; and the other doses and time frames did not lead to the same conclusion. Studies addressing the antioxidant effects of chicory and its metabolites have sometimes produced contradictory results [64]. For example, chicoric acid, a constituent of chicory, has been reported to alleviate oxidative stress and inflammation in endothelial cells, as well as the lungs [27] while it was shown to increase ROS production and generate proapoptotic state, dose- and time-dependently, in 3 T3-L1 preadipocytes [65]. Chicory root extract (50 mg/ml) has been found to lower H2O2-induced oxidative stress in C2C12 myoblasts during 24 h [21] whereas furfural, a chicory root ingredient, was found to induce ROS accumulation and cellular damage in Saccharomyces cerevisiae [66].
Although CSE led to increased production of NO by HUVECs, in line with a previous study from our laboratory [15], chicoric acid, an individual component of chicory, has been found to have both NO-enhancing [27, 67] and NO-diminishing effects [28]. Conflicting results have been obtained also for compounds such as resveratrol that was reported to increase NO production by endothelial cells and platelets [68, 69] but seemed to lower it in hepatocytes [70]; and a different study has suggested that the effect of resveratrol on NO production may be dose-dependent, with low and high doses leading to decreased and increased production of NO, respectively, by endothelial cells [71]. A comparison of our results with those of other studies suggests that factors as the duration of exposure [72], the dose of PA or drug [73] and cell type [53] may influence the outcome of exposing HUVECs to PA or a drug like CSE but it is also possible that at any combination of time and dosage certain component(s) of CSE and their integrated action may prevail over other combinations.
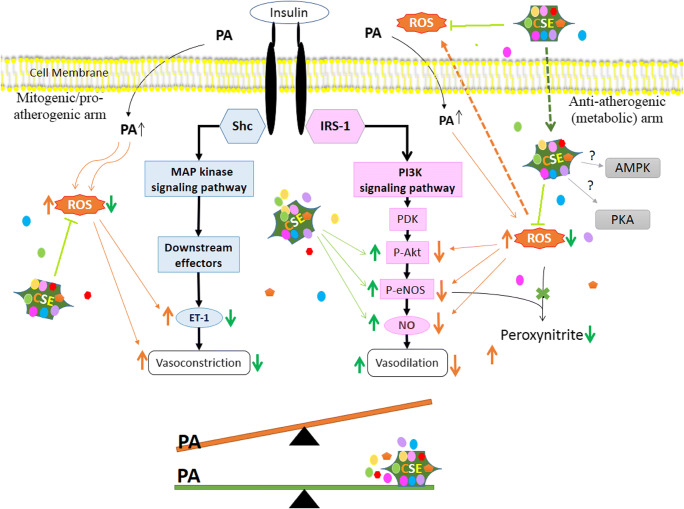
The insulin signaling cascade and its relation to endothelial dysfunction have been summarized in Fig. 7. In physiological conditions, the binding of insulin to the transmembrane INSR leads to parallel and balanced activation of these pathways. However, certain physicochemical factors such as changes in pH, hypertension, obesity, atherosclerosis, myocardial infarction, congestive heart failure, aging, and even air pollution can cause an imbalance in the activity of these pathways by favoring the MAP-kinase branch of insulin signaling [74, 75]. In the state of endothelial dysfunction and IR, the MAPK/ERK/ET-1 pathway is either preserved or augmented while the IRS-1/PI3K/AKT/ eNOS signaling pathway deteriorates [6]. ET-1 induces pro-atherogenic effects and vasoconstriction by binding to endothelin (ETA and ETB) receptors, and there is a vicious circle between oxidative stress and ET-1 production; oxidative stress leads to upregulation of ET-1, and in return, ET-1 stimulates ETA-mediated NADPH oxidase-dependent ROS generation, which inhibits endothelial NO bioavailability [2].
Fig. 7.
