Abstract
Purpose
Spexin, a novel 14-amino acid peptide, has multiple physiological functions. The purpose of this paper was to systematically evaluate the current literature on the role of Spexin neuropeptide in obesity and its related comorbidities, food intake and overall metabolic status in human, animal and in vitro studies.
Methods
Multiple databases, including PubMed, EMBASE, ProQuest, Scopus and Google Scholar were searched for English-language papers published since inception until December 2018, that investigated Spexin levels in relation to chronic metabolic diseases, overall metabolism control and feeding-related behaviors.The quality of the included observational studies was assessed by a version of the Newcastle Ottawa Scale (NOS) designed for non-randomized studies and SYRCLE’s assessment tool for animal models.
Results
Out of 224 records screened, search results led to a total of 24 related studies (12 human studies (ten cross-sectional studies, one cohort study, and one longitudinal study) and 12 studies in either animals or in vitro).Nine of the included cross-sectional studies and one Longitudinal study had moderate to good study quality, and one cross-sectional and one cohort study had high-quality (or low risk of bias).
Conclusion
It appears that Spexin has a positive impact on overall metabolic status. As a novel appetite-regulating peptide, Spexin can act as an anorexigenic factor. Information about Spexin is very limited, and well-designed randomized controlled clinical trials are warranted for replicating, validating, and extending the current findings.
PROSPERO registration number
CRD42018117198).
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00636-8) contains supplementary material, which is available to authorized users.
Keywords: Spexin, Neuropeptide Q, Appetite, Feeding-related behaviors, Systematic review
Introduction
Spexin, a novel 14-amino acid neuropeptide, also referred to as neuropeptide Q, was discovered in 2007 [1]. Spexin is encoded by the C12orf29 gene, which is located in the 12thchromosome of the human genome [2].This neuropeptide is predominantly produced in human white adipose tissue [1],but can also be expressed in other various tissues/organs, including the brain, heart, lung, liver, thyroid, adrenal, muscle, ovary, testis, pancreas, stomach, and diverse sections of the gastrointestinal (GI) tract, as reported by the studies in either fish (e.g. goldfish) or mammals (e.g. rodents & human) [1, 3, 4].
The role of Spexin is not well-established yet; however, results from the preliminary studies indicate a probable role for it in regulation of obesity, energy homeostasis, appetite control, satiety, glucose and lipid metabolism, fatty acid uptake, cardiovascular/renal functions, endocrine homeostasis, reproduction and GI function [5].Spexin expression in adipose tissue and its circulating levels have been found to be remarkably decreased in obese individual [6, 7]. Furthermore, according to the serum data collected from obese vs. non-obese subjects, a negative correlation has been detected between Spexin and leptin [8, 9], and a low serum level of Spexin has been postulated to be a biomarker for childhood [6] and adult obesity [7]. On the other hand, based on the results from animal studies, Spexin might also act as an anorexigenic factor. In goldfish, injection of Spexin to the brain, inhibited both basal and neuropeptide Y (NPY)-induced food consumption, and decreased the expression of orexigenic factors, while augmented that of the anorexigenic factors, and increased brain levels of Spexin mRNA in the post-prandial state [3].
The limited studies conducted in humans thus far, have revealed that Spexin has an inverse correlation with blood glucose, hemoglobin A1C (HbAlc), triglyceride and low-density lipoprotein (LDL)-cholesterol; lower spexin levels have been found in type 2 diabetes mellitus (T2DM) patients as compared to non-diabetic individuals, which suggests that Spexin might affect glucose and lipid metabolism [11, 12]. Since obesity contributes substantially to the prevalence of diabetes, which adversely affects public health [13], investigating the role of Spexin in glucose homeostasis might help discover novel functions for it. Human pancreatic islets express Spexin; this implies that Spexin might be released together with insulin [14].
In obese women, serum Spexin had a negative correlation with serum levels of the hormones insulin and glucagon; this observation raises the possibility that Spexin might be implicated in insulin resistance and glucose metabolism [8].This idea is also in accord with the findings from a study in obese type 2 diabetic mouse model, in which Spexin administration not only reduced body weight but also enhanced glucose tolerance by decreasing insulin resistance as well as HbAlc [15]. However, no correlations between serum Spexin and diabetes, serum levels of the insulin sensitivity-related parameters, or blood lipids were demonstrated in adolescents afflicted with type 2 diabetes [16]. On the other hand, a study by Kumar et al., showed significantly lower Spexin concentrations in obese in comparison with lean adults and adolescents, and suggested a probable satiety-inducing role for the neuropeptide in humans [6].
The role of Spexin in humans is yet to be discovered. Recent findings suggest Spexin as a favorable candidate biomarker for the assessment of cardio-metabolic risk. Because progression of CVD is silent and the final end-points of CVD are not frequently seen in childhood,surrogate biomarkers are required to assess and modify these end-points [17]. In one study, the serum levels of high-sensitivity C-reactive protein (hs-CRP) revealed a positive correlation with low Spexin, and high leptin levels in obese adolescents, indicating that the Spexin signal might be associated with the risk of cardiovascular disease [9]. Similarly to adult data, C-reactive protein (CRP) is probably the most studied biomarker for inflammation in children. An independent association between CRP and raised lesions in the abdominal aorta and right coronary artery in the PDAY study also suggested CRP’s role in the early phases of preclinical atherosclerosis [18]. Spexin levels have also been shown to be significantly and positively correlated with Interleukin IL-1ß; this association warrants further investigation as to the probable role of Spexin in modulation of immune function [19]. This review is aimed to appraise the findings of all studies that have evaluated the role of the Spexin neuropeptide in obesity and its related comorbidities, food intake and overall metabolic status in human, animal, and in vitro studies.
Methods
Data sources and search strategy
A systematic literature search was carried out based on MOOSE reporting checklist(Meta-analyses of Observational Studies in Epidemiology) [20] and PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [21], using PubMed, EMBASE, ProQuest, Scopus, and Google Scholar electronic databases. The keywords searched included “spexin” or “neuropeptide Q” as text word and/or medical subject heading (MeSH) terms. The search was limited to human studies, animal studies, and in vitro original studies. The initial search was conducted in March 2018; as the project lasted more than six months, an update search was performed in December 2018 to find the relevant articles published between March and December. The review protocol was registered at the PROSPERO database of Systematic Reviews (registration number: CRD42018117198).
Eligibility criteria
Peer-reviewed studies published in the English language were included; book chapters, conference publications, letters, and review articles were excluded. Studies were eligible if they met the following criteria: all epidemiologic studies (including cohort, case-control, and cross-sectional studies)(2) clinical trials (3) animal studies (4) and in vitro studies that evaluated the role of spexin in feeding-related behaviors, metabolic state and other conditions that related to obesity and its related disorders. Papers with insufficient information were excluded.
Data extraction
The systematic search in electronic databases was done by a librarian specializing in medical information. Two of the co-authors independently screened the titles and abstracts of the retrieved articles, according to the inclusion and exclusion criteria; the studies which did not meet the criteria were excluded at this step.
Quality assessment
The full texts of the eligible papers were retrieved for quality assessment and data extraction. These papers were analyzed according to a checklist of aims, research question, and inclusion and exclusion criteria. Then, a third reviewer assessed the quality of the included studies; any disagreements between the reviewers were resolved through a discussion of the article among all the three reviewers. Data extracted from the eligible studies are summarized in Tables 1 and 2.Authors, Participants and number, Study design, aim and main outcomes for human studies and authors, animal model/ Cell line, Intervention/ duration, aim and main outcomes for animal/cell line studies are presented in these tables.
Table 1.
