Abstract
Objective
Several trials investigated the efficacy of L-carnitine administration on markers of inflammation and indicators of oxidative stress; however, their findings are controversial. The aim of this study was to conduct a comprehensive meta-analysis and a critical review, which would analyze all randomized controlled trials (RCTs) in order to determine the effects of L-carnitine supplementation on inflammatory markers and oxidative stress.
Methods
An electronic search was performed using Scopus, Cochrane Library, PubMed, Google scholar and Web of Science databases on publications from 1990 up to May 2020. Human RCTs conducted in healthy subjects or participants with certain disorders which investigating the efficacy of L-carnitine supplementation compared to control (placebo, usual treatment or no intervention) on inflammation and oxidative markers were included. Data were pooled applying a random-effects model and as the overall effect size, weighted mean difference (WMD) was presented. Between heterogeneity among studies was computed using Cochran’s Q test and I-square (I2). Quality of studies assessed using the Jadad scale. Dose-response analysis was measured using meta-regression. The funnel plot, as well as the Egger’s regression test was applied to determine the publication bias.
Results
44 trials (reported 49 effect sizes for different outcomes of interest) met the inclusion criteria for this meta-analysis. According to the findings, L-carnitine supplementation resulted in a significant reduction in C-reactive protein (CRP) (WMD: -0.10; 95% CI: -0.14, -0.06), interleukin 6 (IL-6) (WMD: -1.87; 95% CI: -2.80, -0.95), tumor necrosis factor-α (TNF-α) levels (WMD: -1.43; 95% CI: -2.03, -0.84), and malondialdehyde (MDA) (WMD: -0.47; 95% CI: -0.76, -0.18) levels, while there was a significant increase in superoxide dismutase (SOD) (WMD: 2.14; 95% CI: 1.02, 3.25). However, no significant effects of L-carnitine on glutathione peroxidase (GPx) (WMD: 0.02; 95% CI: -0.01, 0.05) and total antioxidant capacity (TAC) (WMD: 0.14; 95% CI: -0.05, 0.33) were found.
Conclusions
L-carnitine supplementation was associated with lowering of CRP, IL-6, TNF-α, and MDA, and increasing SOD levels, but did not affect other inflammatory and oxidative stress biomarkers.
Keywords: Carnitine, Inflammatory markers, C-reactive protein, Oxidative stress, Meta-analysis
Introduction
Inflammation is a complex biological process related to infection, irritation etc. [1]. During chronic inflammation and oxidative stress, there is an elevated levels of free radicals and reactive oxygen species (ROS), which may cause structural damage to the cells [2]. Inflammation and oxidative stress are associated with a wide range of chronic diseases including atherosclerosis, diabetes mellitus (DM), neurological disorders, pulmonary diseases, cancer, and rheumatoid arthritis (RA) [3].
Carnitine is effective in preventing accumulation of end-products related to lipid peroxidation due to its anti-inflammatory and antioxidant effects [4]. The major physiologic role of carnitine is transferrin of long-chain fatty acids through the mitochondrial membrane and contribution in the oxidative release of energy [5]. It also helps to remove the short- and medium-chain fatty acids from mitochondria [6]. Any change in carnitine homeostasis may have an impact on metabolism and function of lipids, red blood cells, and cardiac muscle cells [7]. The influences of carnitine supplementation on many biomarkers of inflammation and oxidative stress have already been investigated, but the findings were not conclusive. In a meta-analysis by Sahebkar et al. [8], a beneficial effect of carnitine supplementation in decreasing circulating levels of C-reactive protein (CRP) were found. Carnitine supplementation at a dosage of 20 mg/kg of body weight during 8 weeks to patients with end-stage renal disease was attributed to a significant decrease in biomarkers of oxidative stress [9]. Moreover, it has been suggested that carnitine administration at a dosage of 1.5 g/day during 2 months to patients with maple syrup urine disease (MSUD) has an antioxidant and anti-inflammatory effect [10]. Taking carnitine supplements during 3 months by patients with age-related macular degeneration (AMD) significantly reduced oxidative damage by reducing a marker of lipid peroxidation malondialdehyde (MDA) and increasing glutathione (GSH) levels [11]. However, Shakeri et al. [12] did not find any favorable effects of carnitine supplementation during 12 weeks on parameters of oxidative stress in hemodialysis subjects with hyperlipoproteinemia. Furthermore, Sawicka et al. [13] indicated that 1,500 mg/day carnitine supplementation for 24 weeks to healthy women older than 65 years had no effects inflammatory variables.
Discrepancies among existed evidence might be related to the differences in several factors such as study design, characteristics of studies populations, comorbidities, duration of intervention, as well as different formulations and dosages of carnitine used. Therefore, we performed this meta-analysis to identify the effect of L-carnitine on inflammation and oxidative stress.
Methods
Search strategy
Two independent authors searched electronic databases including Scopus, Cochrane Library, Web of Science, PubMed and Google scholar databases from 1990 to May 2020 for relevant randomized controlled trials (RCTs) investigating the associations between L-carnitine supplementation and indicators of oxidative stress and inflammatory profiles. Search strategy was limited to RCTs in humans with written in English or Persian. The following keywords were used to identify primary articles: intervention (“L-carnitine” OR “L-carnitine -L-tartrate” OR “propionyl L-Carnitine” OR “Acetyl-L-carnitine” OR “carnitine orotate complex”), and outcomes [“tumor necrosis factor-α (TNF-α)” OR “C-reactive protein (CRP)” OR “interleukin 6 (IL-6)” OR “total antioxidant capacity (TAC)” OR “malondialdehyde (MDA)” OR “glutathione peroxidase (GPx)” OR “Superoxide dismutase (SOD)”]. In order to detect further articles that were not captured in our primary search, we searched the reference lists of related RCTs and previous reviews manually.
Inclusion and exclusion criteria
For this meta-analysis, we included RCTs, which fulfilled the following criteria: (1) Participants: healthy subjects or individuals with different underlying conditions and disorders. (2) Intervention: oral or intravenous carnitine supplementation for a duration more than 2 weeks. (3) Comparisons: control (placebo, usual treatment or no intervention). (4) Outcomes: inflammatory biomarkers and indicators of oxidative stress. (5) Study design: parallel design or cross-over. Due to the unfamiliarity with other languages, relevant articles which were written English or Persian were included. Data were extracted from RCTs presented mean/median with standard deviation (SD) or standard error or interquartile range or related 95% confidence intervals (CIs) for the both intervention and control groups. Other studies such as in vitro studies, animal model investigations, case reports and observational studies were excluded. In addition, investigations with healthy control (case control studies) or without control group and trials with two weeks or less duration were not included.
Data extraction and quality assessment
Two authors (HF and AM) independently screened the articles based on the eligibility criteria. In the first step the title and abstract of studies were reviewed. Then, the full-text of relevant studies was assessed to ascertain the suitability of a study for including in the meta-analysis. Any disagreement was resolved by the judgment of the concealment of third author (JH).
The following data were extracted from selected studies: the name of first authors, publication year, study location, study design, sample size, age, health status and type of disease, duration of the intervention, dosage of supplementation, the mean and SD for biomarkers of inflammation and oxidative stress in each intervention group. The same two authors also assessed the studies’ quality independently using the Jada tool.
Data synthesis and statistical analysis
The pooled effects of L-carnitine supplementation on the each indicator of inflammation and oxidative stress were calculated applying change score method. Weighted mean difference (WMD) with related 95% CI was used for pooling data to determine the overall effect sizes by using the random-effect model.
Heterogeneity and publication bias
Using Cochran’s Q test (with significant P-value < 0.1) and I-square test (I2 greater than 50 percent showing significant heterogeneity) heterogeneity across included trials was evaluated. We conducted different subgroup analyses to reveal potential sources of between-study heterogeneity. These subgroup analyses were done based on participants’ age, health condition of participants, study location, dosage of supplements, study duration, and sample size. In order to determine the publication bias, funnel plot, as well as the test of Egger’s regression was used. For data analysis, both STATA 11.0 (Stata Corp., College Station, TX) and Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) software were applied.
