Abstract
Introduction
Although type 2 diabetes mellitus (DM) is a global public health problem, the diabetes-associated dermatological (non-infectious) manifestations (DADM) remain poorly understood and under-diagnosed. We aimed to evaluate the prevalence of 7 known DADM in a primary care setting, and their association macro/microvascular complications.
Methods
Cross-sectionnal study included patients consulting in general practice for DM-follow up, from November 2016 to January 2017. Patients aged <18 years old or consulting for other reason than DM follow up were excluded. Each patient were screened for diabetic dermopathy (DD), Huntley’s papules (HP), necrobiosis lipoidica diabeticorum (NL), acanthosis nigricans (AN), cheiroarthropathy (CA, or stiff hand syndrom), scleredema adultorum of Buschke (SB) and bullosis diabeticorum (BD).
Results
213 diabetic patients were included over a period of 3 months. We found a prevalence of 17.8% (38 patients) for DD, 8.5% (18) for HP, 2.8% (6) for NL, 2.3% (5) for AN, 1.9% (4) for CA, 1.4% (3) for SB and 1.4% (3) for BD. DADM seems to be a risk factor for vascular complications (OR 1.97, p ≤ 0.001). Association with vascular involvement was stronger with DD and macroangiopathy (OR 1.86, p ≤ 0.001), and with NL and microangiopathy (OR 9.7, p ≤ 0.001).
Conclusion
In primary care, DM-associated dermatological manifestations present similar prevalence rates to a tertiary care setting, based on litterature. Complete dermatological examination of diabetic patients is essential and could lead to a better overall management of the pathology, as diabetic cutaneous manifestations appear as a sign of vascular involvement.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00594-1) contains supplementary material, which is available to authorized users.
Keywords: Dermatological manifestations, Diabetes mellitus, Primary care, Vascular complications, Diabetic dermopathy, Necrobiosis lipoidica diabeticorum
Introduction
The number of patients with diabetes mellitus (DM) rose from 108 million in 1980 to 422 million in 2014 [1, 2], far exceeding all previous estimates [3]. From 2002 to today, DM, along with HIV infections, are the two diseases having recorded the largest rise in prevalence among the world’s population [4].
The prevalence of DM has increased more rapidly in low- and middle-income countries. The situation in France remains worrysome. Between 1999 and 2016, the number of patients treated for type 2 DM increased significantly (+ 44%). Several factors explain this growth, such as a significant current increase in the total population in France, aging of the population and the problem of “junk food” causing an increase in obesity. A recent study showed that obesity among childrens and adults has doubled in 70 countries worldwide, and continuously increased in most countries, over the last 25 years [5]. In this context, the training of physicians in clinical examination of the diabetic patient is a key element. However, clinical examination is relatively limited as these types of clinical illnesses are often the responsibility of a medical specialist, and / or biology results. It therefore seems appropriate to focus on dermatological lesions, potentially informative on common diabetic complications, particularly the associated vascular risks. To this day, DM-associated dermatological manifestations (DADM) have been evaluated only in tertiary or quaternary care settings (Figures 1, 2, 3 and, 4).
Fig. 1.
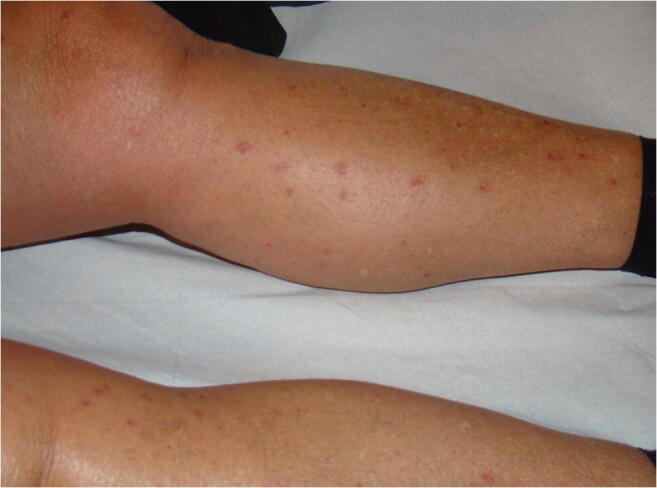
Diabetic Dermopathy [6]
Fig. 2.
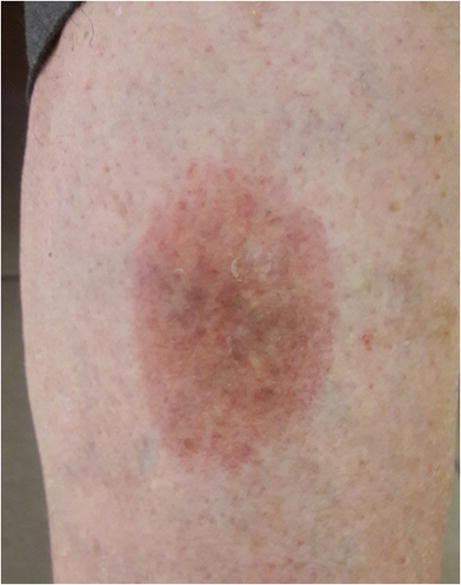
Necrobiosis Lipoidica Diabeticorum [6]
Fig. 3.