Characteristics of the included human studies
| Author Year, (Reference) | Participants and number | Study design | aim | Main Outcomes |
|---|---|---|---|---|
| Karaca et al. 2018 [11] | 29 type 1 and 30 type 2 diabetic patients and a control group of 23 healthy subjects | cross-sectional study | to measure levels of spexin in lean type 1 diabetic patients and its relevance to glycemic parameters without the presence of obesity or insulin resistance |
spexin levels: - significantly lower in patients with diabetes than in control subjects - not correlated with glycemic parameters, lipids, BMI, cortisol levels and thyroid stimulating hormone - no association between age and spexin levels in Regression models - no association with spexin levels within each group in Regression models including cortisol, BMI, HbA1c |
| Lin et al. 2018 [30] | 68 healthy adult women aged are in a wide range (minimum: 23; median: 38.5; maximum: 64 | cross-sectional study | the relationship between circulating spexin levels and age and to study their interaction effects on body mass index (BMI), fasting glucose, and -lipids |
Serum spexin levels: - significantly correlated with age, BMI, fasting glucose and TG - independently predict the risk of high BMI and high fasting glucose - No interaction effects of spexin and age on BMI and fasting glucose |
| Lin et al. 2018 [30] | 91 healthy adults)age of 18–65 years( | Cross sectional | Association between spexin and bile acid metabolism. |
serum spexin: - negatively correlated with TBA and TC levels,serum levels of GCDCA or TCDCA |
| Al-Daghi et al. 2018 [12] | 102 Saudi pregnant women (63 non-GDM and 39 GDM | prospective study | to determine whether circulating spexin (SPX) is modified during the course of pregnancy and whether it is affected by the presence of glucose intolerance, i.e., Gestational Diabetes Mellitus (GDM) |
In GDM patients, SPX levels: - increased significantly after 6-months, in parallel with a borderline significant increase in glucose In non-GDM patients SPX levels: - decreased from baseline to 6-months and this change was not associated with changes in glucose levels. Change in glucose from baseline to 6-months was positively associated with change in SPX in GDM patients only |
| Al-Daghri et al. 2018 [12] | 124 participants (41 males and 83 females; aged 42.4 ± 10.3 y) (MetS group) and 136 (21 male and 115 females; aged 33.1 ± 8.7 y) (non-MetS group) | cross-sectional study | to determine whether circulating levels of spexin (SPX) is associated with components of metabolic syndrome (MetS) |
SPX levels - significantly lower in participants with MetSvs. non-MetS - associated with MetS only in women with Stratification according to sex. - this significance was lost after adjustment for age and BMI |
| Al-Daghri et al. 2018 [12] | N = 117 (n = 63 women with gestational diabetes mellitus (GDM) N = 54 without GDM) | Cross-sectional study. | Relationship between sepxin and glucose hemostasis, lipid profile and inflammatory factors in GDM |
levels of SPX: - not significantly different in pregnant women with and without GDM. - positive associations with total cholesterol, LDL-cholesterol, 25(OH)D, and IL1b. - modest associations with glucose and HOMA in all subjects |
| Kumar et al. 2018 [9] | (12 girls with severe obesity; age = 16.7 ± 1.5 years; BMI = 51.6 ± 2.9 kg/m 2). | Longitudinal study | effect of Roux-en-Y gastric bypass (RYGB) surgery on endogenous spexin concentration and various risk factors of type 2 diabetes and cardiovascular disease in youth with severe obesity. |
Serum spexin - increased at 6 months after surgery and stabilized afterward - correlated negatively with: homeostatic model assessment insulin resistance, HOMA - correlated positively with: high molecular weight adiponectin. - The change in spexin, from baseline to 6 months after surgery, inversely correlated with: the corresponding change in BMI - the 6-month changes in spexin and HOMA-IR, inversely correlated even after adjusting for the change in BMI |
| Kolodziejski et al. 2018 [8] |
healthy female (n = 15, meanBMI 22.50 ± 0.58) and obese patients (n = 15, mean BMI 40.23 ± 1.31) |
cross-sectional study | Relationship between sepxin and KISS with obesity and glucose hemostasis |
Serum spexin and KISS - significantly lower in obese patients compared to non-obese. - SPX negatively correlated with:BMI, HOMA-IR, insulin, glucagon,active ghrelin and leptin - positively correlated with: serum levels of obestatin, GLP-1 and adiponectin and orexin-A |
| Kumar et al. 2018 [9] |
N = 19 adolescents with obesity Mean ages = 15.8 ± 1.7 years) |
Cross-sectional study | Relationship between sepxin and adiposity and inflammatory hormones |
Spexin inversely related with leptin. the odds of having ‘low spexin and high leptin’ in participants with higher hs-CRP (≥ 3 mg/L) were 12.25 times higher than those of participants with lowerhs-CRP (<3 mg/L). |
| Hodges et al. 2017 [16] |
12healthy normal weight and 10 obese adolescents mean age = 16 years 12obese with T2DM |
Cross sectional | Relationship between spexin and obesity | Spexin was not significantly correlated with any of the body composition, fitness, or blood biochemical measurements |
| Kumar et al. 2016 [6] | 51 obese and18 normal weight; Mean age = 15.3 ± 0.26. N = 69 | cross-sectional study | Relationship between sepxin and weight and cardio-metabolic hormones |
Spexin levels: - were significantly lower in obese vs normal-weight children, - not correlate with other adipokines and/or insulin and glucose levels. |
| Gu et al. 2015 [14] |
121 subjects with T2DM and 105 healthy individuals 12 healthy volunteers (aged: 52.8 ± 9.2 years with BMI: 23.3 ± 1.8 kg/m2) including 5 males and 7 females |
Cross sectional | to examine circulating spexin levels after glucose load in T2DM patients. |
- Serum spexin level: significantly lower inT2DM - significant negative correlation: between spexin and FBG, HbA1c, TG and LDL-C - During the OGTT: - negative correlation of blood glucose with spexin - spexin exhibited a progressive and significant decrease from time 0 to 60 min and returned gradually to the basal values at 180 min |
- Abbreviation: TBA: total bile acid, GCDCA:glycocheno-deoxycholate, TCDCA:tauro-chenodeoxycholate, SPX: spexin, GDM: Gestational diabetes mellitus, MetS:Metabolic syndrome, KISS:kisspeptin, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, OGTT:oral glucose tolerance test
Table 2.