Results
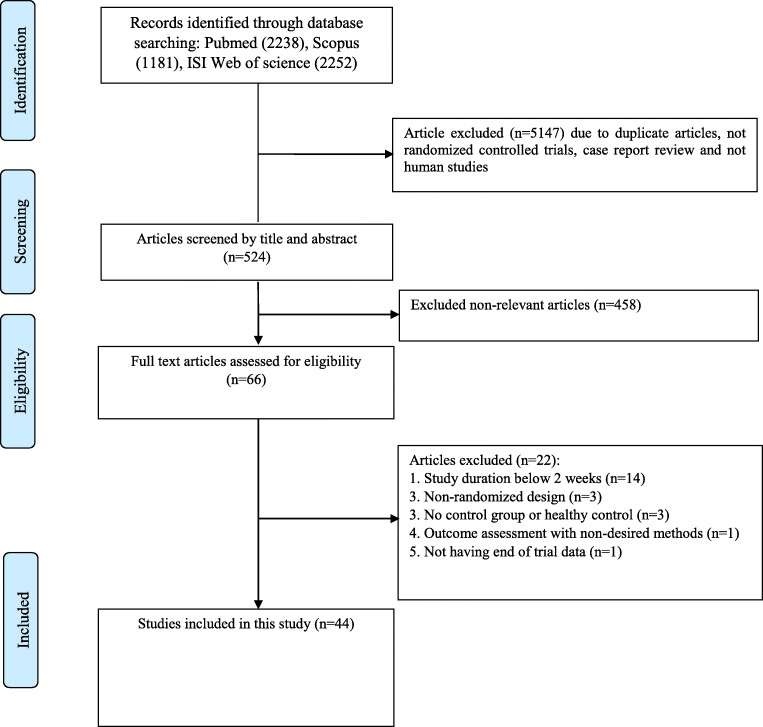
Following an initial search in mentioned databases, 5671 articles were recognized. Among them, 5147 articles were duplicates, review, case report, not RCT and not human studies and then removed. Then, 524 articles were screened based on title and abstract. After screening, 458 publication were excluded due to non-relevant studies. Then, 66 articles were eligible to evaluate their full-text. Of these, 22 articles were excluded due to duration equal or below 2 weeks (n = 14), Non-randomized design (n = 03), healthy control or not having control group (n = 03), outcome assessment with non-desired methods (n = 01), not having end of trial data (n = 01). Finally, 44 articles were included to present systematic review and meta-analysis. Flowchart of procedure for study selectin is presented in Fig. 1.
Fig. 1.
Literature search and review flowchart for selection of studies
In Table 1, general characteristics of included studies are given. In total, 44 studies which reported 49 effect sizes were included with a total of 2742 subjects (1429 subjects in intervention and 1313 subjects in control groups). These studies were published between 2000 and 2019 and were conducted in Turkey, India, Italy, USA, Japan, Iran, Greece, Egypt, Taiwan, Spain, Malaysia, Poland, and Korea. The duration of supplementation varied between 4 and 48 weeks. L-carnitine, carnitine orotate complex, propionyl L-carnitine, Glycine propionyl-L-carnitine, Acetyl-L-carnitine, and L-carnitine -L-tartrate were used as supplements in the included studies; one study used L-carnitine enriched bread and one another a fruit-flavored drink. Thirty two studies were at a quality score ≥ 3 and 12 others had a quality score < 3 (Table 4).
Table 1.
Summary of included randomized controlled studies
| Authors (Ref) | Publication year | Country | Sample size (intervention/control) |
Duration (wk) | Age (y) (intervention/control) |
Intervention (type and dosage) |
Total quality score |
|---|---|---|---|---|---|---|---|
| Gurlek et al.[14] | 2000 | Turkey | 31/20 | 4 | 64.3 ± 7.8, 66.2 ± 8.7 | 2000 mg L-carnitine | 2 |
| Vesela et al.[15] | 2001 | Germany | 12/12 | 24 | 28–71 | 15 mg/kg L-carnitine three times/week | 1 |
| Mosca et al.[16] | 2002 | Italy | 9/6 | 16 | 29–40 | 100 mg N-acetl-L-carnitine + 100 mg L-carnitine + selenomethionin + αtocopherol + Q10 + α lipoic acid | 2 |
| Duranay et al.[17] | 2006 | Turkey | 21/21 | 24 | 44.0 ± 13.9, 43.4 ± 13.9 | 20 mg/kg L-carnitine three times/week | 3 |
| Kumar et al.[18] | 2007 | India | 29/29 | 12 | 52.9 ± 6.4, 50.4 ± 7.7 | 2250 mg L-carnitine + 270 mg ubiquinol | 5 |
| McMackin et al.[19] | 2007 | USA | 36/26 | 8 | ≥ 55 | 1000 mg acetyl-L-carnitine + 200 mg α-lipoic acid | 5 |
| Yonei et al.[20] | 2007 | Japan | 18/17 | 8 | 40–69 | 1000 mg L-carnitine + 700 mg conjugated linoleic acid | 3 |
| Ates et al.[21] | 2008 | Turkey | 30/30 | 12 | 55–70 | 200 mg L-carnitine | 2 |
| Yonei et al.[22] | 2008 | Japan | 18/17 | 8 | 48.3 ± 6.9 |
600 mg L-carnitine + 500 mg Garcinia cambogia extract |
3 |
| Bloomer et al.[23] (a) | 2009 | USA | 10/4 | 8 | 18–44 | 1000 mg GPLC + aerobic exercise | 4 |
| Bloomer et al.[23] (b) | 2009 | USA | 11/5 | 8 | 18–44 | 3000 mg GPLC + aerobic exercise | 4 |
| Bloomer et al.[24] | 2009 | USA | 14/15 | 8 | 20–55 | 3000 mg acetyl L-carnitine arginate | 4 |
| Bloomer et al.[25] (a) | 2009 | USA | 11/4 | 8 | 20–40 | 1000 mg propionyl L-carnitine + 348 mg glycine + aerobic exercise | 4 |
| Bloomer et al.[25] (b) | 2009 | USA | 12/5 | 8 | 20–40 | 3000 mg propionyl L-carnitine + 1044 mg glycine + aerobic exercise | 4 |
| Derosa et al.[26] | 2011 | Italy | 114/113 | 48 | 51 ± 4, 53 ± 6 | 2000 mg + 360 mg orlistat | 5 |
| Hakeshzadeh et al.[27] | 2010 | Iran | 18/18 | 12 | 20–74 | 1000 mg L-carnitine | 3 |
| Malaguarnera et al.[28] | 2010 | Italy | 36/38 | 24 | 47.9 ± 5.4, 47.8 ± 5.8 | 2000 mg L-carnitine + diet | 5 |
| Mantovani et al.[29] | 2010 | Italy | 88/44 | 16 | 62.4 ± 9.4, 61.5 ± 9.7 | 4000 mg L-carnitine + a prostagelandin agent + EPA + thalidomide | 2 |
| Shakeri et al.[12] | 2010 | Iran | 18/18 | 12 | 24–80 | 1000 mg L-carnitine | 2 |
| Fatouros et al.[9] | 2010 | Greece | 12/12 | 8 | 53.8 ± 7.96 | 20 mg/kg L-carnitine three times/week | 4 |
| Derosa et al.[30] | 2010 | Italy | 113/110 | 12 | ≥ 18 | 2000 mg L-carnitine + 10 mg sibutramine | 5 |
| Suchitra et al.[31] | 2011 | India | 20/15 | 24 | 50.2 ± 8.6, 53.4 ± 9.6 | 1000 mg L-carnitine three times a week | 3 |
| Mortazavi et al.[32] | 2011 | Iran | 24/24 | 36 | 50.66 ± 17, 57.91 ± 13 | 750 mg L-carnitine | 5 |
| Macciò et al.[33] | 2012 | Italy | 61/63 | 24 | 35–76 | 4000 mg L-carnitine + megestrol acetate + celecoxib + antioxidants | 2 |
| Karl et al.[34] (a) | 2012 | USA | 21/7 | 8 | 58 ± 15, 63 ± 9 | fruit-flavored drink containig 300 mg L -carnitine + other nutrients | 4 |
| Karl et al.[34] (b) | 2012 | USA | 23/7 | 8 | 60 ± 13, 63 ± 9 | fruit-flavored drink containig 300 mg L -carnitine + other nutrients + red yeast rice | 4 |
| Rondanelli et al.[35] | 2013 | Italy | 41/45 | 8 | 25–45 | 300 mg L-carnitine + botanical extracts | 4 |
| Fukami et al.[36] | 2013 | Japan | 32/38 | 24 | 68.±12.4, 67 ± 13.2 | 900 mg L-carnitine | 2 |