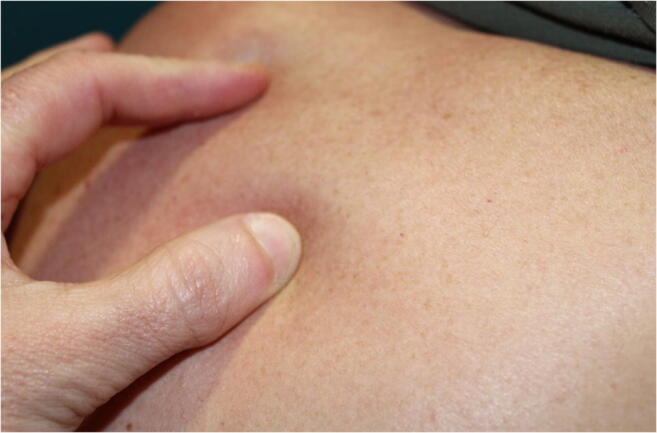
Scleredema Adultorum of Buschke [6]
Fig. 4.
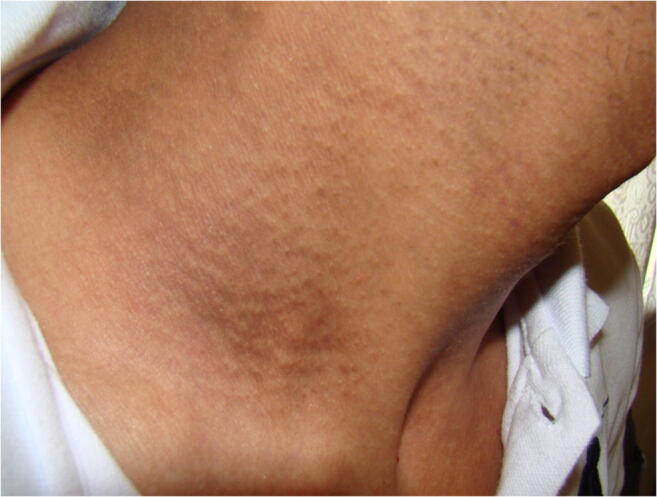
Acanthosis Nigricans [6]
The aim of the study was to evaluate the prevalence of 7 known DADM in a primary care population, and, secondly, their association to macro and/or micro-vascular complications.
Materials and methods
We conducted an monocentric cross-sectionnal study, in a general practice setting to determine the prevalence of selected DM-associated dermatological manifestations in primary care and their association with vascular complications of DM. Data was collected over 3 months (from November 2016 to January 2017), according to the diabetic pathology follow-up scheme recommended by French national societies [11].
We successively included all type-2 diabetic patients, aged 18 years-old or more, consulting in general practice for a recommended DM follow-up. Inclusions were carried out by 2 practitioners. We excluded any diabetic patient consulting for another reason than scheduled DM follow-up (related to diabetic disease or not), patients of 18 years old or less and patients unable to express consent. A complete dermatological examination was performed for each patient at time of inclusion to identify DM-associated dermatological manifestations. Then each patient informations were extracted from medical record, and a standardised questionnaire was used to collect the following data: inclusion date, identity, medical history including glycated haemoglobin (HbA1c), treatment received at time of inclusion, known history of macro or microvascular [cerebrovascular accident/stroke (CVA), diabetic retinopathy (DR), diabetic nephropathy (DN), Lower extremity arterial disease (LEAD), supra-aortic trunk occlusive disease, coronary artery disease (CAD)]. To be retained, the macro or microvascular complications had to be confirmed by a physician of the corresponding medical specialty (with the exception of diabetic nephropathy which is diagnosed based on biological criteria). CVA was defined by a history of stroke or transient ischemic attack (TIA) diagnosed by a neurologist and/or sequelae of stroke established on a brain imaging. CAD was defined by a history of myocardial infarction and/or coronary angioplasty. Supra-aortic trunks aortic disease was defined by history of significant stenosis (as specified by the World Federation of Neurology [12]) of at least one supra-aortic trunk, including common carotid, carotid bulb, carotid artery, vertebral artery and subclavian artery. LEAD was defined by ankle-brachial index outside normal ranges (0.9 to 1.3). Diagnosis of DR included either mild to severe non-proliferative DR, or proliferative DR, or macula edema [13]. DN was defined by microalbuminuria or macroalbuminuria, with or without decreased renal clearence [14, 15].