Summary of PA and CSE action on insulin signal transduction pathway and endothelial dysfunction. The insulin signaling cascade is comprised of the PI3K-Akt pathway (metabolic arm) and the Sch-MAPK pathway (mitogenic arm). When insulin binds to INSR, a conformational change and autophosphorylation of the receptor lead to the recruitment and phosphorylation of receptor substrates such as IRS and Shc proteins. Shc activates the MAPK/ERK/ET-1 pathway, which leads to the secretion of ET-1 and adhesion molecule expression in vascular endothelium, promotion of growth and differentiation, and hormone production leading to the promotion of inflammation and atherogenesis (these are collectively known as the mitogenic, pro-inflammatory effects). In the PI3-kinase arm, tyrosine phosphorylation of IRS proteins, allows them to bind to PI3K. PI3K activates phosphoinositide-dependent kinase (PDK) which promotes the phosphorylation of Akt (p-Akt) at Thr308 and Ser473. Activated Akt phosphorylates eNOS which regulates NO production and vasodilatation in vascular endothelium. Akt also plays a role in the control of glucose transport, lipid synthesis, gluconeogenesis, glycogen synthesis, cell cycle, and survival (these effects are broadly described as “anti-inflammatory effects”). In the PA-treated cells, the pro-inflammatory effects overtake the anti-inflammatory pathway whereas in the presence of CSE this imbalance is prevented. When CSE is added to the culture medium, its components dissolve and segregate. A portion of phytochemicals may enter the cell cytoplasm. Inside or out, CSE components neutralize ROS as much as possible. The preferential increase in NO production occurs via augmentation of the phosphorylation of Akt and eNOS proteins due to activation of PI3K/NO pathway, and attenuated production of ET-1 is achieved via the prevention of MAPK/ET-1 pathway. Akt (Protein kinase B); AMPK: 5′ Adenosine Monophosphate-Activated Protein Kinase; CSE: Chicory Seed Extract (aqueous); eNOS: Endothelial Nitric Oxide Synthase; ERK1/2: Extracellular Regulated Kinase-1 and -2; ET-1: Endothelin-1; INSR: Insulin Receptor; IRS-1: Insulin Receptor Substrate 1; MAPK: Mitogen-Activated Protein Kinase; NO: Nitric Oxide; PA, an abbreviation for palmitate-BSA; PDK1: Phosphoinositide-Dependent Kinase 1; PI3K: Phosphatidylinositol 3-Kinase; PKA: Protein Kinase A (cAMP-Dependent Protein Kinase); ROS: Reactive Oxygen Species; Shc: Src-Homology-2-Containing Proteins
In the present study, stimulation of HUVECs with PA, in the presence of insulin, increased the ET-1 protein expression in agreement with many reports [34, 76, 77]. In co-treatment groups, CSE prevented insulin-induced ET-1 production implying that the MAPK/ERK/ET-1 pathway was triggered by PA and impeded by CSE. The inhibition of Akt phosphorylation by PA was in line with other studies that showed decreased phosphorylation of Akt at Ser473 by PA in human HepG2 cells and rat hepatocytes [78, 79]. It has been reported that in C2C12 cells, PA decreases Akt phosphorylation at both Ser473 and Thr308 [80, 81]. The present study showed that the inhibitory effect of PA on Akt phosphorylation at Thr308 was prevented dose-dependently by the simultaneous treatment of HUVECs with PA + CSE, and all CSE doses were able to induce further Akt phosphorylation in normal HUVECs that were not treated with PA. Chicoric acid has been reported to stimulate Akt phosphorylation in C2C12 myotubes even independent of insulin [82], whereas it was found to decrease insulin-induced Akt phosphorylation in L6 myotubes at Ser473 [83].
Concerning eNOS phosphorylation, 12 h exposure of HUVECs to PA caused a decrease in eNOS phosphorylation without a change in the expression of total eNOS protein (Ser1177) (p < .01). Similar results have been obtained within 30 min and 16 h of PA exposure by others [46, 84]. Another study has reported that PA-driven increase in eNOS phosphorylation, in endothelial cells within 3 and 6 h exposure, disappeared by 12 h [85]. The impaired eNOS expression in the PA-treated group can be attributed to oxidative stress [86] because superoxide due to hyperglycemia has been shown to reduce eNOS expression in endothelial cells [87], and oxidative stress due to smoking leads to inactivation of eNOS [88]. Therefore, compounds with antioxidant properties should be expected to counteract the oxidative stress-induced imbalance in the insulin signaling cascade. In support of this notion, previous findings have shown that anthocyanins and chicoric acid are capable of increasing eNOS phosphorylation [27, 89]. One study, however, reported that chicory and one of its components, protocatechuic acid, did not affect eNOS phosphorylation [90].
