Abstract
Purpose
The management of diabetes comprises diet, pharmacological therapy, lifestyle counseling, patient education, and physical exercising, to achieve change in health behavior and control of the disease. However, a large proportion of diabetes patients does not adhere to treatment recommendations, mainly in the lifestyle aspect, which remains sedentary. Considering that self-efficacy is an essential determinant of health behaviors such as exercise practicing, the objective of the study was to investigate the psychometric properties of the Brazilian Portuguese version of Bandura’s Exercise Self-Efficacy Scale (BESES) to be used in diabetes patients.
Methods
The BESES was initially completed by thirty diabetes patients to confirm the feasibility of the answers be provided by themselves. The psychometric properties (i.e., internal consistency, test-retest reproducibility, convergent validity, and ceiling and floor effects) were tested in other two-hundred diabetes patients (≥18 years old).
Results
The BESES achieved significant internal consistency (Cronbach’s alpha coefficient = 0.92), substantial test-retest reproducibility (intraclass correlation coefficient = 0.83). The convergent validity was confirmed by negative correlations between BESES total scores and barriers to exercise total scores (ρ = −0.333; P = 0.018) and rate of perception exercise corrected by distance covered in the incremental shuttle walking test (ρ = −0.426; P = 0.002). Ceiling and floor effects were not found. In addition, physically active patients had BESES total scores higher compared to sedentary (56.8 ± 21.4 vs. 47.9 ± 20.0; P = 0.003).
Conclusion
The Brazilian Portuguese version of the BESES showed adequate psychometric properties and proved to be valid for assessing the exercise self-efficacy in diabetes patients in Brazil.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00581-6) contains supplementary material, which is available to authorized users.
Keywords: Brazil, Diabetes, Exercise, Psychometric properties, Self-efficacy
Introduction
Diabetes is considered an isolated risk factor for the development of cardiovascular diseases in adults [1], which are among the leading causes of death in the world [2]. According to the International Diabetes Federation, the world is awakening to the diabetes epidemic, and the increase in people living with this disease worldwide has devastating health, social and financial consequences and imposes a high human, social and economic cost on countries at all income levels [3]. Currently, Brazil is in the fifth position among the ten countries in the world, with the most significant number of people living with diabetes (16.8 million), with an expectation of 26.0 million in 2045, considering the age group of 20 to 79 years [3]. According to the Brazilian health care management, the diabetes prevalence increased in the past decade, with the percentage of adults living with diabetes rising from 5.5% to 7.7%. [4].
Since physical exercise is a core component of diabetes care, the diabetes guidelines recommend accumulating at least 150 min per week of moderate to vigorous-intensity aerobic training besides resistance exercises of moderate to the vigorous-intensity two-three times per week to contribute to disease control [1, 2, 5–7]. Despite this, a large proportion of diabetes patients maintains a sedentary or poorly active lifestyle [8, 9] since there are several barriers to adhere to healthy lifestyles [10] such as living in places with poor conditions and opportunities for physical activity [11] and lack of time and motivation [12]. In such a manner, behavioral intentions are essentials in the process of changing unhealthy habits and consequently help disease control and treatment [6, 13].
In this context, self-efficacy has been considered a powerful determinant of behavioral intentions, including health behaviors such as physical exercising, and its concept refers to an individual’s beliefs in his or her ability to perform a specific task [14]. Exercise self-efficacy is defined as a belief in the ability to keep practicing physical exercises even in the face of barriers that may arise and has been shown to play an essential role in starting and maintaining physical exercise [15, 16]. Studies have shown that exercise self-efficacy is a critical predictor of the physical exercise adoption and maintenance in diabetes patients, and it should be part of the clinical and research outcomes to support the planning of therapeutic approach strategies [17, 18].
The exercise self-efficacy can be assessed through specific scales that emerged to evaluate this concept [19–21]. Among them, the Bandura’s Exercise Self-Efficacy Scale (BESES) is the most popular worldwide. It has been widely used in several countries after the process of translation, cross-cultural adaptation and evaluation of its psychometric properties, which has proved to be a valid instrument for assessing confidence in the ability to exercise regularly under different circumstances in distinct populations [22–26]. The original version of BESES was translated to Brazilian Portuguese and validated in individuals with cardiovascular risk, mostly hypertension and obesity [27]. However, the validity of the Brazilian Portuguese version of BESES was not tested in diabetes patients up until now. The objective of the study was to test the psychometric properties of the Brazilian Portuguese version of BESES in people living with diabetes.