From various articles in the literature [7–10, 45], we selected seven non-infectious dermatological manifestations considered as the most specific to DM: diabetic dermopathy (DM), necrobiosis lipoidica diabeticorum (NL), scleredema adultorum of Buschke (SB), acanthosis nigricans (AN), Huntley’s papules (or finger peebles) (HP), bullosis diabeticorum (BD) and cheiroarthropathy (CA). Descriptions of these conditions are detailed in Appendix 1.
The authors did not retain xerosis as a DADM in this study because its prevalence in the global population remains high even in non diabetic patients. A recent study showed a prevalence of xerosis of 60% among 5547 healthy middle-aged and elderly patients [52]. Multivariable logistic regression showed a significant but low odds ratio for localised xerosis and diabetes (OR = 1.22 (1.04–1.45)). Furthermore, clinical assesments of xerosis rely on ordering grading scales, most of them suffering from vague and overlapping definitions [53].
Informed oral consent to participate in this study was obtained from the patients, as was signed agreement to waiver their image rights in relation to any collected images. This study received ethics approval from general practice department of the University of Medicine of Montpellier, as the usual care of patients wasn’t modified and oral consent was necessary.
The Student’s test was used to compare means following a normal distribution. In the opposite case, the Mann-Whitney test was preferred. Normality of distributions was assessed by the Agostino & Pearson test. For qualitative variables, comparaison of variables was assessed with chi-squared test, with Yates correction if necessary. Association between two variables was assessed by univariate odds-ratio (OR). The level of significance (α, as first species risk) was set at 5%.
Results
During 3 months, we included 213 consecutive DM-patients. No patient refused to participate. Demographic data of the population is described and summarised in Table 1. 93 patients (43.7%) had had DM for more than 10 years, and the average diabetes mellitus duration was 10.6 years. The average HbA1c level in this cohort was 7.2%, and a satisfactory level of diabetic control was observed in 75.6% of subjects (according to control criteria of French recommendations at time of inclusion) [13].
Table 1.
Clinical and biochemical characteristics of T2DM-patients
| Variables | T2DM-related cutaneous signs | All (n = 213) | ||
|---|---|---|---|---|
| Presence (n = 77) | Absence (n = 136) | p value | ||
| Age (years; mean[SD]) | 66.2 [12.64] | 67.9 [11.35] | 0.30 | 67.3 [11.9] |
| Sex ratio (male-to-female ratio) | 1.41 | 1.18 | 0.68 | 1.27 |
| HbA1c (%; mean[SD]) | 7.34 [0.93] | 7.05 [0.95] | 0.03 | 7.16 [0.96] |
| Patients with optimal glycemic management (n(%)) | 52 (67.5) | 109 (80.1) | 0.07 | 161 (75,6) |
| Duration of diabetes (years, mean[SD]) | 10.9 [7.31] | 9.9 [6.83] | 0.17 | 10.6 [7.22] |
| Patients with duration of diabetes ≥10 years old (n(%)) | 41 (53.2) | 52 (38.2) | 0.03 | 93 (43.7) |
| Treatment | ||||
| Lifestyle & dietary measures (n,%) | 3 (3.9) | 14 (10.3) | 0.10 | 17 (8.0%) |
| Single therapy (n, %) | 49 (63.6) | 70 (51.5) | 119 (55.9%) | |
| Dual therapy (n, %) | 16 (20.8) | 41 (30.1) | 57 (26.8%) | |
| Triple therapy (n, %) | 8 (10.4) | 11 (8.1) | 19 (8.9%) | |
| Others (n,%) | 1 (1.3) | 0 (0) | 1 (0.5%) | |
Results are expressed by (mean[standard deviation]) or (number(percentage))
T2DM = Type 2 Diabetes Mellitus, HbA1c: Glycated Hemoglobin
Regarding treatments, in patients on single oral treatment, the majority were being treated with biguanides (37.7%), with sulphonamides being used if any intolerance to the former (5.2%). Insulin therapy alone was used by 13.1% of patients. The only patient on quadriple therapy was under diabetic specialist follow-up.
We identified 77 patients (36,1%) presenting at least one DADM (Table 2). The two DADM with highest prevalence were DD with 38 (17.8%) patients and HP with 18 (8.5%) patients. Prevalences of the seven DADM are summarized in Table 2. DD and AN were significantly associated with macrovascular disease, with OR = 1.83 (p ≤ 0.001) and 2,57 (p = 0.024), respectively. Necrobiosis lipoidica diabeticorum (NL) was associated to microvascular complications with OR = 9.7 (p ≤ 0.001). Some cutaneous manifestations (CA, BD, and SB) were not numerous enough to allow satisfactory statistical tests.
Table 2.