| Barzegar et al.[37] | 2013 | Iran | 30/30 | 8 | 20–50 | 2000 mg L-carnitine + low-calorie diet | 2 |
| Higuchi et al.[38] | 2014 | Japan | 67/64 | 48 | 20–85 | 20 mg/kg L-carnitine | 2 |
| Hong et al.[39] | 2014 | Korea | 24/24 | 12 | 30–75 | 900 mg carnitine orotate complex + metformin | 3 |
| Samadi et al.[40] | 2014 | Iran | 9/11 | 8 | 20–30 | 2000 mg L-carnitine | 4 |
| Soare et al.[41] | 2014 | USA | 28/26 | 12 | 38–55 | 500 mg acetyl-L‐carnitine + other nutrients | 4 |
| Lee et al.[42] | 2014 | Taiwan | 20/19 | 12 | 71.9 ± 10.6, 72.7 ± 10.1 | 1000 mg L-carnitine | 3 |
| Lee et al.[43] | 2015 | Taiwan | 20/19 | 12 | 71.9 ± 10.6, 72.7 ± 10.1 | 1000 mg L-carnitine | 3 |
| Bañuls et al.[44] (a) | 2015 | Spain | 15/13 | 12 | 51.7 ± 7.7 | 2325 mg L-carnitine enriched bread + soluble fiber | 4 |
| Bañuls et al.[44] (b) | 2015 | Spain | 11/15 | 12 | 53.8 ± 10.7 | 2325 mg L-carnitine enriched bread + soluble fiber | 4 |
| Malek Mahdavi et al.[45] | 2015 | Iran | 33/36 | 8 | 40–60 | 750 mg L-carnitine | 5 |
| Badrasawi et al.[46] | 2016 | Malaysia | 26/24 | 10 | 68.2 ± 6.3, 68.8 ± 6.5 | 1500 mg L-carnitine | 5 |
| Malek Mahdavi et al.[47] | 2016 | Iran | 33/36 | 8 | 40–60 | 750 mg L-carnitine | 5 |
| Jamilian et al.[48] | 2017 | Iran | 30/30 | 12 | 18–40 | 250 mg carnitine | 5 |
| Mohammadi et al.[49] | 2017 | Iran | 26/26 | 8 | 41.0 ± 9.65, 40.6 ± 9.90 | 2000 mg L-carnitine tartrate | 4 |
| Sharifi et al.[50] (a) | 2017 | Iran | 32/31 | 12 | 40–65 | 1200 mg L-carnitine + 150 mg Q10 | 3 |
| Sharifi et al.[50] (b) | 2017 | Iran | 32/32 | 12 | 40–65 | 1200 mg L-carnitine + 150 mg Q10 + TLC diet | 3 |
| Koozehchian et al.[51] | 2018 | Iran | 12/11 | 9 | 25.5 ± 1.5, 24.5 ± 1.5 | 2000 mg L-carnitine | 4 |
| Talari et al.[52] | 2019 | Iran | 30/30 | 12 | 18–40 | 250 mg carnitine | 5 |
| Samulak et al.[53] | 2019 | Poland | 11/9 | 24 | 65–70 | 1500 mg L-carnitine-L-tartrate | 1 |
| Jamilian et al.[54] | 2019 | Iran | 26/27 | 12 | 18–40 | 1000 mg L-carnitine + 200 µg chromium | 5 |
| El-Sheikh et al.[55] | 2019 | Egypt | 31/27 | 24 | ≥ 30 | 2000 mg L-carnitine + glimepiride | 2 |
| Y, Year; EPA, eicosapentaenoic acid; GPLC, Glycine propionyl-L-carnitine. | |||||||
Table 4.
Methodological quality scores for included studies using Jadad scale
| Study | Randomization | Blinding | Account of all patients | Total Score |
|---|---|---|---|---|
| Gurlek et al.2000 | 1 | 0 | 1 | 2 |
| Vesela et al.2001 | 0 | 0 | 1 | 1 |
| Mosca et al.2002 | 1 | 0 | 1 | 2 |
| Duranay et al.2006 | 2 | 0 | 1 | 3 |
| Kumar et al.2007 | 2 | 2 | 1 | 5 |
| McMackin et al.2007 | 2 | 2 | 1 | 5 |
| Yonei et al.2007 | 1 | 1 | 1 | 3 |
| Ates et al.2008 | 1 | 0 | 1 | 2 |
| Yonei et al.2008 | 1 | 1 | 1 | 3 |
| Bloomer et al.2009 | 1 | 2 | 1 | 4 |
| Bloomer et al.2009 | 1 | 2 | 1 | 4 |
| Bloomer et al.2009 | 1 | 2 | 1 | 4 |
| Derosa et al.2011 | 2 | 2 | 1 | 5 |
| Hakeshzadeh et al.2010 | 1 | 1 | 1 | 3 |
| Malaguarnera et al.2010 | 2 | 2 | 1 | 5 |
| Mantovani et al.2010 | 1 | 0 | 1 | 2 |
| Shakeri et al.2010 | 1 | 0 | 1 | 2 |
| Fatouros et al.2010 | 2 | 1 | 1 | 4 |
| Derosa et al.2010 | 2 | 2 | 1 | 5 |
| Suchitra et al.2011 | 1 | 1 | 1 | 3 |
| Mortazavi et al.2011 | 2 | 2 | 1 | 5 |
| Macciò et al.2012 | 1 | 0 | 1 | 2 |
| Karl et al.2012 | 2 | 1 | 1 | 4 |
| Rondanelli et al.2013 | 1 | 2 | 1 | 4 |
| Fukami et al.2013 | 1 | 0 | 1 | 2 |
| Barzegar et al.2013 | 1 | 0 | 1 | 2 |
| Higuchi et al.2014 | 1 | 0 | 1 | 2 |
| Hong et al.2014 | 1 | 1 | 1 | 3 |
| Samadi et al.2014 | 1 | 2 | 1 | 4 |
| Soare et al.2014 | 1 | 2 | 1 | 4 |
| Lee et al.2014 | 1 | 1 | 1 | 3 |
| Lee et al.2015 | 1 | 1 | 1 | 3 |
| Bañuls et al.2015 | 1 | 2 | 1 | 4 |
| Malek Mahdavi et al.2015 | 2 | 2 | 1 | 5 |
| Badrasawi et al.2016 | 2 | 2 | 1 | 5 |
| Malek Mahdavi et al.2016 | 2 | 2 | 1 | 5 |
| Jamilian et al.2017 | 2 | 2 | 1 | 5 |
| Mohammadi et al.2017 | 1 | 2 | 1 | 4 |
| Sharifi et al.2017 | 1 | 1 | 1 | 3 |
| Koozehchian et al.2018 | 1 | 2 | 1 | 4 |
| Talari et al.2019 | 2 | 2 | 1 | 5 |
| Samulak et al.2019 | 0 | 0 | 1 | 1 |
| Jamilian et al.2019 | 2 | 2 | 1 | 5 |
| El-Sheikh et al.2019 | 1 | 0 | 1 | 2 |
Ten studies used a carnitine dosage < 500 mg [9, 15–17, 21, 34, 35, 38, 48, 52]. In seven trials [22, 32, 36, 39, 41, 45, 47] and one effect size [23] (a) a dosage ≥ 500–1000 mg was administrated. A dosage ≥ 1000–2000 mg was provided in 12 studies [12, 19, 20, 26, 27, 31, 42, 43, 46, 50, 53, 54] and one effect size [25] (a). A dosage ≥ 2000 mg was reported in 13 studies [14, 18, 24, 28–30, 33, 37, 40, 44, 49, 51, 55], and 2 effect sizes [23] (b) and [25] (b).
Based on the most included studies, carnitine supplementation was well tolerated and no significant side effects were observed in the intervention compared to the control groups [9, 14, 16, 22–24, 26, 28–30, 32–35, 39, 41–46, 48, 49, 51, 52, 54, 55]. In 2 studies the reason of withdrawal in the carnitine group were as follow: diarrhea which resolved after gastroenterology concealment and halving the intervention dosage for 1 week [21] and pruritic rash and nausea which were resolved after discontinuation of study intervention [19]. However, 15 studies did not mention or assess the adverse effects related to carnitine supplementation [12, 15, 17, 18, 20, 25, 27, 31, 36–38, 40, 47, 50, 53].