Characteristics of the animal model/ Cell linestudies
| Author Year, (Reference | animal model/ Cell line | Intervention/ duration | aim | Main Outcomes |
|---|---|---|---|---|
| Sassek et al. 2018 [33] | male Wistar rats/INS-1E cells and pancreatic islets | treated for 3 days with spexin | To identify the role, connections, and potential functions of spexin in endocrine pancreas. |
Insulin secretion: - was reduced by spexin at 16 mM glucose level from cultured cells and isolated - Was decreased after injection with spexin in obese rats Spexin treatment: - increase in cultured cells and pancreatic islets cell viability and proliferation and proliferating cell nuclear antigen protein level. - decrease in insulin and Pdx gene expression. |
| Sassek et al. 2018 [33] | A pancreas obtained from a pig | three concentrations of glucose (1, 6, and 16 mM) and two periods of incubation (90 min and 24 h) | To identify the potential role of spexin in the physiology of the endocrine pancreas |
1- presence of spexin inside the endocrine structures of the pancreas 2- glucose administration: - increase in spexin release from islets after a shortterm - decrease in spexin release after a long term - negative feedback loops between spexin andinsulin were found |
| Kolodziejski et al. 2018 [35] | murine 3 T3-L1 cell adipocytes and/or isolated human adipocytes. | treated with different concentrations of SPX (1.0, 10, 100, 1000 nM) | To investigate the effects of SPX on proliferation, cell viability, lipogenesis and lipolysis |
SPX: - inhibits adipogenesis - down-regulates mRNA expression of pro-adipogenic genes such as PPARγ, C/EBPα, C/EBPβ and Fabp4. - Stimulates: lipolysis by increasing the phosphorylation of (HSL). - inhibits lipogenesis and glucose uptake in human adipocytes and murine 3 T3-L1 cells. - no effect on murine 3 T3-L1 cell proliferation and viability |
| Deng et al. 2018 [24] | spotted scat fish | fasting and refeeding periods(for 2 or 7 days) | To clarify the roles of Spx in the regulation of food-intake and reproduction in the spotted scat |
A significant increase of: - spx expression in hypothalamus was observed after 2 and 7 days of food deprivation. A significant decrease of: - the spx transcript levels in the 7 day fasting group after refeeding 3 h after the scheduled feeding time |
| Wang et al. 2018 [25] | Tongue sole fish |
- fasting and refeeding periods(for 2, 7, or 35 days) -tongue sole SPX14 (100 and 1000 ng/g BW dosage) |
(1)to study the expression profiles of spexin mRNA in various tissues, (2) to evaluate the effects of feeding status on spexin mRNA levels, (3) to investigate the potential role of SPX in the control of appetite and reproduction of tongue sole |
spexin mRNA: - could be detected in various tissues, notably in the brain. - Stimulated by fasting(the hypothalamic expression of SPX) |
| He et al. 2017 | goldfish (hepatocytes and brain cell culture) | IP injection of D-(+)-glucose or human insulin | To study the Functional Link Between Food Intake and Spexin Expression |
feeding could elevate: - plasma levels of glucose, insulin, and SPX with concurrent rises in insulin and SPX (mRNA) expression in the liver. Injection of glucose and insulin: - elevate in SPX mRNA level in the liver and brain areas involved in appetite control respectively insulin signal induced by glucose shows dual role in SPX regulation - (1) acting as an autocrine/paracrine signal to trigger SPX mRNA expression in the liver - (2) serving as an endocrine signal to induce SPX gene expression in the brain |
| Zheng et al. 2017 [27] | zebrafishspx1−/− mutant lines | Intracranial administration of SPX1 | Spexin role in Food Intake in zebrafish tissue |
the spx1−/− mutant fish: - higher food intake than the wild type (WT) fish. - Higher level of (AgRP1), - higher glucose, triacylglycerol and cholesterol in the serum than WT fish Intracranial administration of SPX1: - reduce the mRNA expression of the AgRP1. |
| Wu et al. 2016 [28] | Ya-fish (Schizothoraxprenanti) | Feeding- re feeding periods | identification, tissue distribution and mRNA expression of spexin responses to peri-prandial and fasting |
SPX mRNA expression: - significantly increased in fed group than unfed groups after a meal, while the unfed group at 1 and 3 h substantially decreased than pre-prandial groups - significantly decreased during fasting for a week and sharply increased after refeeding on the 7th day, and then return to normal level on the 9th day. |
| Li et al. 2016 [10] | orange-spotted grouper fish (Epinepheluscoioides) |
- IP administration of spexin-14(100 and 1000 ng/g BW) -Feeding- re feeding periods |
Molecular cloning and functional characterization of spexininorange-spotted grouper |
Tissue expression analysis: -spexin is highly expressed in the brain, liver and ovary Real time-PCR analysis: - up-regulation of hypothalamic expression of spexin by food deprivation. IP administration of spexin-14: -significantly elevated the mRNA levels of (POMC) - suppressed the orexin expression in the hypothalamus |
| Ge et al. 2016 [15] | Mouse Models of Fatty Liver Disease | IPadministrationof spexin (25 μg/kg) daily for 28–29 days | To examined the effects of spexin, on obesity, type 2 diabetes mellitus (T2DM), and HS in thigh fat diet induced obesity mice |
spexin treatment cause: - weight loss in DIO mice - improved glucose tolerance - decrease insulin resistance and HbAlc - reduce: hepatic lipids by 60%, serum ALT and AST Incubation with spexin; - inhibited LCFA uptake by hepatocytes isolated from DIO mice - Spexin treatment |
| Walewsk et al. 2014 [7] | C57BL/6 J mice with DIO,Obese (DIO) adult female Wistarrat,omental and subcutaneous human fat samples | Spexin (35 mg/kg/day SC) in rats (25 mg/kg/day IP) in mice |
Effects of Spexin on Adipocyte Uptake of Long Chain Fatty Acids and Weight in Rodents with Diet-Induced Obesity |
Spexin injection in rats: - reduction in caloric intake 32% with corresponding weight loss - meal pattern was not affected Spexin injection in mice: - significantly reduction in the (RER) at night, and increased locomotion. Spexin incubation in vitro: - significantly inhibition of facilitated fatty acid (FA) uptake into DIO mouse adipocytes. |
| Wong et al. 2013 [3] | Goldfish (Carassiusauratus) | ICV injection of Goldfish SPX |
To clarify the function of SPX as a satiety factor in gold fish |
SPX mRNA levels: - elevated by food intake in the telencephalon, optic tectum, and hypothalamus brain injection of SPX: - inhibited both basal and NPY- or orexin induced feeding behavior and food consumption. - reduction in expression of NPY, AgRP, and apelin, - rises in CCK, CART, POMC, MCH, and CRH mRNA levels in brain areas |
Abbreviation:Spx; Spexin,IP: Intraperitoneal, SC:subcutaneous, ICV: Intracerebroventricular, POMC;pro opiomelanocortin, DIO: Diet Induced Obesity, HSL: hormone sensitive lipase,AgRP1:agouti-relate protein 1, LCFA: long chain fatty acid,RER: respiratory exchange ratio CCK: cholecystokinin, CART: Cocaine-andamphetamine-regulated transcript, MCH: melanin-concentrating hormone, CRH: corticotropin-releasing hormone
The quality of the included observational studies was assessed by a version of the Newcastle Ottawa Scale (NOS) designed for non-randomized studies [22]. Based on this scale, each study can get a maximum of 9 scores: 4 for selection and assessment of exposure, 2 for comparability, and 3 for assessment of outcome. Studies with scores of 5 points or more are considered to be of moderate to good study quality, and 7 scores or moreare considered to be of high study quality. The quality of animal studies was evaluated bySYRCLE’s assessment tool for animal models [23]. According to this scale, a “yes” judgment shows a low risk of bias; a “no” judgment indicates high risk of bias; the judgment will be “unclear” if insufficient details have been reported to assess the risk of bias properly.Any discrepancies were resolved by consultant with principal investigator. The quality of the in vitro study (Kolodziejski et al. 2018) was not assessed, since no valid tools have been suggested, thus far. The results are presented in Supplementary Tables 1 and 2.
Results
Study and participant characteristics
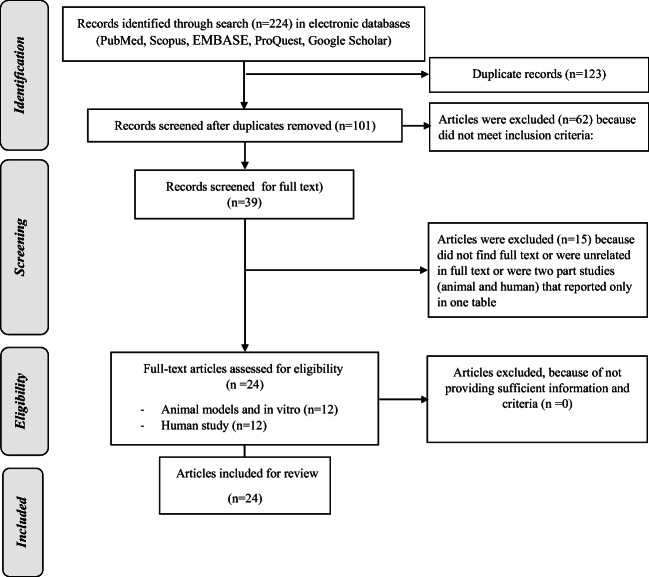
Figure 1 illustrates the flowchart of the study selection process for the present systematic review. In the primary search conducted in March2018, a total of 168 potentially eligible articles were retrieved by the search strategy: EMBASE (n = 37), ProQuest(n = 66), PubMed(n = 29), Scopus (n = 24), and google scholar(n = 12). After removal of the duplicate records,87 titles and abstracts were left for further screening. Fifty-nine of these were excluded, because they did not meet the inclusion criteria; the full texts of the other 28 articles were searched for their full text. Fourteen articles were excluded, as they were unrelated to the study aims, or their full texts could not be retrieved. Finally, 14 relevant full-text studies were reviewed.