T2DM-related cutaneous signs: prevalence and association with vascular complications
| All (n = 213) | Macrovascular Disease | Microvascular Disease | |||
|---|---|---|---|---|---|
| Odd Ratio (IC 95%) | p | Odd Ratio (IC 95%) | p | ||
| Diabetic Dermopathy | 38 (17.8%) | 1.86 (1.38–2.45) | ≤ 0.001 | 1.20 (0.45–3.08) | 0.72 |
| Huntley’s Papules | 18 (8.5%) | 0.42 (0.16–1.03) | 0.10 | – | – |
| Necrobiosis Lipoidica Diabeticorum | 6 (2.8%) | 1.71 (0.75–2.57) | 0.35 | 9.7 (3.47–18.78) | ≤ 0.001 |
| Acanthosis Nigricans | 5 (2.3%) | 2.57 (1.42–25.3) | 0.024 | – | – |
| Stiff Hand Syndrom | 4 (1.9%) | – | – | – | |
| Bullosis Diabeticorum | 3 (1.4%) | – | – | ||
| Scleredema Adultorum of Buschke | 3 (1.4%) | – | – | ||
Results are expressed with (n(%)) and (OR (IC 95%)) in univariate analysis
T2DM: Type 2 Diabetes Mellitus, OR = Adjusted Odd-Ratio, IC 95% = 95% Confidence Interval
Concerning vascular complications, data are summarized in Table 3. CAD and LEAD are the 2 most common complications with 16.4% and 20.2% of the total population, respectively. LEAD seems to be the complication most closely associated to these dermatological disorders (OR 2.7, p ≤ 0.001). From a more global point of view, DM-associated dermatological manifestations present an association with macro and/or microvascular complications with an OR of 1.97 (p < 0.001).
Table 3.
Macrovascular and microvascular complications, stratified by presence of T2DM-related cutaneous signs
| Cutaneous signs of T2DM (n = 77) |
No cutaneous signs of T2DM (n = 136) |
All (n = 213) |
Odd-Ratio (IC 95%) | p | |
|---|---|---|---|---|---|
| CAD | 19 (24.7%) | 16 (11.8%) | 35 (16.4%) | 2.1 (1.15–3.80) | 0.024 |
| PAD | 34 (44.2%) | 31 (22.8%) | 65 (30.5%) | 1.94 (1.30–2.88) | 0.002 |
| ➔ LEAD | 26 (33.8%) | 17 (12.5%) | 43 (20.2%) | 2.7 (1.58–4.63) | < 0.001 |
| ➔ Carotid Artery Disease | 8 (10.4%) | 14 (10.3%) | 22 (10.3%) | 0.98 (0.44–2.17) | 0.846 |
| DR | 6 (7.8%) | 3 (2.2%) | 9 (4.2%) | _ | _ |
| DN | 4 (5.2%) | 7 (5.1%) | 11 (5.2%) | _ | _ |
| CVD | 6 (7.8%) | 5 (3.7%) | 11 (5.2%) | 2.12 (0.71–6.34) | 0.326 |
| Total | 69 (89.6%) | 62 (45.6%) | _ | 1.97 (1.62–2.42) | < 0,001 |
Results are expressed with (n (%)) and (OR (IC95%)) with p as level of significance
T2DM: Type 2 Diabetes Mellitus, OR = Odd-Ratio, CAD = Coronary Artery Disease, DR = Diabetic Retinopathy, DN = Diabetic Nephropathy, CVD = Cerebrovascular Disease, LEAD = Lower Extremities Artery Disease, IC95% = 95% Confidence Interval, PAD = Peripheral Artery Disease
Discussion
To our knowledge, this study provides the first estimation of DM- associated dermatological manifestations in a primary care setting. The highest prevalence was found for dermopathic dermopathy with 38 patients (17.8%). DD and AN seems to be markers for macrovascular involvement (p ≤ .001 and p = .024, respectively), and NL, a marker for microvascular involvement (p < .001).
Our included population was consistent with data from the literature, including the mean age [16] and sex ratio [17, 18]. Male predominance is indeed frequent (SR = 1.27). Men tend to develop type 2 DM with a lower BMI than women [19]. As concerns to the characteristics of diabetic disease, our population data are representative of national data [20]. Taking national recommendations into account (“at risk” and / or dependent patients have higher HbA1c targets than the general population) [11], 75.6% of patients in this study had DM that was considered controlled, with average HbA1c of 7.2%. In a similar hospital-based study, the rate of patients with controlled DM was only 40% [21]. These data underscore the recruitment bias of studies with exclusive hospital recruitment. By contrast, our study reduced this bias with a representative population of diabetic outpatients, and is consequently closer to the entire French diabetic population [16]. In our study, duration of DM has a mean of 10.6 years, which correlates to the French average of 11 years, according to the most recent estimates [22, 23].
DADM seems to appear more frequently in patients with 10 years or more of diabetes (p = 0.03) and with higher HbA1c (p = 0.03).