The effects of L-carnitine administration on inflammatory cytokines
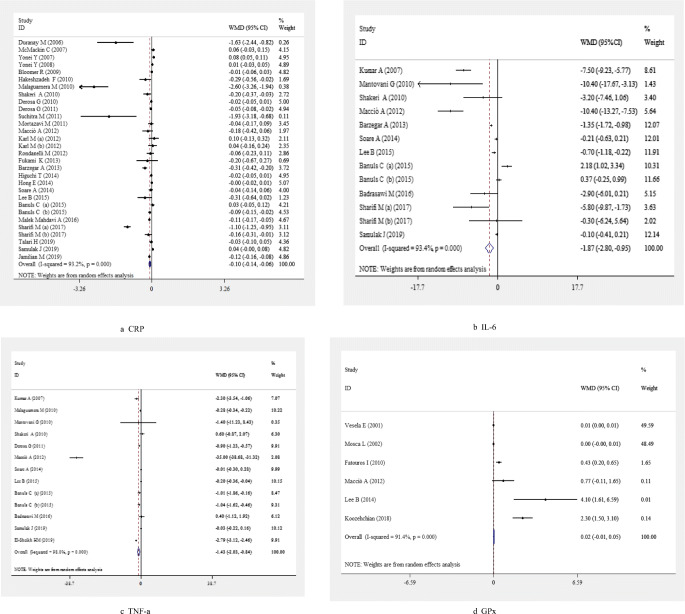
A pooled analysis of 30 effect sizes revealed that following L-carnitine supplementation significant reduction in CRP concentrations was achieved (WMD: -0.10; 95% CI: -0.14, -0.06) (Fig. 2A and Table 2). This finding did not remain significant in studies performed in patients aged ≥ 65 years (WMD: 0.01; 95% CI: -0.00, 0.02), Western countries (WMD: -0.02; 95% CI: -0.04, -0.00), patients with renal disease (WMD: -0.03; 95% CI: -0.06, -0.00), or liver diseases (WMD: -0.00; 95% CI: -0.02, 0.01), and cancer disease (WMD: -0.18 95% CI: -0.42, 0.06), studies which used dosages of 500–1000 mg/day (WMD: -0.01; 95% CI: -0.02, 0.01), those with a duration of 6–12 months (WMD: 0.00; 95% CI: -0.03, 0.04), and studies with a sample size of < 50 (WMD: 0.01; 95% CI: -0.00, 0.02). Carnitine supplementation increased CRP levels in healthy individuals (WMD: 0.02; 95% CI: 0.01, 0.04) and studies which used dosages of < 500 mg/day (WMD: 0.06; 95% CI: 0.03, 0.09) (Table 3).
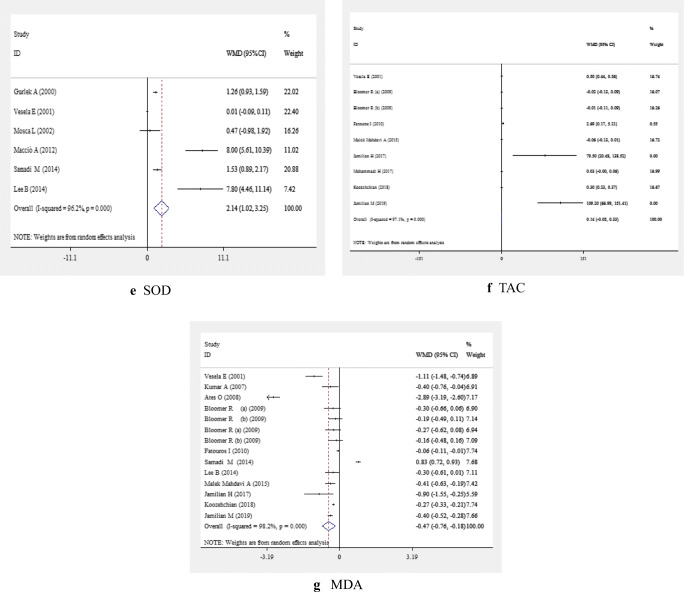
Fig. 2.
Meta-analysis biomarkers of inflammation and oxidative stress weighted mean difference estimates for A) CRP, B) IL-6, C) TNF-α, D) GPx, E) SOD, F) TAC, G) MDA in the L-carnitine supplements and placebo groups (CI=95%)
Table 2.
The effects of L-carnitine supplementation on biomarkers of inflammation and oxidative stress
| Variables | Number of effect sizes | Weighted mean difference | CI 95% | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | P- value heterogeneity | ||||
| CRP | 30 | -0.10 | -0.14, -0.06 | 93.2 | < 0.001 |
| IL-6 | 13 | -1.87 | -2.80, -0.95 | 93.4 | < 0.001 |
| TNF-α | 13 | -1.43 | -2.03, -0.84 | 98.0 | < 0.001 |
| GPx | 6 | 0.02 | -0.01, 0.05 | 91.4 | < 0.001 |
| SOD | 6 | 2.14 | 1.02, 3.25 | 96.2 | < 0.001 |
| TAC | 9 | 0.14 | -0.05, 0.33 | 97.1 | < 0.001 |
| MDA | 14 | -0.47 | -0.76, -0.18 | 98.2 | < 0.001 |
CRP: C-reactive protein; IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-α; GPx: Glutathione Peroxidase; SOD: Superoxide dismutase; TAC: Total Antioxidant Capacity; MDA: Malondialdehyde
Table 3.
Subgroup analyses for the effects of L-carnitine supplementation on biomarkers of inflammation and oxidative stress
| Variables | Subgroups | Number of effect sizes | Pooled WMD | 95% CI | I2 (%) | Between-study I2 (%) |
|
|---|---|---|---|---|---|---|---|
| CRP | Participants’ age | < 45 year | 6 | -0.05 | -0.07, -0.03 | 84.0 | < 0.001 |
| 45–65 year | 14 | -0.06 | -0.04, -0.02 | 95.7 | |||
| ≥ 65 years | 10 | 0.01 | -0.00, 0.02 | 80.3 | |||
| Country | Eastern | 16 | -0.02 | -0.03, -0.01 | 95.4 | < 0.001 | |
| Western | 14 | -0.02 | -0.04, -0.00 | 86.4 | |||
| Health condition | Healthy | 9 | 0.02 | 0.01, 0.04 | 72.4 | < 0.001 | |
| Renal disease | 7 | -0.03 | -0.06, -0.00 | 81.1 | |||
| CVD | 4 | -0.23 | -0.30, -0.16 | 98.3 | |||
| Chronic metabolic diseases | 6 | -0.05 | -0.07, -0.03 | 87.6 | |||
| Liver diseases | 2 | -0.00 | -0.02, 0.01 | 98.3 | |||
| Cancers | 1 | -0.18 | -0.42, 0.06 | - | |||
| Other diseases | 1 | -0.11 | -0.17, -0.05 | - | |||
| Administration dosage | < 500 mg | 4 | 0.06 | 0.03, 0.09 | 53.3 | < 0.001 | |
| 500–1000 mg | 7 | -0.01 | -0.02, 0.01 | 78.5 | |||
| 1000–2000 mg | 11 | -0.06 | -0.09, -0.04 | 96.6 | |||
| ≥ 2000 mg | 8 | -0.04 | -0.06, -0.02 | 92.1 | |||
| Study duration | < 6 month | 21 | -0.02 | -0.03, -0.01 | 94.2 | 0.09 | |
| 6–12 month | 7 | 0.00 | -0.03, 0.04 | 93.2 | |||
| ≥ 12 month | 2 | -0.03 | -0.06, -0.01 | 37.6 | |||
| Sample size | < 50 | 16 | 0.01 | -0.00, 0.02 | 79.8 | < 0.001 | |
| 50–100 | 9 | -0.15 | -0.17, -0.12 | 96.6 | |||
| ≥ 100 | 5 | -0.03 | -0.05, -0.01 | 8.5 | |||
| IL-6 | Participants’ age | < 65 year | 8 | -0.69 | -0.93-0.44 | 94.4 | 0.10 |
| ≥ 65 years | 5 | -0.39 | -0.65, -0.13 | 92.8 | |||
| Country | Eastern | 7 | -1.33 | -1.62, -1.04 | 90.3 | < 0.001 | |
| Western | 6 | -0.06 | -0.28, 0.17 | 93.3 | |||
| Study duration | < 6 month | 11 | -0.96 | -1.22, -0.71 | 93.8 | < 0.001 | |
| 6–12 month | 2 | -0.14 | -0.39, 0.11 | 00.0 | |||
| Sample size | < 50 | 5 | -0.09 | -0.33, 0.15 | 84.0 | < 0.001 | |
| 50–100 | 6 | -1.05 | -1.32, -0.78 | 93.6 | |||
| ≥ 100 | 2 | -10.40 | -13.07, -7.73 | 00.0 | |||
| Health condition | Healthy | 4 | -0.08 | -0.32, 0.15 | 46.0 | < 0.001 | |
| Renal disease | 1 | -3.20 | -7.46, 1.06 | - | |||
| CVD | 4 | -1.25 | -1.71, -0.79 | 95.0 | |||
| Chronic metabolic diseases | 2 | -1.02 | -1.38, -0.67 | 96.9 | |||
| Cancers | 2 | -10.40 | -13.07, -7.73 | 00.0 | |||
| TNF-α | Country | Eastern | 4 | -0.22 | -0.38,-0.06 | 76.3 | 0.12 |