According to our results, in the PA + CSE groups where the HUVEC cells were simultaneously treated with PA and CSE, the speed of ROS buffering activity was slow (an optimal reduction in ROS levels and ROS: NO ratios took 12 h to achieve) and seemed to lag behind the speed of ROS production (which began as quickly as 3 h). Besides, the doses of CSE that could increase Akt and eNOS phosphorylation (Fig. 5) and decrease ET-1 concentration (Fig. 6) did not match up exactly with doses that exerted significant ROS scavenging effects (Fig. 2a & b). Therefore, it is possible to suggest that the enhancement of Akt and eNOS phosphorylation and the attenuation of ET-1 production by CSE occurred independently of its ROS neutralizing activity. Hence, it is possible that when CSE is added to the culture medium, its components dissolve and segregate and a portion may enter the cell cytoplasm (Fig. 7). Both inside and outside of the cells, CSE components start buffering the ROS. Some of the components of CSE individually or synergistically act to increase insulin-stimulated Akt phosphorylation and lower ET-1 production. The eNOS phosphorylation is known to be regulated by p-Akt and although the results suggested the presence of a correlation between CSE dosage and Akt phosphorylation (Fig. 5), such a correlation between CSE dosage and eNOS phosphorylation seemed to be vague, which could imply that high doses of CSE may be able to regulate eNOS phosphorylation through other protein kinases such as AMPK or PKA (cyclic AMP-dependent enzyme). Regarding the attenuation of ET-1 production, the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) have been shown to ameliorate endothelial function by inhibiting pre-proET-1 mRNA expression in a concentration- and time-dependent fashion. Statins such as atorvastatin and simvastatin were able to reduce immunoreactive ET-1 levels with no significant modification of pre-proET-1 mRNA half-life [91] whereas pravastatin could significantly reduce ET-1 protein secretion without ameliorating ET-1 expression in HUVECs [92]. Further study is needed to determine if CSE components act as ET-1 antagonists, that block one or both receptors, or they act to lower ET-1 expression at the gene level.
It should be mentioned that the attenuated production of eNOS is usually expected to be accompanied by the activation of the inducible nitric oxide isozyme (iNOS) [73, 93] but as so many functions, such as the survival, and vaso-relaxing properties, of the endothelial cells depend on eNOS, the effect of PA or CSE on changes of iNOS expression were not considered in the present study. As endothelial dysfunction and IR are phenomena that develop over time and need chronic treatments, the efficacy of long-term (chronic) treatment with CSE should be tackled in future studies. According to our data on ROS production and cell viability at 24 h time, chronic treatment with CSE might be detrimental in vitro (Fig. 1); and high concentrations of NO due to CSE can be of concern [94, 95]; but the human body is an open system capable of easily eliminating most of the unwanted water-soluble compounds. Also, recently there has been a controversy over the benefit of continued consumption of antioxidant supplements as they may deprive the body of free radicals and oxidative stress’s beneficial effects and render it prone to cancer and metastasis. Although more study is needed, our results seemed to suggest that the free radical scavenging properties of plant components do not last for long and are not the sole determinants of the outcomes of medicinal plant consumption. Also, medicinal plants may present an advantage over single drugs because they possess many phytochemical ingredients in naturally correct proportions, whilst the supplemental consumption of individual antioxidants, such as vitamin E or C, has failed to produce clinical protection against different diseases.
Conclusion
In the state of endothelial IR, where ROS levels are high, the PI3K/NO pathway is selectively inhibited, and the MAPK/ET-1 pathway is preserved or even increased, CSE can improve the endothelial dysfunction not only through free radical scavenging activity, but also through selective augmentation of the protective arm and attenuation of the harmful arm of the insulin signaling pathway, most probably by increasing vascular eNOS protein expression via PI3K/Akt dependent phosphorylation, and decreasing ET-1 secretion, respectively.
Acknowledgments
The authors would like to thank Mr. Ali-Akbar Hasseli of the Institute of Biochemistry and Biophysics (IBB) for his help with fluorimeter and fluorescence microscope. This article is the product of the MSc thesis of the first author.