Specific dermatological manifestations and diabetes mellitus
In the literature, diabetic dermopathy is the most common DM - specific dermatosis, ranging from 10 to 25% of cases according to studies according to hospital-based studies [8, 10]. A Belgian study found this condition in 30 to 60% of diabetic patients [7]. While it may be an overestimate, it illustrates the great difficulty of individualising this dermatosis, even if the criteria have been precisely established [8, 24]. The prevalence we report is therefore consistent with previous data, and shows prevalence of diabetic dermopathy to be similar between ambulatory and hospital settings. Some studies show a male predominance [22, 25] which we did not find in our cohort (Sex-ratio = 1.23 in the “ diabetic dermopathy “ subgroup versus a sex-ratio = 1.27 in the overall group).
With regard to Huntley’s papules (finger pebbles), few studies have investigated its prevalence. Only 2 of them, including H.
untley’s original study [23–27] found a prevalence between 70 and 75% in small hospitals cohorts. In our study, the prevalence of Huntley’s papules is much lower (8.5%). As ours is the largest descriptive cohort in the literature studying Huntley’s papules, we consider that this prevalence should be reduced. A difference in prevalence between a hospital and outpatient population of diabetic patients is to be considered.
Necrobiosis lipoidica diabeticorum, a rare dermatological condition, has estimated prevalence of between 0.3 and 2% [25, 28]. The largest cohort reported a prevalence of 0.98% in 1528 patients [25, 29]. Our prevalence of 2.8% is slightly higher. While our results may be the result of statistical variation; it remains consistent with the data in the literature to consider necrobiosis lipoidica diabeticorum as rare.
Acanthosis nigricans has a prevalence that varies according to ethnicity: 1 to 5% in Caucasian subjects as opposed to 13% in Black African or Hispanic subjects [27, 30, 27, 31]. AN usually indicates an insulin resistance (a decrease in functional insulin receptors) [32]. Its development could be explained by excess insulin binding insulin-like growth factor receptors, which are present in keratinocytes and dermal fibroblasts [33, 31]. Our prevalence is therefore consistent with a predominantly Caucasian population. In the literature, acanthosis nigricans is an independent cardiovascular risk factor [21, 31]. In our study, despite the small inclusions, the presence of acanthosis nigricans has been shown to be a risk factor for macroangiopathy (OR 2.57).
Regarding cheiroarthropathy, prevalence is estimated between 8 to 20% in patients with a long history of DM (duration ≥10 years). Authors often take “pseudo-sclerodermiform states” into account [9, 21, 34], including various clinical manifestations and not cheiroarthropathy alone. Our cohort presented a lower prevalence (1.8%). We therefore estimate that, for the overall diabetic population (regardless of duration of evolution), the specific prevalence of cheiroarthropathy is lower than the data found in the literature.
Bullosis diabeticorum is a rare condition moslty affecting patients with a long history of DM but the existing estimates remain poorly understood [35]. A previous Indian study estimated its prevalence at 1% [36], and another study at 0.5% in the USA [37]. Our study finds a prevalence that confirms the data of the literature and the rarity of this pathology.
The prevalence of Scleredema adultorum of Buschke in our study is 1.0%. This is lower than data in the literature, which estimate prevalence at 2 to 5% in the overall diabetic population [21, 38], and up to 14% according to some authors [39], but also confirms its rarity. In our study, Scleredema adultorum of Buschke occurs in patients with a long history of DM (OR 18.7%), which has previously been reported in the literature [40].
Specific cutaneous manifestations and vascular involvement
Concerning macro or microvascular complications, we were able to observe that specific dermatological lesions of DM were significantly associated with macro and/or microvascular involvements: OR = 1.97 (p ≤ 0.001), in univariate analysis. Although our study is a pilot study and lacks the power to acquire statistical significance on all variables, we observed that DM-specific dermatoses appear to be associated with elevated overall vascular risk. In this study, specific cutaneous involvement of DM represents a significant risk factor for LEAD (OR = 2.7, p ≤ 0.001).
Concerning diabetic dermopathy (DD), we found in the literature a link between this dermatosis and microangiopathy: retinopathy, nephropathy and neuropathy [41] or large vessel disease [50], despite the fact that this link is still disputed [21]. Our study did not reaffirm the link with microangiopathy; however, we found that DD was significantly correlated with the presence of macrovascular disease (OR = 1.86, p ≤ 0.001). This result reinforces a theory put forward by a majority of authors, recognising decreased cutaneous vascularisation as a factor favoring DD. The risk of developing macrovascular involvement of the lower limbs is 4 times higher in diabetic patients compared to the non-diabetic population [42], with marked mediacalcosis mainly leading to infra-popliteal arterial stenosic lesions [43]. To our knowledge, our study is the first to associate, as a risk factor, DD and macrovascular involvement (particularly LEAD). Indeed, the vast majority of studies have focused on microvascular disorders. Demonstration of a correlation between macrovascular involvement and DD seems logical considering that macrovascular disease can lead to aggravation of microcirculatory cutaneous involvement. Although preliminary, this study corroborates the hypothesis that DD is an independent sign of macrovascular as well as microvascular involvement, as supported by other authors [44].