Fig. 1.
Flow diagram of the literature search and study selection process
The complementary search in December 2018resulted in an extra 56 potentially eligible articles: EMBASE (n = 13), ProQuest (n = 9), PubMed (n = 9), Scopus (n = 16), and google scholar (n = 9). Following removal of duplicate and unrelated records, 10 full-text studies were reviewed. Finally, a total of 24 articles (12 animal studies and 12 human studies) were selected for qualitative synthesis. It should be noted that a number of articles consisted of two phases (human plus animal study, or animal plus cell line study);we reported the results of these studies in one table, and included the data regarding the other phase, below the corresponding study. Participants of the selected human studies included healthy male and females and subjects with obesity, DM, or metabolic syndrome (MetS); the age of the subjects in various studies ranged between 15 and 52 years.
Of the 12 selected human studies, eight evaluated the Spexin status in relation with glucose homeostasis (fasting blood glucose (FBG),insulin resistance, Homeostatic model assessment of insulin resistance(HOMA-IR), insulin, and HbA1c).Nine studies examined the relationship between obesity and Spexin. Five human studies reported spexin and a number of biochemical factors assumed to be involved in food intake. Eight studies reported the correlation between Spexin and inflammation, and seven studies reported lipid profile in association with Spexin serum levels.
From the 12 selected animal studies, eight studies investigated the Spexin role in feeding- related factors (NPY, Agouti-related protein (AgRP), apelin, etc.).Five studies evaluated the relationship between Spexin and glucose homeostasis parameters, five reported lipid profile in association with Spexin serum level, and two studies reported the role of Spexin in weight management and obesity.
Methodological quality and risk of bias
Out of 224 records screened, search results led to a total of 24 related studies (12 human studies (ten cross-sectional studies, one cohort study, and one longitudinal study) and 12 studies in either animals or in vitro. Nine of the included cross-sectional studies and one Longitudinal study had moderate to good study quality(NOS ≥ 5) and one cross-sectional and one cohort study had high-quality (or low risk of bias; NOS ≥ 7).Supplementary Table 1 presents further details on the quality of human studies. Details on the quality of animal studies, based on the bySYRCLE’s assessment tool, are presented in Supplementary Table 2.
Spexin and regulation of food intake
As mentioned above, eight animal [3, 7, 10, 24–28] and five human studies [6, 8, 9, 19, 29]investigated the role of Spexin in regulation of food intake. Several human studies showed an inverse relationship between Spexin and feeding hormones like leptin, and some studies showed a direct association between Spexin and ghrelin, obestatin, and adiponectin. Kolodziejski et al reported that obese patients had low Spexin and kisspeptin (KISS) levels compared to normal-weight subjects, and that Spexin was inversely correlated with BMI, leptin, glucagon and active ghrelin. In his study, a positive correlation was found between Spexin and serum levels of obestatin, glucagon-like peptide 1 (GLP-1), adiponectin and orexin-A [8].In a study by Kumar et al in 2016, no significant correlation was observed between Spexin levels and leptin and total adiponectin [6]. By contrast, her study in 2018 showed that Spexin was inversely correlated with leptin [9].In another study by the same research group, six months after Roux-en-Y gastric bypass (RYGB) surgery in women with severe obesity, Spexin level positively correlated with high molecular weight adiponectin (Spearman correlation r = 0.691, P = 0.011) [6, 29].Al-Daghri et al. Reported that Spexin levels were significantly associated with leptin (R = -0.43,P < 0.01)in pregnant women; this association was not significant for adiponectin [19]. In the report by Deng et al. a significant increase in Spexin expression in hypothalamus was found after two and seven days of food deprivation. However, on the seventh day of fasting, refeeding the animals 3 h after the scheduled feeding time resulted in a reduction in the Spexin transcript levels [24]. In another study, fasting stimulated the expression of Spexin mRNA in hypothalamus of Tongue sole fish [25].
In one animal study on goldfish, the authors indicated that feeding could raise plasma levels of glucose, insulin, and Spexin; this was concurrent with rises in insulin and Spexin mRNA expression in the liver. Likewise, increased Spexin mRNA levels was observed in the liver and brain areas that are involved in appetite control in goldfish, following intra-peritoneal injection of glucose and insulin, respectively [26].Zheng and his colleagues showed that the spx1−/− mutant fish had greater food intake than the wild type (WT). AgRP1 (a significant appetite stimulant) expression levels were significantly higher in spx1−/− mutant fish after feeding, as well. Intracranial administration of Spexin could also diminish mRNA expression of the AgRP1 [27]. Hongwei et al. reported the same result in Ya-fish. In addition, Spexin mRNA expression in forebrain was considerably decreased (P < 0.01) during a one-week fasting and abruptly augmented (P < 0.01) after re-feeding on the seventh day, and then returned to its normal level on the ninth day [28].
Wong et al. study revealed that food intake led to elevated Spexin mRNA levels in the telencephalon, optic tectum, and hypothalamus of goldfish brain. However, injection of goldfish Spexin into the brain hindered both basal and NPY/orexin-induced feeding behavior and food intake. Transcript expression of NPY, AgRP, and apelin were decreased by a similar intervention; rises in cholecystokinin (CCK), Cocaine-and amphetamine-regulated transcript (CART), POMC, melanin-concentrating hormone (MCH), and corticotropin-releasing hormone (CRH) mRNA levels were found in different brain areas assessed, simultaneously. The differential effects of Spexin administration on expression of NPY, CCK, and MCH transcripts could be noted in vitro in brain cell culture of goldfish, as well [3]. Li et al. demonstrated that the hypothalamic expression of Spexin was up-regulated by food deprivation. Intra-peritoneal administration of Spexin-14 peptides to grouper fish caused a significant increase in the mRNA levels of POMC, and suppressed the orexin expression in the hypothalamus [10]. Spexin also could reduce appetite in an animal model; 35 mg/kg/day subcutaneous (SC) injection of Spexin led to an approximately 32% reduction in caloric intake with corresponding weight loss in rats. Similarly, intra-peritoneal injection of Spexin at a dose of 25 mg/kg/day significantly decreased the respiratory exchange ratio (RER) at night, in mice [7].
Spexin, obesity and glucose homeostasis
Totally Nine human studies, and two animal studies reported obesity-related outcomes [6–8, 11, 12, 15, 16, 19, 29, 30].Three cross-sectional studies and one prospective study have focused on Spexin in association with diabetes in patients with type 1, type 2 and gestational diabetes mellitus (GDM) parameters [11, 14, 19, 31]. and Four studies reported fasting glucose, insulin concentration and HOMA-IR status in relation with Spexin in obese subjects [6, 8, 29, 30].