Abbreviations
- Akt
(Protein kinase B)
- AMPK
5' Adenosine Monophosphate-Activated Protein Kinase
- BSA
Bovine Serum Albumin
- CSE
Chicory Seed Extract (aqueous)
- DMEM
Dulbecco's Modified Eagle's Medium
- eNOS
Endothelial Nitric Oxide Synthase
- ET-1
Endothelin-1
- FBS
Fetal Bovine Serum
- FFA
Free Fatty Acids
- HUVECs
Human Umbilical Vein Endothelial Cells
- HRP
Horseradish Peroxidase
- IKKβ
Inhibitor of Nuclear Factor Kappa-B Kinase Subunit Beta
- iNOS
Inducible Nitric Oxide Synthase
- INSR
Insulin Receptor
- IR
Insulin Resistance
- MTT
3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2H-Tetrazolium Bromide
- MAPK
Mitogen-Activated Protein Kinase
- PES
Polyethersulfone
- NO
Nitric Oxide
- NF-κB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- PA
an abbreviation for palmitate-BSA
- PI3K
Phosphatidylinositol 3-Kinase
- PKA
Protein Kinase A
- ROS
Reactive Oxygen Species
Authors’ contributions
RA conceptualized the idea and performed the experiments; AN conceptualized the idea and wrote the paper; MBK provided technical assistance; AM assisted with statistical analysis; SA helped drafting the manuscript.
Funding
This work was financially supported by Tehran University of Medical Sciences and Health Services under Grant No. 95–02–30-32269.
Compliance with ethical standards
Ethical approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raziyeh Abdolahipour, Email: r.abdollahi1370@yahoo.com.
Azin Nowrouzi, Email: anowrouzi@tums.ac.ir.
Masoumeh Babaei Khalili, Email: m_b_khalili@ut.ac.ir.
Alipasha Meysamie, Email: Meysamie@tums.ac.ir.
Samin Ardalani, Email: Samin_ardalan@yahoo.com.
References
- 1.Citi V, Martelli A, Gorica E, Brogi S, Testai L, Calderone V. Role of hydrogen sulfide in endothelial dysfunction: pathophysiology and therapeutic approaches. J Adv Res. 2020. 10.1016/j.jare.2020.05.015. [DOI] [PMC free article] [PubMed]
- 2.Daiber A, Chlopicki S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Rad Biol Med. 2020. 10.1016/j.freeradbiomed.2020.02.026. [DOI] [PubMed]
- 3.Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, de Haan JB. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol. 2018;9:114. doi: 10.3389/fphys.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masaki N, Ido Y, Yamada T, Yamashita Y, Toya T, Takase B, Hamburg NM, Adachi T. Endothelial insulin resistance of freshly isolated arterial endothelial cells from radial sheaths in patients with suspected coronary artery disease. J Am Heart Assoc. 2019;8(6):e010816. doi: 10.1161/jaha.118.010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richey JM. The vascular endothelium, a benign restrictive barrier? NO! Role of nitric oxide in regulating insulin action. Diabetes. 2013;62(12):4006–4008. doi: 10.2337/db13-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinol. 2007;148(1):160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 9.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 10.Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840(9):2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Tao L, Hai CX. Redox-regulating role of insulin: The essence of insulin effect. Molecular and Cellular Endocrinol. 2012;349(2):111–127. doi: 10.1016/j.mce.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Aghili Khorasani MH. Makhzan-al-Adviah. Rewritten by shams Ardakani MR, Rahimi R, Farjadmand F. 1st ed. 1771. Tehran University of Medical Sciences.
- 13.Balbaa SI, Zaki AY, Abdel-Wahab SM. el-Denshary ES, Motazz-Bellah M. preliminary phytochemical and pharmacological investigations of the roots of different varieties of Cichorium intybus. Planta Med. 1973;24(2):133–144. doi: 10.1055/s-0028-1099480. [DOI] [PubMed] [Google Scholar]
- 14.Nowrouzi A, Pourfarjam Y, Amiri A. Cichorium intybus L. (CI) as an insulin sensitizer. Diabetes. 2013;(62, Suppl. 1):A304.