Regarding acanthosis nigricans, it could be a marker of macrovascular complications of type 2 DM [21]. Acanthosis nigricans is strongly correlated with diabetes mellitus and obesity, as both are responsible for insulin resistance. The two entities have been connected for many years to increased cardiovascular mortality. A recent study in obese adolescents has shown that of acanthosis nigricans is an independent cardiovascular risk factor, as are BMI, abdominal circumference, total cholesterol, blood pressure, and type 2 DM family history [45]. Our study reinforces data demonstrating strong association between acanthosis nigricans and macrovascular complications (including CAD) (OR = 2.57, p = 0.024). Therefore, it would be interesting to study more precisely the link between acanthosis nigricans and cardiovascular mortality by prospectively comparing cardiovascular mortality in a diabetic and/or obese population according to acanthosis nigricans. This would allow patients with acanthosis nigricans to have appropriate care including early detection of coronary artery disease.
In the literature, necrobiosis lipoidica diabeticorum (NL) is generally recognised as associated with microvascular complications (especially retinopathy and nephropathy) and tabacco use [29, 46]. An Italian team even found this link in a paediatric cohort [47]. Although it included fewer patients with NL, our study confirms this connection by reporting an increased risk (OR 9.7, p ≤ 0.001)) of presenting microvascular disease (retinal or nephrological) in patients with NL in comparison to those without NL.
Cheiroarthropathy seems strongly associated with microvascular involvement [48], particularly retinopathy [49]. It has prognostic value for the occurrence of microvascular complications. Our small number of patients presenting this dermatosis did not make it possible to highlight a link with macro and / or microvascular complications. However all of our patients had a long history of DM (> 10 years), up to 40 years of evolution, reinforcing the link with microvascular complications of which frequency increases proportionally with the duration of DM. This raises the question of possible overestimation of the frequency of this dermatosis by recruitment bias, because all the studies on this subject, to date, have been carried out in hospitals. It is therefore legitimate to think that patients requiring hospital care are those with a long history of diabetes mellitus or difficult to control.
This study has several limitations. First, the 2 physicians who carried inclusions were general practionniers, leading to potential bias in diagnostic. However, they both received years of dermatology training and one is an active publishing author regarding dermatological diagnosis and management. Second, this study is monocentric with a general practice based in a semi-urban area; hence, the included patients may not be reprensentative of the entire ambulatory population. Third, no causality links can be affirmed between DM-associated dermatological manifestations and vascular involvement as the study design is cross-sectionnal.
Conclusion
This study shows the importance of a complete skin examination of each diabetic patient followed in primary care, as DM-associated dermatological manifestations seems be have similar prevalence rates compared to a tertiary setting (based on data from litterature). These manifestations are frequent with a global prevalence of 35.7%, and seems associated to vascular complications. To our knowledge, this study provides the first estimate of prevalence rates of DM-associated dermatological manifestations in a primary care setting.
Electronic supplementary material
(DOCX 2206 kb)
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest relevant to this article to disclose.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Highlights
• Diabetes-associated dermatological manifestations (DADM) are frequent and underdiagnosed.
• This study provides prevalence rates of 7 frequent DADM in primary care.
• Prevalence rates in primary care seems similar to available data on tertiary care.
• They are associated with both macro- and microvascular involvement.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation . Global status report on noncommunicable diseases 2014: attaining the nine global noncommunicable diseases targets; a shared responsibility. Geneva: World HealthOrganization; 2014. [Google Scholar]
- 2.World Health Organisation Report on diabetes in the world. 2016
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Loncar D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377(1):13–27 [DOI] [PMC free article] [PubMed]
- 6.Trihan J-E, Laneelle D, Perez-Martin A, Metclafe N, Frances P. Prévalence des manifestations cutanées non infectieuses spécifiques du diabète de type 2 en secteur de soins primaires. Médecine. 2018;14(9):401–6.