Kumar et al in 2018 found that Spexin levels decreased after gastric bypass surgery, and had an inverse correlation with the corresponding change of BMI (Spearman correlation r = −0.573, P = 0.051) in girls with severe obesity. In this sudy, serum Spexin concentrations increased six months after Roux-en-Y gastric bypass (RYGB) surgery(P = 0.01),but stabilized afterwards and Circulating levels of Spexin inversely correlated with HOMA-IR (Spearman correlation r = −0.796, P < 0.001).The changes in Spexin and HOMA-IR observed six months after the surgery, were negatively correlated (slope [standard error; SE] = − 0.0084 (0.0019), P = 0.001)], even when the analyses were adjusted for the change in body mass index (BMI) [29]. The same authors had previously shown that Spexin levels were significantly lower in obese children compared to their normal-weight peers Median (inter quartile range; IQR): 0.33 ng/mL [0.27–0.44] vs. 0.42 ng/mL [0.33–0.55]; P = 0.024).When analyzed as ordinal categorical variable, Spexin showed a strong reverse association with obesity. Cluster analysis of Spexin and BMI z scores allowed for categorizing the study participants into normal-weight and obese groups with great accuracy and in Contrary to Kumar’s new study there were not any significant correlation between Spexin levels and fasting insulin or glucose levels in children, [6].In agreement with the study by Kumar et al.(2018), Kolodziejski reported that Spexin was negatively correlated with HOMA-IR [8] in this study, obese patients had lower Spexin and KISS levels as compared to non-obese participants [8].Lin et al. indicated that serum Spexin levels were correlated with age (Spearman r = −0.277, P = 0.022), BMI (Spearman r = −0.45, P < 0.001), fasting glucose (Spearman r = −0.302, P = 0.014) and TG (Spearman r = −0.324, P = 0.008). Spexin levels could predict the risk of high BMI, independently of other factors. The authors observed no interaction effects of Spexin and age on BMI or FBG [30]. They concluded that Spexin serum concentrations independently predicted the risk of developing high FBG [30].
Karaca et al. in 2018 reported that Spexin levels were not associated with BMI in neither healthy subjects nor diabetic patients. and in this study Spexin level was significantly lower in patients with diabetes(type 1 and type 2) than in the control subjects(P = 0.008, and P = 0.041, respectively);but Spexin level was not correlated with glycemic parameters (fasting plasma glucose, postprandial plasma glucose, Insulin and HbA1c), though (P > 0.05) [11]. A similar study reported the same results (Spexin level in subjects with diabetes:2.04 ± 0.70 ng/mL versus the control group:3.65 ± 0.73 ng/mL, P < 0.001); however, a significant negative correlation was reported between Spexin and fasting glucose level(r = −0.686, P < 0.001), HbA1C(r = −0.632, P < 0.001), TG (r = −0.236, P < 0.001), and LDL-C(r = −0.382, P < 0.001) in this study[14]I[32]n another cross-sectional study, Spexin was not significantly correlated with body composition, fitness, or blood biomarkers [16]. Al-Daghri et al. in 2018 reported that Spexin concentrations were significantly lower in participants with MetS in comparison with non-MetS (0.18 ng/ml (0.13–0.24) vs. 0.26 ng/ml (0.17–0.50); P < 0.001). Stratification based on sex showed that Spexin was associated with MetS only in females, the significance of which was lost after adjusting for age and BMI [12].
In another study, Al-Daghri, found no significant difference in the Spexin levels between pregnant women with and without GDM. In all the study participants, Spexin had modest associations with glucose and HOMA-IR. Spexin concentration elicited significant positive associations with BMI in non-GDM pregnant women(R = 0.40; P < 0.01) [19]. Based on the results of another study led by the same authors, Spexin circulating levels significantly augmented in GDM patients after six months, with a parallel borderline significant rise in glucose levels (P = 0.07);changes in glucose from baseline to 6-months was positively associated with changes in Spexin in GDM patients only (R = 0.37; p < 0.05) [31].
Only two animal studies have directly addressed the role of spexin in weight loss; Walewsk et al. reported that Spexin (35 mg/kg/day SC) decreased caloric intake by 32% with consistent weight loss in rats [7].In another research, Spexin treatment (25 μg/kg/day)caused weight loss in mice with Diet-Induced Obesity(DIO) [15].
Among the included animal studies, five reported glucose homeostasis-related biomarkers (FBG, glucose tolerance test, insulin resistance and HbAlc). Sassek reported in 2018 that insulin secretion from both cultured cells and isolated islets was decreased by Spexin injection at the glucose level of 16 mM. In obese rats, insulin secretion was diminished after Spexin injection. Spexin treatment consequently led to increased viability and proliferation of both cultured and pancreatic islets cell, and also higher levels of proliferating cell nuclear antigen protein. On the other hand, a reduction in insulin and gene expression of Pdx (a gene that encodes insulin promoter factor 1), were revealed [33].
In another study, the same group reported that Spexin was present inside beta cells, and described three main outcomes:1)in response to glucose administration, Spexin release from the islets increased in short term(90 min incubation) but diminished in long term (24 h); 2)At 6 mM glucose levels, a remarkable decline in Spexin secretion was detected after 90 min of incubation with three varying concentrations of insulin(1, 10, and 100 nM);when the incubation was prolonged to 24 h, a statistically significant diminution was observed in Spexin secretion at insulin concentrations of 10 and 100 nM); 3) when 6 mM glucose was used in incubations, 100 and 1000 nM of Spexin resulted in a statistically significant reduction in insulin secretion; in Spexin-free medium, insulin concentrations were1.4- and 1.3- fold higher, respectively [32] .Similar to this study, Ge et al .reported that in mice fed a high fat diet for 30 days, Spexin treatment for four weeks led to improved/normalized oral glucose tolerance,insulin resistance (HOMA-IR)(P < 0.05), and HbA1C (P = 0.024) [15].
Ma et al. found that feeding goldfish could increase plasma levels of glucose, insulin and Spexin as well as hepatic mRNA expression of insulin and Spexin. Similar elevation was observed in the liver and brain (which are involved in appetite control) of goldfish after intra-peritoneal injection of glucose and insulin, respectively. In experiments with goldfish hepatocytes and brain cell cultures, insulin signal induced by glucose exerted a dual role in regulation of Spexin:(1) an autocrine/paracrine signal to trigger hepatic mRNA expression of Spexin (2) an endocrine signal to up regulate the Spexin gene in the brain [26].In another study, spx1−/− mutant fish showed higher serum glucose than wild type [27].
Spexin and lipid metabolism
Lin et al .in 2018reported that serum Spexin was inversely correlated with total bile acid (TBA) (P = 0.0042), and total cholesterol(TC) levels (P = 0.0031). Explicitly, significant negative correlations were found between Spexin and circulating levels of glycocheno-deoxycholate (GCDCA) and tauro-chenodeoxycholate(TCDCA)in the 91 healthy volunteers [34].In another study by the same group, Spexin levels were reported to be correlated with TG in healthy adult women (Spearman r = 0.324, P = 0.008) [30].Al-Daghri et al. reported that serum Spexin level was associated with lipid profile (except for high-density lipoprotein(HDL)-cholesterol) in women [19]. In a study carried out on patients with MetS in 2018,low levels of Spexin showed an inverse relationship with high levels of triglycerides (1.8 mmol/l, P < 0 001).Lower Spexin levels were also associated with low HDL-cholesterol(1.0 ± 0.3 mmol/l, P < 0 001) in the MetS group [12].Gu et al. reported that serum Spexin levels were lower in T2DM, and had a significant correlation with circulating glucose and lipid levels [TG (r = −0.236, P < 0.001), LDL-C (r = −0.382, P < 0.001] [14].However, Karaca et al. failed to find any correlation between Spexin levels and lipids in diabetic patients [11].Hodges et al .reported that Spexin was not significantly correlated with lipid profile (TC, HDL-C, LDL-C, TG, non-esterified fatty acids(NEFA)), or total fat mass in adolescents [16].five animal studies have assessed lipid metabolism in association with Spexin [7, 15, 27, 35].Kolodziejski et al found that Spexin obstructs adipogenesis and reduces mRNA expression of pro-adipogenic genes. Spexin was also found to stimulate lipolysis by enhancing the phosphorylation of hormone sensitive-lipase (HSL). This neuropeptide hinders lipogenesis and glucose uptake in human adipocytes and murine3 T3-L1 cells [35].Zheng et al. reported that spx1−/− mutant fish demonstrated higher serum TG and cholesterol than the wild type fish [27].