- 15.Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru. 2012;20(1):56. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26(4):285–292. doi: 10.1016/j.numecd.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Zhao B, Wang Y, Wu D, Wang Y, Yu Y, Yan Y, Zhang W, Liu Z, Liu X. Cichoric acid prevents free-fatty-acid-induced lipid metabolism disorders via regulating Bmal1 in HepG2 cells. J Agric Food Chem. 2018;66(37):9667–9678. doi: 10.1021/acs.jafc.8b02147. [DOI] [PubMed] [Google Scholar]
- 18.Hazra B, Sarkar R, Bhattacharyya S, Roy P. Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia. 2002;73(7–8):730–733. doi: 10.1016/S0367-326X(02)00232-0. [DOI] [PubMed] [Google Scholar]
- 19.Karthikesan K, Pari L, Menon VP. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem Biol Interact. 2010;188(3):643–650. doi: 10.1016/j.cbi.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Kumar G, Mukherjee S, Paliwal P, Singh SS, Birla H, Singh SP, Krishnamurthy S, Patnaik R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedeberg's Arch Pharmacol. 2019;392(10):1293–1309. doi: 10.1007/s00210-019-01670-x. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Kim DH, Kim YS, Kim TJ. Prevention of oxidative stress-induced apoptosis of C2C12 myoblasts by a Cichorium intybus root extract. Biosci Biotechnol Biochem. 2013;77(2):375–377. doi: 10.1271/bbb.120465. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu Z, Liu X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-kappaB. FASEB J. 2017;31(4):1494–1507. doi: 10.1096/fj.201601071R. [DOI] [PubMed] [Google Scholar]
- 23.Milala J, Grzelak-Błaszczyk K, Król B, Juśkiewicz J, Zdunczyk Z. Composition and properties of chicory extracts rich in fructans and polyphenols. Pol J Food Nutr Sci. 2009;59(1):35–43. [Google Scholar]
- 24.Papetti A, Daglia M, Grisoli P, Dacarro C, Gregotti C, Gazzani G. Anti- and pro-oxidant activity of Cichorium genus vegetables and effect of thermal treatment in biological systems. Food Chem. 2006;97(1):157–165. doi: 10.1016/j.foodchem.2005.03.036. [DOI] [Google Scholar]
- 25.Singh H. Anti-diabetic activity of Methanolic extract of chicory roots in streptozocin induced diabetic rats. Int J Pharm. 2013;3:211–216. [Google Scholar]
- 26.Suzuki A, Yamamoto N, Jokura H, Yamamoto M, Fujii A, Tokimitsu I, Saito I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J Hypertens. 2006;24(6):1065–1073. doi: 10.1097/01.hjh.0000226196.67052.c0. [DOI] [PubMed] [Google Scholar]
- 27.Tsai KL, Kao CL, Hung CH, Cheng YH, Lin HC, Chu PM. Chicoric acid is a potent anti-atherosclerotic ingredient by anti-oxidant action and anti-inflammation capacity. Oncotarget. 2017;8(18):29600–29612. doi: 10.18632/oncotarget.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu D, Wang Y, Du Q, Liu Z, Liu X. Cichoric acid reverses insulin resistance and suppresses inflammatory responses in the glucosamine-induced HepG2 cells. J Agric Food Chem. 2015;63(51):10903–10913. doi: 10.1021/acs.jafc.5b04533. [DOI] [PubMed] [Google Scholar]
- 29.Gehrmann W, Wurdemann W, Plotz T, Jorns A, Lenzen S, Elsner M. Antagonism between saturated and unsaturated fatty acids in ROS mediated lipotoxicity in rat insulin-producing cells. Cell Physiol Biochem. 2015;36(3):852–865. doi: 10.1159/000430261. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Lim JY, Choi SJ. Oleate prevents palmitate-induced atrophy via modulation of mitochondrial ROS production in skeletal myotubes. Oxidative Med Cell Longev. 2017;2017:2739721–2739711. doi: 10.1155/2017/2739721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdomo L, Beneit N, Otero YF, Escribano O, Diaz-Castroverde S, Gomez-Hernandez A, et al. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc Diabetol. 2015;14:75. doi: 10.1186/s12933-015-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeong SO, Son Y, Lee JH, Cheong YK, Park SH, Chung HT, et al. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep. 2015;12(1):937–944. doi: 10.3892/mmr.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fratantonio D, Cimino F, Molonia MS, Ferrari D, Saija A, Virgili F, Speciale A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim Biophys Acta. 2017;1862(3):351–357. doi: 10.1016/j.bbalip.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Ye M, Qiu H, Cao Y, Zhang M, Mi Y, Yu J, Wang C. Curcumin improves palmitate-induced insulin resistance in human umbilical vein endothelial cells by maintaining proteostasis in endoplasmic reticulum. Front Pharmacol. 