- 7.Flagothier C, Quatresooz P, Bourguignon R, Piérard-Franchimont C, Piérard GE. Cutaneous Stigmata of Diabetes Mellitus. Rev Med Liege. 2005;60(5‑6):553–559. [PubMed] [Google Scholar]
- 8.Bessis D, Bessis D, Francès C, Guillot B. Manifestations dermatologiques des maladies d’ organes. Dermatologie et Médecine. 2011. Diabète sucré; pp. 76.1–76.10. [Google Scholar]
- 9.Senet P, Chosidow O. Manifestations cutanéomuqueuses du diabète. EMC - Dermatologie. 2011;6(4):1–8. [Google Scholar]
- 10.Françès C. Peau et diabète: a-t-on vraiment avancé? Réalités Thérapeutiques en Dermatologie - Vénérologie. 2014;237:7–12. [Google Scholar]
- 11.HAS. Guide de parcours de soins : Diabète de type 2 de l’adulte. 2014
- 12.Von Reutern G-M, Goertler M-W, Bornstein NM, Del Sette M, Evans DH, Hetzel A, et al. Grading carotid stenosis using ultrasonic methods. Stroke. 2012;43(3):916–921. doi: 10.1161/STROKEAHA.111.636084. [DOI] [PubMed] [Google Scholar]
- 13.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 14.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 15.Doshi SM, Friedman AN. Diagnosis and Management of Type 2 Diabetic Kidney Disease. CJASN. 2017;12(8):1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier C, Chabert A, Mosnier-Pudar H, Aujoulat I, Fagot-Campagna A, Gautier A ENTRED Study 2007-2010. 2011
- 17.Kusnik-Joinville O, Weill A, Ricordeau P, Allemand H. Diabète traité en France en 2007: un taux de prévalence proche de 4% et des disparités géographiques croissantes. BEH. 2008;43(2008):409–413. [Google Scholar]
- 18.Institut de veille sanitaire (France) Prévalence et incidence du diabète, et mortalité liée au diabète en France: synthèse épidémiologique. Saint-Maurice: Institut de veille sanitaire; 2010. [Google Scholar]
- 19.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricci P, Blotière P-O, Weill A, Simon D, Tuppin P, Ricordeau P, et al. Diabète traité: quelles évolutions entre 2000 et 2009 en France. Bull Epidemiol Hebd. 2010;42(43):425–431. [Google Scholar]
- 21.Diris N, Colomb M, Leymarie F. Dermatoses non infectieuses au cours du diabète sucré. Ann Dermatol Venereol. 2003;109:1009–1014. [PubMed] [Google Scholar]
- 22.Verny C, Noaillon M, Baudry E. Treatment of Elderly Patients with Type 2 Diabetes Mellitus. Rev Prat. 2015;65(8):1046–1049. [PubMed] [Google Scholar]
- 23.Fagot-Campagna A, Romon I, Fosse S, Roudier C Prévalence et incidence du diabète, et mortalité liée au diabète en France. Synthèse épidémiologique. Institut de Veille Sanitaire. 2019
- 24.McCash S, Emanuel PO. Defining diabetic dermopathy: Defining diabetic dermopathy. J Dermatol. 2011;38(10):988–992. doi: 10.1111/j.1346-8138.2011.01251.x. [DOI] [PubMed] [Google Scholar]
- 25.Melin H. An Atrophic Circumcised Skin Lesion In The Lower Extremities Of Diabetics. Acta Med Scand. 1964;176(Suppl 423):1–75. [PubMed] [Google Scholar]
- 26.Huntley AC. Finger pebbles: A common finding in diabetes mellitus. J Am Acad Dermatol. 1986;14(4):612–617. doi: 10.1016/s0190-9622(86)70078-9. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgibbons PG, Weiss A-PC. Hand Manifestations of Diabetes Mellitus. J Hand Surg. 2008;33(5):771–775. doi: 10.1016/j.jhsa.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Peyrí J, Moreno A, Marcoval J. Necrobiosislipoidica. Semin Cutan Med Surg. 2007;26(2):87–89. doi: 10.1016/j.sder.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Kelly WF, Nicholas J, Adams J, Mahmood R. Necrobiosis lipoidica diabeticorum: association with background retinopathy, smoking and proteinuria. A case controlled study. Diabet Med. 1993;10(8):725–728. doi: 10.1111/j.1464-5491.1993.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 30.Rafalson L, Pham TH, Willi SM, Marcus M, Jessup A, Baranowski T. The association between acanthosis nigricans and dysglycemia in an ethnically diverse group of eighth grade students. Obesity. 2013;21(3):E328–E333. doi: 10.1002/oby.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48–53. doi: 10.1016/j.clindermatol.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Humbert P, Nguyen N. Physiopathology of acanthosis nigricans. Ann Dermatol Venereol. 1998;125(11):851–855. [PubMed] [Google Scholar]
- 33.De Schepper S, Naeyaert J-M. Acanthosis Nigricans. Encyclopédie Médico Chirurgicale. 2006
- 34.Cabo HA. Thick skin syndrome in diabetes mellitus. J Eur Acad Dermatol Venereol. 2000;14(2):143–144. doi: 10.1046/j.1468-3083.2000.00037-4.x. [DOI] [PubMed] [Google Scholar]