| Western | 9 | -0.35 | -0.40, -0.30 | 98.6 | |||
| Sample size | < 50 | 5 | -0.18 | -0.30, -0.06 | 74.3 | < 0.001 | |
| 50–100 | 5 | -0.35 | -0.41, -0.29 | 98.2 | |||
| ≥ 100 | 3 | -1.17 | -1.50, -0.84 | 99.4 | |||
| GPx | Country | Eastern | 2 | 2.47 | 1.71, 3.23 | 45.1 | < 0.001 |
| Western | 4 | 0.01 | 0.00, 0.01 | 81.6 | |||
| SOD | Participants’ age | < 65 year | 2 | 1.36 | 0.77, 1.94 | 41.7 | < 0.001 |
| ≥ 65 year | 4 | 0.13 | 0.03, 0.22 | 97.4 | |||
| Country | Eastern | 2 | 1.75 | 1.12, 2.38 | 92.3 | < 0.001 | |
| Western | 4 | 0.12 | 0.03, 0.22 | 96.8 | |||
| TAC | Country | Eastern | 5 | 0.05 | 0.02, 0.08 | 95.8 | < 0.001 |
| Western | 4 | 0.29 | 0.24, 0.34 | 97.2 | |||
| Sample size | < 50 | 5 | 0.29 | 0.25, 0.33 | 96.3 | < 0.001 | |
| ≥ 50 | 4 | 0.01 | -0.01, 0.04 | 84.3 | |||
| < 500 mg | 2 | 0.50 | 0.44, 0.56 | 85.5 | < 0.001 | ||
| 500–1000 mg | 2 | -0.06 | -0.12, 0.01 | 78.1 | |||
| 1000–2000 mg | 2 | -0.02 | -0.13, 0.10 | 96.1 | |||
| ≥ 2000 mg | 3 | 0.06 | 0.04, 0.09 | 96.1 | |||
| MDA | Participants’ age | < 45 year | 8 | -0.10 | -0.14, -0.05 | 98.1 | < 0.001 |
| 45–65 year | 3 | -0.08 | -0.13, -0.03 | 83.5 | |||
| ≥ 65 years | 3 | -1.53 | -1.71, -1.34 | 98.6 | |||
| Country | Eastern | 7 | -0.11 | -0.15, -0.07 | 98.4 | < 0.09 | |
| Western | 7 | -0.16 | -0.21, -0.12 | 98.4 | |||
| Administration dosage | < 500 mg | 3 | -2.04 | -2.26, -1.83 | 97.1 | < 0.001 | |
| 500–1000 mg | 2 | -0.08 | -0.13, -0.03 | 89.1 | |||
| 1000–2000 mg | 4 | -0.37 | -0.47, -0.27 | 00.0 | |||
| ≥ 2000 mg | 5 | -0.04 | -0.08, 0.01 | 98.8 | |||
| Sample size | < 50 | 9 | -0.06 | -0.10, -0.03 | 97.8 | < 0.001 | |
| ≥ 50 | 5 | -0.66 | -0.75, -0.56 | 98.4 | |||
CRP: C-reactive protein; IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-α; GPx: Glutathione Peroxidase; SOD: Superoxide dismutase; TAC: Total Antioxidant Capacity; MDA: Malondialdehyde
L-carnitine also reduced IL-6 levels, as shown in the meta-analysis of 13 effect sizes (WMD: -1.87; 95% CI: -2.80, -0.95) (Fig. 2B and Table 2). This finding did not change in our subgroup analyses, except for studies done in Western countries (WMD: -0.06; 95% CI: -0.28, 0.17), studies with a duration of 6–12 months (WMD: -0.14; 95% CI: -0.39, 0.11), studies with a sample size of < 50 (WMD: -0.09; 95% CI: -0.33, 0.15), and those done on healthy subjects (WMD: -0.08; 95% CI: -0.32, 0.15) or patients with renal disease (-3.20, 95%CI -7.46, 1.06 (Table 3).
Pooling findings of 13 studies showed a significant reduction in TNF-α concentrations (WMD: -1.43; 95% CI: -2.03, -0.84) (Fig. 2C and Table 2) which did not alter in all subgroup analyses (Table 3). Moreover, after excluding one study (Maccio et al.) which had greatly different results from the other studies, overall finding did not change (WMD: -0.73; 95% CI: -1.14, -0.31).
The effects of L-carnitine on antioxidant enzymes
L-carnitine supplementation had no significant on the levels of GPx enzyme (WMD: 0.02; 95% CI: -0.01, 0.05) (Fig. 2D and Table 2). However, a significant elevation of GPx levels following L-carnitine supplementation was seen in subgroup analysis based on study location (Table 3). L-carnitine supplementation significantly increased the levels of SOD enzyme in the pooled analysis (WMD: 2.14; 95% CI: 1.02, 3.25) (Fig. 2E and Table 2) and in all subgroup analyses (Table 3).
The effects of L-carnitine on endogenous antioxidants
After combining 9 effect sizes, no significant effect of L-carnitine supplementation on TAC enzyme levels was found (WMD: 0.14; 95% CI: -0.05, 0.33) (Fig. 2F and Table 2). A significant increase in TAC levels was seen after subgroup analysis based on study location. Such an increase was also found in studies with a sample size of < 50 (WMD: 0.29; 95% CI: 0.25, 0.33) and studies used a dosage < 500 mg/day (WMD: 0.50; 95% CI: 0.44, 0.56) (Table 3).
The effects of L-carnitine administration on other biomarkers of oxidative stress
L-carnitine supplementation was associated with a significant decrease in MDA (WMD: -0.47; 95% CI: -0.76 -0.18) (Fig. 2G and Table 2). Findings of subgroup analyses were similar to the pooled analyses. However, L-carnitine supplementation did not alter MDA levels in studies which used L-carnitine supplements at daily dosages more than 2000 mg/day (WMD: -0.04; 95% CI: -0.08, 0.01) (Tables 3 and 4).
Meta-regression
Meta-regression showed A significant inverse association between carnitine supplementation dosage and serum levels of TNF-α (P = 0.02) and IL-6 (P = 0.01). However, no significant association was found between supplementation dosage and serum CRP (P = 0.70), TAC (P = 0.36) and MDA (P = 0.05) concentrations.