2017;8:148. doi: 10.3389/fphar.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Tang Z, Xia N, Yuan X, Zhu X, Xu G, Cui S, Zhang T, Zhang W, Zhao Y, Wang S, Shi B. PRDX1 is involved in palmitate induced insulin resistance via regulating the activity of p38MAPK in HepG2 cells. Biochem Biophys Res Commun. 2015;465(4):670–677. doi: 10.1016/j.bbrc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Park G, Sim Y, Lee W, Sung SH, Oh MS. Protection on skin aging mediated by antiapoptosis effects of the water lily (Nymphaea Tetragona Georgi) via reactive oxygen species scavenging in human epidermal keratinocytes. Pharmacology. 2016;97(5–6):282–293. doi: 10.1159/000444022. [DOI] [PubMed] [Google Scholar]
- 38.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Fratantonio D, Speciale A, Ferrari D, Cristani M, Saija A, Cimino F. Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-κB pathways. Toxicol Lett. 2015;239(3, 152):–60. 10.1016/j.toxlet.2015.09.020. [DOI] [PubMed]
- 41.Zhang M, Wang CM, Li J, Meng ZJ, Wei SN, Li J, Bucala R, Li YL, Chen L. Berberine protects against palmitate-induced endothelial dysfunction: involvements of upregulation of AMPK and eNOS and downregulation of NOX4. Mediat Inflamm. 2013;2013:260464–260468. doi: 10.1155/2013/260464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JE, Song SE, Kim YW, Kim JY, Park SC, Park YK, Baek SH, Lee IK, Park SY. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase a and AMP-activated protein kinase. J Endocrinol. 2010;207(1):35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- 43.Mahmoud AM, Wilkinson FL, McCarthy EM, Moreno-Martinez D, Langford-Smith A, Romero M, et al. Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J. 2017;31(10):4636–4648. doi: 10.1096/fj.201601244RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, et al. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J Mol Cell Cardiol. 2015;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Wu C, Li S, Chen X. Adiponectin protects palmitic acid induced endothelial inflammation and insulin resistance via regulating ROS/IKKbeta pathways. Cytokine. 2016;88:167–176. doi: 10.1016/j.cyto.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Storniolo CE, Roselló-Catafau J, Pintó X, Mitjavila MT, Moreno JJ. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014;2:971–977. doi: 10.1016/j.redox.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadeghi A, Seyyed Ebrahimi SS, Golestani A, Meshkani R. Resveratrol ameliorates palmitate-induced inflammation in skeletal muscle cells by attenuating oxidative stress and JNK/NF-kappaB pathway in a SIRT1-independent mechanism. J Cell Biochem. 2017;118(9):2654–2663. doi: 10.1002/jcb.25868. [DOI] [PubMed] [Google Scholar]
- 49.Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46(3):823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 50.Wang S-L, Li Y, Wen Y, Chen Y-F, Na L-X, Li S-T et al. Curcumin, a Potential Inhibitor of Up-regulation of TNF-alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-kappaB and JNK Pathway. Biomed Environ Sci. 2009;22(1):32–9. 10.1016/S0895-3988(09)60019-2. [DOI] [PubMed]
- 51.Shirasuna K, Takano H, Seno K, Ohtsu A, Karasawa T, Takahashi M, Ohkuchi A, Suzuki H, Matsubara S, Iwata H, Kuwayama T. Palmitic acid induces interleukin-1beta secretion via NLRP3 inflammasomes and inflammatory responses through ROS production in human placental cells. J Reprod Immunol. 2016;116:104–112. doi: 10.1016/j.jri.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Zhou BR, Zhang JA, Zhang Q, Permatasari F, Xu Y, Wu D, Yin ZQ, Luo D. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha via a NF-kappaB-dependent mechanism in HaCaT keratinocytes. Mediat Inflamm. 2013;2013:530429–530411. doi: 10.1155/2013/530429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volpe CM, Abreu LF, Gomes PS, Gonzaga RM, Veloso CA, Nogueira-Machado JA. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid Medicine Cellular Longev. 2014;2014:479587–479512. doi: 10.1155/2014/479587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto KI, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284(22):14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du J, Fan LM, Mai A, Li JM. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptors and endothelium in dietary obesity of middle-aged mice. Br J Pharmacol. 