Ge et al. reported that incubation with Spexin directly repressed long chain fatty acid (LCFA) uptake by70% in hepatocytes obtained from DIO mice with HS/NAFLD (Mouse Models of Fatty Liver Disease). Spexin treatment in vivo for four weeks diminished hepatic lipids by 60%, and decreased serum alanine and aspartate aminotransferases [15].In another report, Spexin incubation in vitro significantly prohibited facilitated fatty acid (FA) uptake by adipocytes of DIO mouse [7]. Lin et al. .reported that, spexin (300 μg/kg) could significantly reduce hepatic and circulating total bile acids (TBA) level compared with the controls and long-term treatment(28 days)by spexin injection at 25 μg/kg significantly slowed down body weight gain and decrease serum TBA levels In rats [34].
Spexin and inflammation
Al-Daghri et al .showed a positive association between Spexin levels and IL-1β (R = 0.41,P < 0.01), 25(OH)D (R = 0.22,P = 0.04),and leptin (R = -0.43,P < 0.01).The association between spexin and TNF-α,IL-6,and adiponectin were not statistically significant. Stepwise regression indicated that IL-1β and leptin were the best predictors of the neuropeptide Spexin [19]. Kumar et al.(2018)showed that the odds of having low spexin and high leptin were 12.25 times higher in subjects with higher hs-CRP (≥3 mg/L) compared to those with hs-CRP < 3 mg/L..However, the authors did not find any significant correlation between Spexin levels and any of the adipokines and CRP [9].
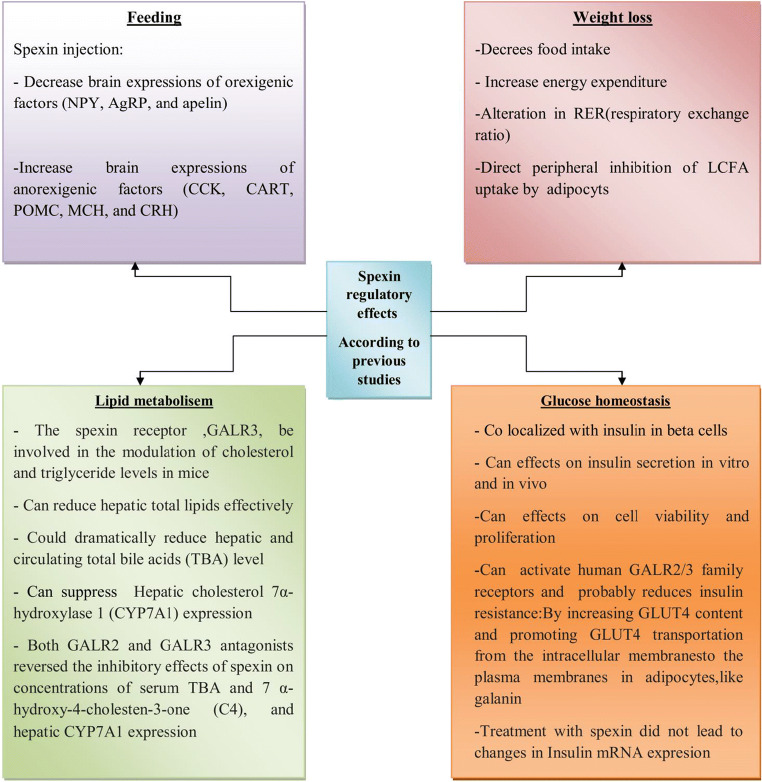
In another study Spexin was not significantly correlated with CRP in adolescents [16].One human study found positive correlations between Spexin and serum levels of adiponectin [8]. In another study conducted among 12 girls with severe obesity six months after a Roux-en-Y gastric bypass (RYGB) surgery, Spexin level correlated positively with high molecular weight adiponectin (Spearman correlation r = 0.691, P = 0.011) [29].By contrast, the study by Kumar et al .in 2016 reported no significant correlation between Spexin levels and leptin or total adiponectin [6].A recent research showed that Spexin levels were significantly associated with leptin (R = -0.43,P < 0.01)in pregnant women; this association was not significant for adiponectin, though [19].Karaca et al. showed that Spexin levels were not correlated with cortisol levels (P > 0.05) [11].Figure 2 presentsthe Spexin regulatory effects on overall metabolic status and feeding related-behaviors in in vivo/in vitro studies, animal models or human subjects with/without conditions, including obesity, diabetes and MetS.
Fig. 2.
Spexin regulatory effects on overall metabolic status and feeding related behaviorsin in vivo/in vitro studies and animal models or human subjects with/without tconditions, including obesity, diabetes and metabolic syndrome
Discussion
To explore the potential effects of Spexin on obesity and related comorbidities, this systematic review was performed. Spexin is a novel neuropeptide chiefly produced in human white adipose tissue, and is now implicated as a potential signal which might affect regulation of body weight, energy and glucose homeostasis, satiety, lipid metabolism and immune responses. Several human studies have reported that obese patients have lower Spexin level compared to non-obese individuals [6, 8, 9].On the other hand, some others have found no significant correlations [11, 16].Microarray studies on fat biopsies surgically isolated, revealed that Spexin gene is the most down-regulated gene in human fat of the obese in comparison with that of non-obese subjects [6].
Spexin has been found to facilitate weight loss in animal models [7].
Several mechanisms have been proposed in this regard; diminished food intake, augmented energy expenditure, changes in the respiratory exchange ratio (promoted burning of fat in preference to carbohydrate), and regulation of adipose tissue function through directly inhibiting LCFA uptake by the adipocytes, are some of these mechanisms [7].Studies suggest that regulation of LCFA uptake by the adipocytes is an important control point for adiposity [36].Administration of Spexin inhibited the uptake of LCFA into adipocytes in one animal study, and the authors speculated that the absence of Spexin might be a key element of the hormonal dysregulation observed in obese fat, and that repletion of the serum Spexin may help re-establish normal feeding behaviors and energy balance in obese animals and man [7]. Actually,feeding behavior is regulated by particular areas in the brain, so-called feeding centers [37].
In mammals, the feeding centers seem to be limited to the hypothalamus, and are under the control of hormones secreted by the brain and the periphery. Neuro-hormones produced by the brain, specifically the hypothalamic area, regulate energy balance by preventing (through anorexigenic factors) or motivating (through orexigenic factors) feeding. Circulating biochemical (e.g. glucose) or endocrine (e.g.GI hormones) factors pass into the brain through the blood brain barrier (BBB), and have a direct action on feeding centers. Peripheral sensory information (mechanical or endocrine) carried by the vagus nerve can also influence the feeding centers, by innervation from the brainstem [37].
Reports on the role of Spexin in glycemic parameters are controversial. Some studies found no significant correlation between Spexin levels and glycemic parameters [6, 11, 16],but others reported significant results [8, 14, 29, 31]. The heterogeneous findings can be due to different study designs, different characteristics of the participants including age, gender and health conditions, as well as difference in quality of the studies. In total, studies which reported significant results had higher quality than others (of the four studies with positive results, two had high quality) [8, 31].
The endocrine part of the pancreas which produces hormones, plays a vital role in regulating metabolic homeostasis. Co-localization of Spexin and insulin in β cells has been established in humans and pigs, but not in rats [4, 14, 32].This means that Spexin could possibly influence pancreas function. Sassek et al. in their study observed that Spexin may be strictly involved in the pathogenesis of DM or its recovery; this is attributed to the probable effects of Spexin on insulin release as well as cell viability and proliferation, observed in in-vitro and in- vivo studies [33].In one study no changes in insulin mRNA expression were observed in pancreatic islet cells after Spexin treatment. This suggests that Spexin affects the secretion, and not the expression of insulin [32].Spexin can activate the human GALR2/3 receptors, which suggests that Spexin is a natural ligand for galanin 2/3. Extensive distribution of spexin, GALR2 and GALR3 in various tissues from central nervous system to peripheral tissues indicates that spexin might have multiple biological functions [38]. A positive association between galanin and T2DM/ MetS has been previously described [39–42]. Endogenous galanin decreases insulin resistance by enhancing glucose transporter type 4 (GLUT4) content, and increasingGLUT4 transportation from the intracellular membranes to the plasma membranes in adipocytes [43]. These results suggest that Spexin may act in the same manner as galanin.