Publication bias
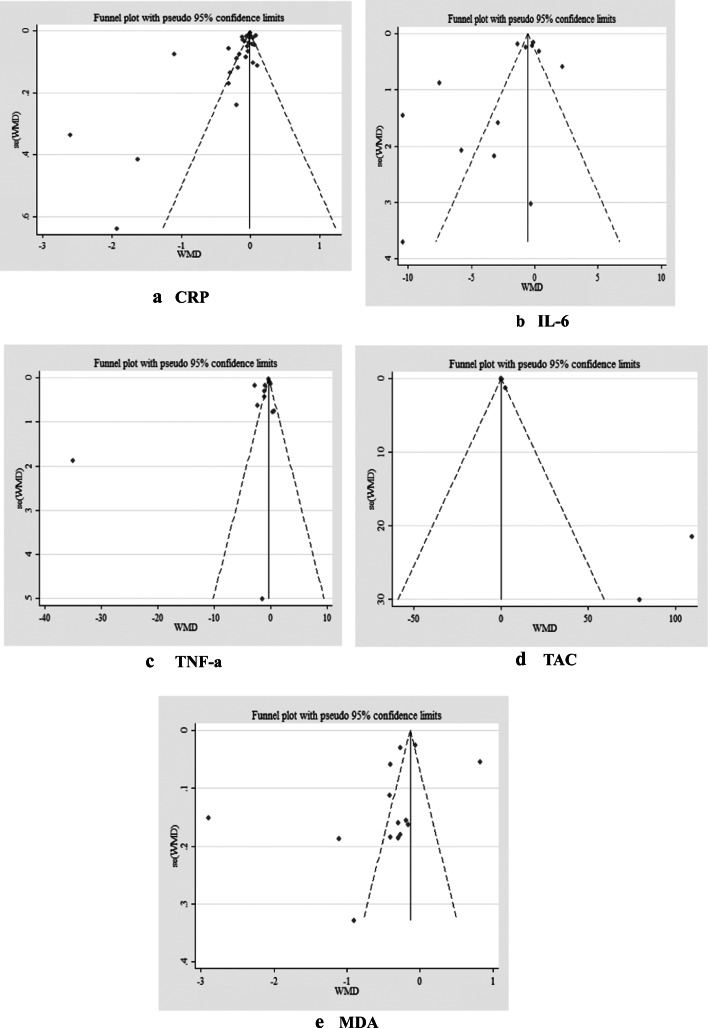
No evidence of publication bias among the included studies was showed in visual inspection of funnel plots for CRP, IL-6, TNF-α, TAC and MDA (Fig. 3A-E).
Fig. 3.
Funnel plots for A) CRP, B) IL-6, C) TNF-a, D) TAC and E) MDA
Discussion
In this meta-analysis, we investigated the effects of L-carnitine supplementation on the biomarkers of inflammation and oxidative stress. Our findings demonstrated that L-carnitine supplementation significantly attenuated CRP, IL-6, TNF-α, and MDA, and increased SOD levels, but did not influence GPx and TAC levels. Also dose-response meta-regression showed a significant inverse association between carnitine supplementation dosage and serum IL-6 and TNF-α concentrations which means reduction in the concentrations of these inflammatory cytokines following carnitine supplementation was more expressed in studies with higher dosage.
Effects on inflammatory profiles
In this meta-analysis, we showed that L-carnitine supplementation significantly lowerd the levels of CRP, IL-6 and TNF-α. In line with our findings, a meta-analysis on 13 trials indicated that oral carnitine supplementation led to decrease in the levels inflammatory variables in subjects with diverse underlying conditions [56]. Similarly, another meta-analysis performed by Sahebkar et al. [8] indicated that oral L-carnitine supplementation was associated with a significant decrease in CRP levels. In addition, another meta-analysis showed that carnitine administration in hemodialysis patients significantly reduced CRP values [57]. However, in some studies carnitine consumption was not effective for the attenuation of inflammation. For example, Sawicka et al. [13] failed to show any significant effects of carnitine supplementation at a dosage of 1,500 mg/day for 24 weeks on IL-6, TNF-α and CRP concentrations in healthy women older than 65 years. In another study, the consumption of carnitine-enriched bread foe three months did not change circulating inflammatory markers among participants with or without metabolic syndrome (MetS), which is opposite to our findings [44]. It is evidenced that elevated inflammatory status contributes to development and progression of atherosclerosis and cardiovascular disease [58]. In addition, higher CRP level is associated with increased mortality in general population and some chronic disorders [59–61]. Anti-inflammatory interventions may provide beneficial effects for targeting atherosclerosis [62]. Carnitine may play several important roles in the amelioration of inflammation, It is well known that ROS are as enhancers of inflammatory environment [63]. Therefore, the role of carnitine in decreasing inflammatory response, in part can be explained by its ability to reducing of ROS production [64]. Carnitine also exert a significant anti-inflammatory role through downregulation of nuclear factor kappa B pathway which leads to decrease in the expression of pro-inflammatory cytokines [65].
Effects on oxidative status
Results of our study indicated that L-carnitine supplementation was capable to the reduction of MDA and increment of SOD levels, but did not change TAC and GPX concentrations. Previously, several clinical studies have searched the effects of carnitine on oxidative stress in different diseases. In agreement with the results of present study, Signorelli et al. [66] reported that 600 mg propionyl L-carnitine supplementation for 12 months in hemodialysis patients with PAD was associated with improvement of oxidative parameters. In addition, taking carnitine supplements for three months by patients with age-related macular degeneration (AMD) significantly decreased MDA concentrations, while GSH levels were significantly increased [11]. Also it has been reported that carnitine administration at a dosage of 2000 mg/day for 2 months to patients with pemphigus vulgaris had favorable effects on TAC levels [49]. However, in a study by Shakeri et al. [12] 12 weeks carnitine supplementation to hemodialysis patients with hyperlipidemia did not show any significant effects on the parameters indicative of oxidative stress. Enhanced ROS generation and insufficient eliminating of free radicals lead to the alternation of function and structure of several molecules like lipids, carbohydrates, proteins, and DNA by peroxidation and glycoxidation [67, 68]. Carnitine supplementation may contribute to the modulation of oxidative stress due to its effects on the increasing of antioxidant system components like glutathione peroxidase [69], chelating of ferrous ions, interfering with the ROS formation [70], and the stabilaztion of free radicals [70, 71]. Based on all these findings as well as the results of present study, carnitine supplementation may be an effective intervention in order to the improvement of oxidative stress and protection against inflammatory status in different conditions, but these findings need to be confirmed by more RCTs in other diseases.
Strengths and limitations of study
This study is a comprehensive systematic review and meta-analysis of trials about the effect of carnitine administration on parameters of inflammation and indicators of oxidative stress. The present study had some strengths. This meta-analysis conducted on trials that investigating the efficacy of carnitine supplementation in participants with different underlying conditions. Previous meta-analyses focused on the anti-inflammatory effects of oral carnitine supplementation, but we included both oral and intravenous forms of carnitine. There are several limitations in the current meta-analysis that need to be considered in order to interpretation of the present results. In some studies, carnitine administration was combined with the supplementation of other nutrients or natural compounds to intervention group. In addition, due to the unfamiliarity with other languages, only English or Persian articles were included in this meta-analysis, which could lead to the language bias. Moreover, carnitine dosage and study duration were different in the included studies. We attempted to minimize these discrepancies trough different subgroup analyses.
Conclusions
In conclusion, carnitine supplementation lowered CRP, IL-6, TNF-α, and MDA levels, and increased SOD levels in studies conducted on healthy subjects and individuals with certain disorders, but did not affect other inflammatory parameters and oxidative stress profiles. Higher doses of L-carnitine might cause more reduction in some inflammatory variables like IL-6 and TNF-α. More RCTs in other diseases are needed to give confirmation for the findings of present work.
Abbreviations
- CRP
C-reactive protein
- IL-6
Interleukin-6
- TNF-α
Tumor Necrosis Factor-α
- GPx
Glutathione Peroxidase
- SOD
Superoxide dismutase
- TAC
Total Antioxidant Capacity
- GSH
Glutathione
- MDA
Malondialdehyde
- ROS
Reactive Oxygen Species
Author contributions
HF, JH contributed in conception, design, statistical analysis and drafting of the manuscript. AM, RZ, EA, ZA and MAM contributed in data collection and manuscript drafting. All authors approved the final version for submission. JH supervised the study.
Data availability
The primary data for this study is available from the authors on direct request.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hadis Fathizadeh, Email: Fathi_zh@yahoo.com.
Alireza Milajerdi, Email: amkhv@yahoo.com.
Željko Reiner, Email: zeljko.reiner@kbc-zagreb.hr.
Elaheh Amirani, Email: e.amirani74@gmail.com.
Zatollah Asemi, Email: Asemi_r@yahoo.com.
Mohammad Ali Mansournia, Email: mansournia_ma@yahoo.com.
Jamal Hallajzadeh, Email: jamal.hallaj@yahoo.com.