2013;170(5):1064–1077. doi: 10.1111/bph.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 57.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29(9):1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao Y, Gou L, Chen L, Zhong X, Zhang D, Zhu H, Lu X, Zeng T, Deng X, Li Y. NADPH oxidase 4 and endothelial nitric oxide synthase contribute to endothelial dysfunction mediated by histone methylations in metabolic memory. Free Radic Biol Med. 2018;115:383–394. doi: 10.1016/j.freeradbiomed.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascular Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Cha SH, Hwang Y, Kim KN, Jun HS. Palmitate induces nitric oxide production and inflammatory cytokine expression in zebrafish. Fish Shellfish Immun. 2018;79:163–167. doi: 10.1016/j.fsi.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 61.Ke J, Wei R, Yu F, Zhang J, Hong T. Liraglutide restores angiogenesis in palmitate-impaired human endothelial cells through PI3K/Akt-Foxo1-GTPCH1 pathway. Peptides. 2016;86:95–101. doi: 10.1016/j.peptides.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Shan Y, Li Y, Luo X, Shi H. Palmitate impairs angiogenesis via suppression of cathepsin activity. Mol Med Rep. 2017;15(6):3644–3650. doi: 10.3892/mmr.2017.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen P, Liu H, Xiang H, Zhou J, Zeng Z, Chen R, Zhao S, Xiao J, Shu Z, Chen S, Lu H. Palmitic acid induced autophagy increases reactive oxygen species via the Ca2+/PKCα/NOX4 pathway and impairs endothelial function in human umbilical vein endothelial cells. Exp Ther Med. 2019;17(4):2425–2432. doi: 10.3892/etm.2019.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2014;72:138–146. doi: 10.1016/j.fct.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 65.Xiao H, Wang J, Yuan L, Xiao C, Wang Y, Liu X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric Food Chem. 2013;61(7):1509–1520. doi: 10.1021/jf3050268. [DOI] [PubMed] [Google Scholar]
- 66.Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, et al. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels. 2010;3:2. doi: 10.1186/1754-6834-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-kappaB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol. 2011;660(2–3):454–459. doi: 10.1016/j.ejphar.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Gresele P, Pignatelli P, Guglielmini G, Carnevale R, Mezzasoma AM, Ghiselli A, Momi S, Violi F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138(9):1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 69.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22(7):2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 70.Kimbrough CW, Lakshmanan J, Matheson PJ, Woeste M, Gentile A, Benns MV, Zhang B, Smith JW, Harbrecht BG. Resveratrol decreases nitric oxide production by hepatocytes during inflammation. Surgery. 2015;158(4):1095–1101. doi: 10.1016/j.surg.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi S, Uchiyama T, Toda K. Differential effect of resveratrol on nitric oxide production in endothelial f-2 cells. Biol Pharm Bull. 2009;32(11):1840–1843. doi: 10.1248/bpb.32.1840. [DOI] [PubMed] [Google Scholar]
- 72.Loaiza A, Carretta MD, Taubert A, Hermosilla C, Hidalgo MA, Burgos RA. Differential intracellular calcium influx, nitric oxide production, ICAM-1 and IL8 expression in primary bovine endothelial cells exposed to nonesterified fatty acids. BMC Vet Res. 2016;12(1):38. doi: 10.1186/s12917-016-0654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Lima TM, de Sa LL, Scavone C, Curi R. Fatty acid control of nitric oxide production by macrophages. FEBS Lett. 2006;580(13):3287–3295. doi: 10.1016/j.febslet.2006.04.091. [DOI] [PubMed] [Google Scholar]
- 74.Barton M. Endothelial dysfunction and atherosclerosis: Endothelin receptor antagonists as novel therapeutics. Curr Hypertens Rep. 2000;2(1):84–91. doi: 10.1007/s11906-000-0064-5. [DOI] [PubMed] [Google Scholar]
- 75.Finch J, Conklin DJ. Air pollution-induced vascular dysfunction: potential role of endothelin-1 (ET-1) system. Cardiovasc Toxicol. 2016;16(3):260–275. doi: 10.1007/s12012-015-9334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwok CF, Shih KC, Hwu CM, Ho LT. Linoleic acid and oleic acid increase the endothelin-1 binding and action in cultured rat aortic smooth muscle cells. Metabolism. 2000;49(11):1386–1389. doi: 10.1053/meta.2000.17719. [DOI] [PubMed] [Google Scholar]