The role of Spexin in lipid metabolism has been reported in several human studies. Some studies have reported a significant negative correlation [12, 14, 19, 30, 34], while the others have not found significant correlations [11, 16]. Differences in the health status of the participants, age ranges and number of the study participants can be the reason of heterogeneous findings. The quality of studies with significant results and non-significant results was similar (moderate to good).The Spexin receptor, GALR3, has been proposed to be involved in modulating cholesterol and triglyceride levels in mouse [44].Moreover, Spexin has been found to efficiently reduce total lipids in the liver [15], suggesting a key role for it in lipid metabolism in mammals. Lin et al. highlighted the role of Spexin in bile acid synthesis in rats. He reported that Spexin could significantly diminish hepatic and circulating TBA levels in comparison with the controls. The gene encoding cholesterol 7α-hydroxylase 1 (CYP7A1) in the liver, was down-regulated by Spexin injection. Both GALR2 and GALR3 antagonists inverted the inhibitory effects of Spexin on serum concentrations of TBA and 7 α-hydroxy-4-cholesten-3-one (C4), as well as hepatic CYP7A1 expression [34].
In another study, Spexin substantially depleted hepatic TG both biochemically and histologicaly, in a mouse model with hepatic steatosis [15].Kolodziejski et al .reported that Spexin impedes adipogenesis and down-regulates pro-adipogenic genes such as PPAR-γ, C/EBPα, C/EBPβ, and FABP4. Spexin induces lipolysis by promoting the phosphorylation of HSL. Spexin has also been shown to inhibit lipogenesis in human adipocytes and murine 3 T3-L1 cells. These results suggest that this neuropeptide regulates fat tissue metabolism in murine 3 T3-L1 adipocytes and human adipocytes [35].
The association between Spexin and some inflammatory biomarkers has been assessed in some studies. Spexin levels were significantly and positively correlated with IL-1β [19]. The significant association between Spexin and IL-1β is significant, since IL-1β is known for its role in autoimmune and inflammatory conditions [45].However, this significant association might merely be an indirect one, due to the inverse association between Spexin and leptin [8, 9, 19], which is a more established cytokine well-known to moderate both the adaptive immune system, and the inflammatory responses as well as development of particular autoimmune disorders [46, 47]. It is generally believed that leptin can directly link nutritional status to pro-inflammatory T helper 1 (Th1) immune responses; leptin has been implicated in the pathogenesis of chronic inflammation, and its elevated serum levels in obesity seems to contribute to the low-grade inflammatory background. It is noteworthy that inflammation makes obese individuals more vulnerable to cardiovascular diseases, T2DM, or degenerative diseases such as autoimmunity and cancer [48].On the other hand, results on the association between Spexin and other biomarkers including adiponectin and hs-CRP are controversial. Some authors have reported significant correlations [8, 9, 29], while the others have not [6, 16, 19]. Decreased plasma and expression levels of adiponectin, and elevated plasma level of hs-CRP may serve as biomarkers of increased metabolic and inflammatory risk [49].Establishing the association of Spexin with inflammatory-related factors requires further studies to assess the role of Spexin in modulation of immune system. The exact mechanisms through which Spexin exerts its effects are still unclear, because there is little information on the Spexin receptors [50].
The results of the current systematic review should be interpreted in light of few limitations. as a wide range of study designs and different study populations were used in the reviewed studies and and limitation in transparency of reviewed studies, it was not possible to apply statistical methods such as meta-analysis to test association between the Spexin and obesity and its related comorbidities.
Conclusions and future directions
Despite the limitations and/or inconsistencies in the current findings, Spexin appears to have a positive impact on overall metabolic status and feeding-related behaviors in human, animals and in vitro. Evidence on the role of Spexin in satiety and metabolism are accumulating, and these suggest multiple physiological functions of the neuropeptide due to its expression in various tissues. e. Once its safety established by further animal studies, clinical trials are warranted to assess the potential therapeutic effects of Spexin. Well-designed cell line and animal studies are also encouraged to shed more light on the probable mechanisms of action.
Electronic supplementary material
(DOCX 34 kb)
(DOC 64 kb)
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contribution
Conceived and designed the systematic review: MB,AO.
Systematic literature search: ZF, MB.
Screened the titles and abstracts of the retrieved articles: VM,SP,EV.
Quality assessment and data extraction: MB,VM,AO.
Wrote the paper: All of authors.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, Birney E, Rosenthal N, Gross C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007;17(3):320–327. doi: 10.1101/gr.5755407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan B, Wang XR, Zhou YB, Zhang X, Huo K, Han ZG. C12ORF39, a novel secreted protein with a typical amidation processing signal. Biosci Rep. 2009;30(1):1–10. doi: 10.1042/BSR20080156. [DOI] [PubMed] [Google Scholar]
- 3.Wong MK, et al. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am J Physiol Endocrinol Metab. 2013;305(3):E348–E366. doi: 10.1152/ajpendo.00141.2013. [DOI] [PubMed] [Google Scholar]
- 4.Porzionato A, Rucinski M, Macchi V, Stecco C, Malendowicz LK, de Caro R. Spexin expression in normal rat tissues. J Histochem Cytochem. 2010;58(9):825–837. doi: 10.1369/jhc.2010.956300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma A, Bai J, He M, Wong AOL. Spexin as a neuroendocrine signal with emerging functions. Gen Comp Endocrinol. 2018;265:90–96. doi: 10.1016/j.ygcen.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Hossain J, Nader N, Aguirre R, Sriram S, Balagopal PB. Decreased circulating levels of Spexin in obese children. J Clin Endocrinol Metab. 2016;101(7):2931–2936. doi: 10.1210/jc.2016-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walewski JL, Ge F, Lobdell H, IV, Levin N, Schwartz GJ, Vasselli JR, Pomp A, Dakin G, Berk PD. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring) 2014;22(7):1643–1652. doi: 10.1002/oby.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolodziejski PA, et al. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res. 2018;67(1):45–56. doi: 10.33549/physiolres.933467. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Hossain MJ, Javed A, Kullo IJ, Balagopal PB. Relationship of circulating spexin with markers of cardiovascular disease: a pilot study in adolescents with obesity. Pediatr Obes. 2018;13(6):374–380. doi: 10.1111/ijpo.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Liu Q, Xiao L, Chen H, Li G, Zhang Y, Lin H. Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus coioides) Comp Biochem Physiol B Biochem Mol Biol. 2016;196-197:85–91. doi: 10.1016/j.cbpb.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Karaca A, Bakar-Ates F, Ersoz Gulcelik N. Decreased Spexin levels in patients with type 1 and type 2 diabetes. Med Princ Pract. 2018;27:549–554. doi: 10.1159/000493482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Daghri NM, et al. Spexin levels are associated with metabolic syndrome components. Dis Markers. 2018;2018:1679690. doi: 10.1155/2018/1679690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol (N Y) 2017;2(7):e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu L, Ma Y, Gu M, Zhang Y, Yan S, Li N, Wang Y, Ding X, Yin J, Fan N, Peng Y. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides. 2015;71:232–239. doi: 10.1016/j.peptides.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Ge JF, et al. Regulation of hepatocellular fatty acid uptake in mouse models of fatty liver disease with and without functional Leptin signaling: roles of NfKB and SREBP-1C and the effects of Spexin. Semin Liver Dis. 2016;36(4):360–372. doi: 10.1055/s-0036-1597248. [DOI] [PubMed] [Google Scholar]
- 16.Hodges SK, Teague AM, Dasari PS, Short KR. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatr Diabetes. 2018;19(2):212–216. doi: 10.1111/pedi.12549. [DOI] [PubMed] [Google Scholar]
- 17.Canas JA, Sweeten S, Balagopal PB. Biomarkers for cardiovascular risk in children. Curr Opin Cardiol. 2013;28(2):103–114. doi: 10.1097/HCO.0b013e32835dd0ce. [DOI] [PubMed] [Google Scholar]
- 18.Zieske AW, Tracy RP, McMahan CA, Herderick EE, Homma S, Malcom GT, McGill HC, Jr, Strong JP. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25(6):1237–1243. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 19.Al-Daghri NM, et al. Associations of Spexin and cardiometabolic parameters among women with and without gestational diabetes mellitus. Saudi J Biol Sci. 2018;25(4):710–714. doi: 10.1016/j.sjbs.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, et al.. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2009. Epub Available from: URL: http://www.ohri. ca/programs/clinical_epidemiology/oxford. htm [cited 2009 Oct 19], 2016.