References
- 1.Wang B-S, Huang G-J, Lu Y-H, Chang L-W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013;138:751–6. doi: 10.1016/j.foodchem.2012.11.106. [DOI] [PubMed] [Google Scholar]
- 2.Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54:29–37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad S, Tyagi AK, Aggarwal BB. Detection of inflammatory biomarkers in saliva and urine: potential in diagnosis, prevention, and treatment for chronic diseases. Exp Biol Med. 2016;241:783–99. doi: 10.1177/1535370216638770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dokmeci D, Akpolat M, Aydogdu N, Doganay L, Turan FN. L-carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacol Rep. 2005;57:481–8. [PubMed] [Google Scholar]
- 5.Matera M, Bellinghieri G, Costantino G, Santoro D, Calvani M, Savica V. History of L-carnitine: implications for renal disease. J Ren Nutr. 2003;13:2–14. doi: 10.1053/jren.2003.50010. [DOI] [PubMed] [Google Scholar]
- 6.Bertelli A, Conte A, Ronca G. L-propionyl carnitine protects erythrocytes and low density lipoproteins against peroxidation. Drugs Under Exp Clin Res. 1994;20:191–7. [PubMed] [Google Scholar]
- 7.Eknoyan G, Latos DL, Lindberg J. Practice recommendations for the use of L-Carnitine in dialysis-related carnitine disorder National Kidney Foundation Carnitine Consensus Conference. Elsevier, Amsterdam; 2003. [DOI] [PubMed]
- 8.Sahebkar A. Effect of L-Carnitine Supplementation on Circulating C-Reactive Protein Levels: A Systematic Review and Meta-Analysis/Uticaj Suplementacije L-Karnitinom Na Nivoe C-Reaktivnog Proteina U Cirkulaciji: Sistematski Pregled I Metaanaliza. Journal of medical biochemistry. 2015;34:151–9. doi: 10.2478/jomb-2014-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, Chatzinikolaou A, et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med Sci Sports Exerc. 2010;42:1809–18. doi: 10.1249/MSS.0b013e3181dbacab. [DOI] [PubMed] [Google Scholar]
- 10.Mescka CP, Guerreiro G, Donida B, Marchetti D, Wayhs CAY, Ribas GS, et al. Investigation of inflammatory profile in MSUD patients: benefit of L-carnitine supplementation. Metab Brain Dis. 2015;30:1167–74. doi: 10.1007/s11011-015-9686-9. [DOI] [PubMed] [Google Scholar]
- 11.Ates O, Alp HH, Mumcu U, Azizi S, Cinici E, Kiziltunc A, et al. The effect of L-carnitine treatment on levels of malondialdehyde and glutathione in patients with age related macular degeneration. Eurasian J Med. 2008;40:1. [PMC free article] [PubMed] [Google Scholar]
- 12.Shakeri A, Tabibi H, Hedayati M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodialysis international International Symposium on Home Hemodialysis. 2010;14:498–504. [DOI] [PubMed]
- 13.Sawicka A, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek R. l-Carnitine supplementation in older women. A pilot study on aging skeletal muscle mass and function. Nutrients. 2018;10:255. [DOI] [PMC free article] [PubMed]
- 14.Gurlek A, Tutar E, Akcil E, Dincer I, Erol C, Kocaturk PA, et al. The effects of L-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. Eur J Heart Fail. 2000;2:189–93. doi: 10.1016/s1388-9842(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 15.Vesela E, Racek J, Trefil L, Jankovy’ch V, Pojer M. Effect of L-carnitine supplementation in hemodialysis patients. Nephron. 2001;88:218–23. doi: 10.1159/000045993. [DOI] [PubMed] [Google Scholar]
- 16.Mosca L, Marcellini S, Perluigi M, Mastroiacovo P, Moretti S, Famularo G, et al. Modulation of apoptosis and improved redox metabolism with the use of a new antioxidant formula. Biochem Pharmacol. 2002;63:1305–14. doi: 10.1016/s0006-2952(02)00867-5. [DOI] [PubMed] [Google Scholar]
- 17.Duranay M, Akay H, Yilmaz FM, Senes M, Tekeli N, Yucel D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3211–4. doi: 10.1093/ndt/gfl356. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Singh RB, Saxena M, Niaz MA, Josh SR, Chattopadhyay P, et al. Effect of carni Q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the Tishcon study. Acta Cardiol. 2007;62:349–54. doi: 10.2143/AC.62.4.2022278. [DOI] [PubMed] [Google Scholar]
- 19.McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, et al. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich Conn) 2007;9:249–55. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.YONEI Y, TAKAHASHI Y, WATANABE M. A double-blind, randomized controlled trial (RCT) of L-carnitine and conjugated linoleic acid-based health food with health claims. Anti-Aging Med. 2007;4:19–27. [Google Scholar]
- 21.Ates O, Alp HH, Mumcu U, Azizi S, Cinici E, Kiziltunc A, et al. The effect of L-carnitine treatment on levels of malondialdehyde and glutathione in patients with age related macular degeneration. Eurasian J Med. 2008;40:1–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Yonei Y, Takahashi Y, Hibino S, Watanabe M, Yoshioka T. Effects on the human body of a dietary supplement containing L-carnitine and garcinia cambogia extract: A study using double-blind tests. J Clin Biochem Nutr. 2008;42:89–103. doi: 10.3164/jcbn.2008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloomer RJ, Tschume LC, Smith WA. Glycine propionyl-L-carnitine modulates lipid peroxidation and nitric oxide in human subjects. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J Int de vitaminologie et de nutrition. 2009;79:131–41. doi: 10.1024/0300-9831.79.3.131. [DOI] [PubMed] [Google Scholar]
- 24.Bloomer RJ, Fisher-Wellman KH, Tucker PS. Effect of oral acetyl L-carnitine arginate on resting and postprandial blood biomarkers in pre-diabetics. Nutr Metab. 2009;6:25. doi: 10.1186/1743-7075-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloomer RJ, Smith WA. Oxidative stress in response to aerobic and anaerobic power testing: influence of exercise training and carnitine supplementation. Res Sports Med. 2009;17:1–16. doi: 10.1080/15438620802678289. [DOI] [PubMed] [Google Scholar]
- 26.Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I, et al. Comparison between orlistat plus l-carnitine and orlistat alone on inflammation parameters in obese diabetic patients. Fundam Clin Pharmacol. 2011;25:642–51. doi: 10.1111/j.1472-8206.2010.00888.x. [DOI] [PubMed] [Google Scholar]
- 27.Hakeshzadeh F, Tabibi H, Ahmadinejad M, Malakoutian T, Hedayati M. Effects of L-Carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren Fail. 2010;32:1109–14. doi: 10.3109/0886022X.2010.510617. [DOI] [PubMed] [Google Scholar]
- 28.Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, et al. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis—a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–11. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, et al. Sibutramine and L-carnitine compared to sibutramine alone on insulin resistance in diabetic patients. Intern Med (Tokyo Japan) 2010;49:1717–25. doi: 10.2169/internalmedicine.49.3401. [DOI] [PubMed] [Google Scholar]
- 31.Suchitra MM, Ashalatha VL, Sailaja E, Rao AM, Reddy VS, Bitla AR, et al. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J Kidney Dis Transplant. 2011;22:1155–9. [PubMed] [Google Scholar]
- 32.Mortazavi M, Asgari S, Ghassami M, Seirafian S, Taheri S, Naini AE, et al. The effect of oral L-carnitine on serum albumin and inflammatory markers levels in patients under peritoneal dialysis: A randomized controlled trial. J Isfahan Med Sch. 2011;29.