- 77.Park JY, Kim YM, Song HS, Park KY, Kim YM, Kim MS, et al. Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-kappa B. Biochem Biophys Res Commun. 2003;303(3):891–895. doi: 10.1016/s0006-291x(03)00436-4. [DOI] [PubMed] [Google Scholar]
- 78.Fayyaz S, Henkel J, Japtok L, Kramer S, Damm G, Seehofer D, et al. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia. 2014;57(2):373–382. doi: 10.1007/s00125-013-3123-6. [DOI] [PubMed] [Google Scholar]
- 79.Ishii M, Maeda A, Tani S, Akagawa M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch Biochem Biophys. 2015;566:26–35. doi: 10.1016/j.abb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 80.Li HB, Yang YR, Mo ZJ, Ding Y, Jiang WJ. Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz J Med Biol Res. 2015;48(5):440–446. doi: 10.1590/1414-431X20144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nieuwoudt S, Mulya A, Fealy CE, Martelli E, Dasarathy S, Naga Prasad SV, Kirwan JP. In vitro contraction protects against palmitate-induced insulin resistance in C2C12 myotubes. Am J Physiol Cell Physiol. 2017;313(5):C575–CC83. doi: 10.1152/ajpcell.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng Y, Sun Q, Park Y. Chicoric acid promotes glucose uptake and Akt phosphorylation via AMP-activated protein kinase α-dependent pathway. J Funct Foods. 2019;59:8–15. doi: 10.1016/j.jff.2019.05.020. [DOI] [Google Scholar]
- 83.Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G, Feillet-Coudray C, Cros G, Cabello G, Magous R, Wrutniak-Cabello C. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS One. 2013;8(11):e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N, Zhao Y, Yue Y, Chen L, Yao Z, Niu W. Liraglutide ameliorates palmitate-induced endothelial dysfunction through activating AMPK and reversing leptin resistance. Biochem Biophys Res Commun. 2016;478(1):46–52. doi: 10.1016/j.bbrc.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 85.Weidong C. Palmitate directly stimulates eNOS via AMPK and PI3-kinase activation in cultured endothelial cells (ECs). Diabetes. 2008; 68th scientific session.
- 86.Carlomosti F, D'Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, Maimone B, di Stefano V, de Santa F, Cordisco S, Antonini A, Ciarapica R, Dellambra E, Martelli F, Avitabile D, Capogrossi MC, Magenta A. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid Redox Signal. 2017;27(6):328–344. doi: 10.1089/ars.2016.6643. [DOI] [PubMed] [Google Scholar]
- 87.Srinivasan VAR, Raghavan VA, Parthasarathy S. Biochemical basis and clinical consequences of glucolipotoxicity: A primer. Heart Fail Clin. 2012;(8, 4):501–11. 10.1016/j.hfc.2012.06.011. [DOI] [PubMed]
- 88.Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun. 2010;393(1):66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horie K, Nanashima N, Maeda H. Phytoestrogenic effects of blackcurrant anthocyanins increased endothelial nitric oxide synthase (eNOS) expression in human endothelial cells and ovariectomized rats. Molecules. 2019;24(7). 10.3390/molecules24071259. [DOI] [PMC free article] [PubMed]
- 90.Liu C, Wang W, Lin W, Ling W, Wang D. Established atherosclerosis might be a prerequisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol Nutr Food Res. 2016;60(10):2141–2150. doi: 10.1002/mnfr.201600002. [DOI] [PubMed] [Google Scholar]
- 91.Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, Sánchez-Pascuala R, Hernández G, Díaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101(12):2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Alwis N, Beard S, Mangwiro YT, Binder NK, Kaitu'u-Lino TJ, Brownfoot FC, et al. Pravastatin as the statin of choice for reducing pre-eclampsia-associated endothelial dysfunction. Pregnancy Hypertens. 2020;20:83–91. doi: 10.1016/j.preghy.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Mao Y, Wang J, Yu F, Li Z, Li H, Guo C, Fan X. Ghrelin protects against palmitic acid or lipopolysaccharide-induced hepatocyte apoptosis through inhibition of MAPKs/iNOS and restoration of Akt/eNOS pathways. Biomed Pharmacother. 2016;84:305–313. doi: 10.1016/j.biopha.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 94.Laurindo FRM, Liberman M, Fernandes DC, Leite PF. Chapter 8 - Endothelium-dependent vasodilation: Nitric oxide and other mediators. In: Da Luz PL, Libby P, ACP C, FRM L, editors. Endothelium and Cardiovascular Diseases. 2018. pp. 97–113. [Google Scholar]
- 95.Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]