- 23.Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng SP, Chen HP, Zhai Y, Jia LY, Liu JY, Wang M, Jiang DN, Wu TL, Zhu CH, Li GL. Molecular cloning, characterization and expression analysis of spexin in spotted scat (Scatophagus argus) Gen Comp Endocrinol. 2018;266:60–66. doi: 10.1016/j.ygcen.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Wang B, Chen S. Spexin in the half-smooth tongue sole (Cynoglossus semilaevis): molecular cloning, expression profiles, and physiological effects. Fish Physiol Biochem. 2018;44(3):829–839. doi: 10.1007/s10695-018-0472-6. [DOI] [PubMed] [Google Scholar]
- 26.Ma A, He M, Bai J, Wong MK, Ko WK, Wong AO. Dual role of insulin in Spexin regulation: functional link between food intake and Spexin expression in a fish model. Endocrinology. 2017;158(3):560–577. doi: 10.1210/en.2016-1534. [DOI] [PubMed] [Google Scholar]
- 27.Zheng B, Li S, Liu Y, Li Y, Chen H, Tang H, Liu X, Lin H, Zhang Y, Cheng CHK. Spexin suppress food intake in Zebrafish: evidence from gene knockout study. Sci Rep. 2017;7(1):14643. doi: 10.1038/s41598-017-15138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Lin F, Chen H, Liu J, Gao Y, Zhang X, Hao J, Chen D, Yuan D, Wang T, Li Z. Ya-fish (Schizothorax prenanti) spexin: identification, tissue distribution and mRNA expression responses to periprandial and fasting. Fish Physiol Biochem. 2016;42(1):39–49. doi: 10.1007/s10695-015-0115-0. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Hossain MDJ, Inge T, Balagopal PB. Roux-en-Y gastric bypass surgery in youth with severe obesity: 1-year longitudinal changes in spexin. Surg Obes Relat Dis. 2018;14(10):1537–1543. doi: 10.1016/j.soard.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Lin CY, Huang T, Zhao L, Zhong LLD, Lam WC, Fan BM, Bian ZX. Circulating Spexin levels negatively correlate with age, BMI, fasting glucose, and triglycerides in healthy adult women. J Endocr Soc. 2018;2(5):409–419. doi: 10.1210/js.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Daghri NM, et al. Circulating spexin levels are influenced by the presence or absence of gestational diabetes. Cytokine. 2019;113:291–295. doi: 10.1016/j.cyto.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Sassek M, Kolodziejski PA, Szczepankiewicz D, Pruszynska-Oszmalek E. Spexin in the physiology of pancreatic islets-mutual interactions with insulin. Endocrine. 2019;63(3):513–519. doi: 10.1007/s12020-018-1766-2. [DOI] [PubMed] [Google Scholar]
- 33.Sassek M, Kolodziejski PA, Strowski MZ, Nogowski L, Nowak KW, Mackowiak P. Spexin modulates functions of rat endocrine pancreatic cells. Pancreas. 2018;47(7):904–909. doi: 10.1097/MPA.0000000000001083. [DOI] [PubMed] [Google Scholar]
- 34.Lin CY, Zhao L, Huang T, Lu L, Khan M, Liu J, Zhong LLD, Cai ZW, Fan BM, Wong AOL, Bian ZX. Spexin acts as novel regulator for bile acid synthesis. Front Physiol. 2018;9:378. doi: 10.3389/fphys.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolodziejski PA, Pruszynska-Oszmalek E, Micker M, Skrzypski M, Wojciechowicz T, Szwarckopf P, Skieresz-Szewczyk K, Nowak KW, Strowski MZ. Spexin: a novel regulator of adipogenesis and fat tissue metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(10):1228–1236. doi: 10.1016/j.bbalip.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Petrescu O, Fan X, Gentileschi P, Hossain S, Bradbury M, Gagner M, Berk PD. Long-chain fatty acid uptake is upregulated in omental adipocytes from patients undergoing bariatric surgery for obesity. Int J Obes. 2005;29(2):196–203. doi: 10.1038/sj.ijo.0802868. [DOI] [PubMed] [Google Scholar]
- 37.Volkoff H. The neuroendocrine regulation of food intake in fish: a review of current knowledge. Front Neurosci. 2016;10:540. doi: 10.3389/fnins.2016.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters SM, Krause JE. Distribution of galanin-1, −2 and −3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95(1):265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- 39.Legakis I, Mantzouridis T, Mountokalakis T. Positive correlation of galanin with glucose in type 2 diabetes. Diabetes Care. 2005;28(3):759–760. doi: 10.2337/diacare.28.3.759. [DOI] [PubMed] [Google Scholar]
- 40.Fang P, Yu M, Shi M, Zhang Z, Sui Y, Guo L, Bo P. Galanin peptide family as a modulating target for contribution to metabolic syndrome. Gen Comp Endocrinol. 2012;179(1):115–120. doi: 10.1016/j.ygcen.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Fang P, Yu M, Guo L, Bo P, Zhang Z, Shi M. Galanin and its receptors: a novel strategy for appetite control and obesity therapy. Peptides. 2012;36(2):331–339. doi: 10.1016/j.peptides.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Fang P, Min W, Sun Y, Guo L, Shi M, Bo P, Zhang Z. The potential antidepressant and antidiabetic effects of galanin system. Pharmacol Biochem Behav. 2014;120:82–87. doi: 10.1016/j.pbb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Shi M, Zhang L, Li G, Zhang L, Shao H, Fang P, Ma Y, Li J, Shi Q, Sui Y. Galanin antagonist increases insulin resistance by reducing glucose transporter 4 effect in adipocytes of rats. Gen Comp Endocrinol. 2011;173(1):159–163. doi: 10.1016/j.ygcen.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Brunner SM, Farzi A, Locker F, Holub BS, Drexel M, Reichmann F, Lang AA, Mayr JA, Vilches JJ, Navarro X, Lang R, Sperk G, Holzer P, Kofler B. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc Natl Acad Sci U S A. 2014;111(19):7138–7143. doi: 10.1073/pnas.1318066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X. Regulatory functions of innate-like B cells. Cell Mol Immunol. 2013;10(2):113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cojocaru M, Cojocaru IM, Siloşi I, Rogoz S. Role of leptin in autoimmune diseases. Maedica (Buchar) 2013;8(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- 47.Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism. 2015;64(1):92–104. doi: 10.1016/j.metabol.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Iikuni N, Kwan Lam Q, Lu L, Matarese G, Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Möhlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52(4):942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 50.Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, Kim K, Vaudry H, Seong JY. Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology. 2014;155(5):1864–1873. doi: 10.1210/en.2013-2106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 34 kb)
(DOC 64 kb)