- 33.Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012;124:417–25. doi: 10.1016/j.ygyno.2011.12.435. [DOI] [PubMed] [Google Scholar]
- 34.Karl M, Rubenstein M, Rudnick C, Brejda J. A multicenter study of nutraceutical drinks for cholesterol (evaluating effectiveness and tolerability) J Clin Lipidol. 2012;6:150–8. doi: 10.1016/j.jacl.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Rondanelli M, Opizzi A, Perna S, Faliva M, Solerte SB, Fioravanti M, et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine. 2013;44:391–401. doi: 10.1007/s12020-012-9863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukami K, Yamagishi S, Sakai K, Kaida Y, Adachi T, Ando R, et al. Potential inhibitory effects of L-carnitine supplementation on tissue advanced glycation end products in patients with hemodialysis. Rejuven Res. 2013;16:460–6. doi: 10.1089/rej.2013.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzegar A, Alipour B, Panahi F, Karamzad N. Effect of L-carnitine supplementation on serum adipokines (leptin and visfatin) levels in obese type II diabetes mellitus women with hypocaloric diet. Life Sci J. 2013;10:359–65. [Google Scholar]
- 38.Higuchi T, Abe M, Yamazaki T, Mizuno M, Okawa E, Ando H, et al. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: a randomized controlled trial. Nutrients. 2014;6:5992–6004. doi: 10.3390/nu6125992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, et al. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014;29:1449–57. doi: 10.1111/jgh.12536. [DOI] [PubMed] [Google Scholar]
- 40.Samadi M, Alinejad HA, Jafari M, Khalagi K, Asjodi F, Falah E. Effect of L-carnitine supplementation on health indicators of untrained men over a period of resistance training: A randomized, placebo-controlled trial. Iran J Health Educ Health Promot. 2014;2:232–41. [Google Scholar]
- 41.Soare A, Weiss EP, Holloszy JO, Fontana L. Multiple dietary supplements do not affect metabolic and cardio-vascular health. Aging. 2014;6:149–57. doi: 10.18632/aging.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BJ, Lin JS, Lin YC, Lin PT. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr J. 2014;13:79. doi: 10.1186/1475-2891-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee BJ, Lin JS, Lin YC, Lin PT. Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition (Burbank, Los Angeles County, Calif). 2015;31:475–9. [DOI] [PubMed]
- 44.Bañuls C, Rovira-Llopis S, Monzó N, Solá E, Viadel B, Víctor VM, et al. The consumption of a bread enriched with dietary fibre and l-carnitine improves glucose homoeostasis and insulin sensitivity in patients with metabolic syndrome. J Cereal Sci. 2015;64:159–67. [Google Scholar]
- 45.Malek Mahdavi A, Mahdavi R, Kolahi S, Zemestani M, Vatankhah AM. L-Carnitine supplementation improved clinical status without changing oxidative stress and lipid profile in women with knee osteoarthritis. Nutr Res. 2015;35:707–15. doi: 10.1016/j.nutres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Badrasawi M, Shahar S, Zahara AM, Nor Fadilah R, Singh DK. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging. 2016;11:1675–86. doi: 10.2147/CIA.S113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malek Mahdavi A, Mahdavi R, Kolahi S. Effects of l-carnitine supplementation on serum inflammatory factors and matrix metalloproteinase enzymes in females with knee osteoarthritis: A randomized, double-blind, placebo-controlled pilot study. J Am Coll Nutr. 2016;35:597–603. doi: 10.1080/07315724.2015.1068139. [DOI] [PubMed] [Google Scholar]
- 48.Jamilian H, Jamilian M, Samimi M, Afshar Ebrahimi F, Rahimi M, Bahmani F, et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Gynecol Endocrinol. 2017;33:442–7. doi: 10.1080/09513590.2017.1290071. [DOI] [PubMed] [Google Scholar]
- 49.Mohammadi H, Djalali M, Daneshpazhooh M, Honarvar NM, Chams-Davatchi C, Sepandar F, et al. Effects of L-carnitine supplementation on biomarkers of oxidative stress, antioxidant capacity and lipid profile, in patients with pemphigus vulgaris: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2017. [DOI] [PubMed]
- 50.Sharifi MH, Eftekhari MH, Ostovan MA, Rezaianazadeh A. Effects of Therapeutic Lifestyle Change Diet and Q10 Plus L-Carnitine Supplementation on Inflammatory Biomarkers of In-Stent Restenosis, Lipid Profile, and Left Ventricular Ejection Fraction in Myocardial Infarction: A Randomized Clinical Trial. Iran Red Crescent Med J. 2017;19.
- 51.Koozehchian MS, Daneshfar A, Fallah E, Agha-Alinejad H, Samadi M, Kaviani M, et al. Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males. J Exerc Nutr Biochem. 2018;22:7–19. doi: 10.20463/jenb.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talari HR, Azad ZJ, Hamidian Y, Samimi M, Gilasi HR, Ebrahimi Afshar F, et al. Effects of Carnitine Administration on Carotid Intima-media Thickness and Inflammatory Factors in Patients with Polycystic Ovary Syndrome: A Randomized, Double-blind, Placebo-controlled Trial. Int J Prev Med. 2019;10:89. doi: 10.4103/ijpvm.IJPVM_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W, et al. L-Carnitine supplementation increases trimethylamine-N-Oxide but not markers of atherosclerosis in healthy aged women. Ann Nutr Metab. 2019;74:11–7. doi: 10.1159/000495037. [DOI] [PubMed] [Google Scholar]
- 54.Jamilian M, Foroozanfard F, Kavossian E, Aghadavod E, Amirani E, Mahdavinia M, et al. Carnitine and chromium co-supplementation affects mental health, hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. J Psychosom Obstet Gynaecol. 2019:1–9. [DOI] [PubMed]
- 55.El-Sheikh HM, El-Haggar SM, Elbedewy TA. Comparative study to evaluate the effect of l-carnitine plus glimepiride versus glimepiride alone on insulin resistance in type 2 diabetic patients. Diabetes Metab Syndr. 2019;13:167–73. doi: 10.1016/j.dsx.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Haghighatdoost F, Jabbari M, Hariri M. The effect of L-carnitine on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. 2019;75:1037–46. doi: 10.1007/s00228-019-02666-5. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Abbate M, Tang L, Cai G, Gong Z, Wei R, et al. L-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: a systematic review and meta-analysis. Am J Clin Nutr. 2013;99:408–22. doi: 10.3945/ajcn.113.062802. [DOI] [PubMed] [Google Scholar]
- 58.Shah PK, Lecis D. Inflammation in atherosclerotic cardiovascular disease. F1000Research. 2019;8. [DOI] [PMC free article] [PubMed]
- 59.Tian R, Tian M, Wang L, Qian H, Zhang S, Pang H, et al. C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: A meta-analysis. Cytokine. 2019;117:59–64. doi: 10.1016/j.cyto.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Leuzzi G, Galeone C, Taverna F, Suatoni P, Morelli D, Pastorino U. C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2017;26. [DOI] [PMC free article] [PubMed]
- 62.Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed]
- 63.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mollica G, Senesi P, Codella R, Vacante F, Montesano A, Luzi L, et al. L-carnitine supplementation attenuates NAFLD progression and cardiac dysfunction in a mouse model fed with methionine and choline-deficient diet. Dig Liver Dis. 2020;52:314–23. doi: 10.1016/j.dld.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Koc A, Ozkan T, Karabay AZ, Sunguroglu A, Aktan F. Effect of L-carnitine on the synthesis of nitric oxide in RAW 264·7 murine macrophage cell line. Cell Biochem Funct. 2011;29:679–85. doi: 10.1002/cbf.1807. [DOI] [PubMed] [Google Scholar]
- 66.Santo Signorelli S, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Conti GO, et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl L-carnitine in patients with peripheral arterial disease requiring haemodialysis. Drugs Aging. 2006;23:263–70. doi: 10.2165/00002512-200623030-00008. [DOI] [PubMed] [Google Scholar]
- 67.Mohammadi H, Djalali M, Daneshpazhooh M, Honarvar N, Chams-Davatchi C, Sepandar F, et al. Effects of L-carnitine supplementation on biomarkers of oxidative stress, antioxidant capacity and lipid profile, in patients with pemphigus vulgaris: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2018;72:99. doi: 10.1038/ejcn.2017.131. [DOI] [PubMed] [Google Scholar]
- 68.Korkmaz S, Erturan İ, Nazıroğlu M, Uğuz AC, Çiğ B, Övey İS. Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of Ca 2 + release and antioxidant system. J Membr Biol. 2011;244:113–20. doi: 10.1007/s00232-011-9404-4. [DOI] [PubMed] [Google Scholar]
- 69.Türker Y, Nazıroğlu M, Gümral N, Çelik Ö, Saygın M, Çömlekçi S, et al. Selenium and L-carnitine reduce oxidative stress in the heart of rat induced by 2.45-GHz radiation from wireless devices. Biol Trace Elem Res. 2011;143:1640–50. doi: 10.1007/s12011-011-8994-0. [DOI] [PubMed] [Google Scholar]
- 70.Molfino A, Cascino A, Conte C, Ramaccini C, Fanelli FR, Laviano A. Caloric restriction and L-carnitine administration improves insulin sensitivity in patients with impaired glucose metabolism. J Parenter Enter Nutr. 2010;34:295–9. doi: 10.1177/0148607109353440. [DOI] [PubMed] [Google Scholar]
- 71.Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G, et al. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-L-carnitine therapy. Hypertension. 2009;54:567–74. doi: 10.1161/HYPERTENSIONAHA.109.132522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.