- 35.Riad H, Al Ansari H, Mansour K, Al Mannai H, Al Sada H, Abu Shaikha S, et al. Pruritic Vesicular Eruption on the Lower Legs in a Diabetic Female, Pruritic Vesicular Eruption on the Lower Legs in a Diabetic Female. Case Reports in Dermatological Medicine, Case Reports in Dermatological Medicine 2013; 2013:e641416. [DOI] [PMC free article] [PubMed]
- 36.Mohanty KC, Singh R, Lyengar B. Bullosis Diabeticorum. Indian J Dermatol Venereol Leprol. 1979;45(2):119–121. [PubMed] [Google Scholar]
- 37.Ghosh S, Bandyopadhyay D, Chatterjee G. Bullosisdiabeticorum: a distinctive blistering eruption in diabetes mellitus. Int J Diabetes Dev Ctries. 2009;29(1):41. doi: 10.4103/0973-3930.50714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole GW, Headley J, Skowsky R. Scleredema Diabeticorum: A Common and Distinct Cutaneous Manifestation of Diabetes Mellitus. Diabetes Care. 1983;6(2):189–192. doi: 10.2337/diacare.6.2.189. [DOI] [PubMed] [Google Scholar]
- 39.Murphy-Chutorian B, Han G, Cohen SR. Dermatologic manifestations of diabetes mellitus: a review. Endocrinol Metab Clin North Am. 2013;42(4):869–898. doi: 10.1016/j.ecl.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Sattar MA, Diab S, Sugathan TN, Sivanandasingham P, Fenech FF. Scleroedema Diabeticorum: a Minor but Often Unrecognized Complication of Diabetes Mellitus. Diabet Med. 1988;5(5):465–468. doi: 10.1111/j.1464-5491.1988.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 41.Verrotti A, Chiarelli F, Amerio P, Morgese G. Necrobiosis Lipoidica Diabeticorum in Children and Adolescents: A Clue for Underlying Renal and Retinal Disease. Pediatr Dermatol. 1995;12(3):220–223. doi: 10.1111/j.1525-1470.1995.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 42.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease an update. Hypertension. 2001;37(4):1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 43.Boursier V, Lazareth I, Pernes J-M, Dadon M, Priollet P. Artériopathie du diabétique : les clés de la prise en charge. Sang Thrombose Vaisseaux. 2001;13(10):599–607. [Google Scholar]
- 44.Houck GM, Morgan MB. A reappraisal of the histologicfindings of pigmented pretibial patches of diabetes mellitus. J Cutan Pathol. 2004;31(2):141–144. doi: 10.1111/j.0303-6987.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 45.Barrett SC, Huffman FG, Johnson P, Campa A, Magnus M, Ragoobirsingh D. A cross-sectionalstudy of Jamaican adolescents’ risk for type 2 diabetes and cardiovascular diseases. BMJ Open. 2013;3(7). [DOI] [PMC free article] [PubMed]
- 46.Sibbald C, Reid S, Alavi A. Necrobiosis Lipoidica. Dermatol Clin. 2015;33(3):343–360. doi: 10.1016/j.det.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Verrotti A, Chiarelli F, Amerio P, Morgese G. Necrobiosis Lipoidica Diabeticorum in Children and Adolescents: A Clue for Underlying Renal and Retinal Disease. Pediatr Dermatol. 1995;12(3):220–223. doi: 10.1111/j.1525-1470.1995.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbloom AL, Silverstein JH, Lezotte DC, Richardson K, McCallum M. Limited Joint Mobility in Childhood Diabetes Mellitus Indicates Increased Risk for MicrovascularDisease. N Engl J Med. 1981;305(4):191–194. doi: 10.1056/NEJM198107233050403. [DOI] [PubMed] [Google Scholar]
- 49.Yosipovitch G, Hodak E, Vardi P, Shraga I, Karp M, Sprecher E, et al. The Prevalence of Cutaneous Manifestations in IDDM Patients and Their Association With Diabetes Risk Factors and Microvascular Complications. Diabetes Care. 1998;21(4):506–509. doi: 10.2337/diacare.21.4.506. [DOI] [PubMed] [Google Scholar]
- 50.Duff M, Demidova O, Blackburn S, Shubrook J. Cutaneous Manifestations of Diabetes Mellitus. Clin Diabetes. 2015;33(1):40–48. doi: 10.2337/diaclin.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14(3):95. [Google Scholar]
- 52.Mekić S, Jacobs LC, Gunn DA, Mayes AE, Ikram MA, Pardo LM, et al. Prevalence and determinants for xerosis cutis in the middle-aged and elderly population: A cross-sectional study. J Am Acad Dermatol. 2019;81(4):963–969.e2. doi: 10.1016/j.jaad.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 53.Piérard GE, Piérard-Franchimont C, Scheen A. Critical assessment of diabetic xerosis. Expert Opinion on Medical Diagnostics. 2013;7(2):201–207. doi: 10.1517/17530059.2013.728585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2206 kb)