Methods
Participants
This study was developed in two steps. In the first one, thirty diabetes patients selected by convenience were invited to participate in a pre-test [28]. In the second step, two-hundred adults with diabetes were enrolled in the study to test the psychometric properties. The sample size calculation for the second step was based on Hair and Anderson recommendation of a minimum sample size of 10 participants per item of the instrument, and/or at least 100 participants [29] also the recommendation of a minimal sample size of 50 participants to test-retest reproducibility analysis [28]. These patients were recruited from health services in a Brazilian city according to the following eligibility criteria: diagnosis of type 1 or type 2 diabetes, ≥ 18 years old, no participation in the pre-test described previously, and able to physically exercise. Before signing the consent form, all patients were asked to answer the six-item screener test (SIS) [30] to screen cognitive status, and only potential participants who achieved score ≥ 4 were invited to sign the consent form and were included in the study. Participants who did not complete all procedures of the study protocol according to the subsample to which it was randomized were excluded.
This study was approved by the Research Ethics Committee of the Hospital of University Federal of Juiz de Fora (CAAE 77831517.0.2002.5133), and it is part of a multi-center randomized controlled trial that will investigate the effects of an exercise and lifestyle education program in adults living with diabetes in Brazil (ClinicalTrials.gov ID NCT03914924).
Procedures
Firstly, the Brazilian Portuguese BESES pre-test was performed using a Likert Scale ranging from 1 (I do not understand completely) to 5 (I understand completely) [31] for each item to confirm the feasibility in obtaining self-reported answers to them.
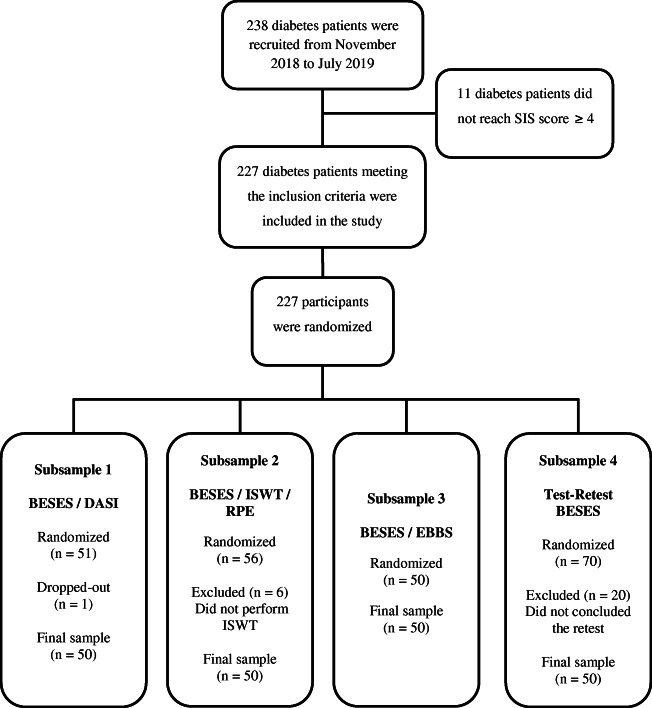
In the sequence, other diabetes patients were recruited to psychometric properties analysis of the Brazilian Portuguese BESES. Before they are enrolled in the study, all patients recruited underwent cognitive status assessment using the SIS to test the validity of the responses to be obtained in the instruments [32]. All participants answered a form to report clinical and sociodemographic and information and information about practicing physical exercise. After that, they were randomized for one of the four subsamples, as described in Fig. 1. The generation of the randomized allocation sequence was performed by the principal investigator (PI) using an online tool, and the allocation sequence was retained a password-protected file. The randomization information was provided from the PI to the assessor only when the assessments were about to be started to ensure allocation concealment.
Fig. 1.
Flow chart of participants’ recruitment, inclusion, and randomization. SIS, Six-Item Screener; DASI, Duke Activity Status Index; BESES, Bandura’s Exercise Self-Efficacy Scale; ISWT, incremental shuttle walking test; RPE, rated perceived exertion
Measurements
The Brazilian BESES was self-administered, and the time for completion was recorded, with neutral monitoring by the assessor who explained in a standardized way, the objective of the assessment to measure the exercise self-efficacy. This instrument (electronic supplementary material) has eighteen items related to situations that may compromise the adherence to regular physical exercising, e. g. when feeling tired or during the holidays. The respondent should indicate from 0 (no confident) to 100 (very confident) how much confidence he or she has about maintaining the physical exercise routine face of the situation described in that item. The total score is the average from the eighteen items scores, and higher total scores indicate great exercise self-efficacy. Besides, the psychometric properties of convergent validity and test-retest reproducibility of the Brazilian Portuguese BESES based in the randomized subsample was performed.
Subsample 1
The participants randomized to subsample 1 responded to the Brazilian version of the Duke Activity Status Index (DASI) questionnaire to assess the perceived functional capacity [33]. DASI is a 12-item instrument to evaluate the respondent’s daily activities such as personal care, walking, household chores, sexual function, and recreation, which presents for each activity a value corresponding to metabolic rate. The total score ranges from zero to 58.2, and higher total scores indicate great perceived functional capacity [34]. This questionnaire was applied as an interview by an experienced assessor, and the DASI total score was considered for convergent validity assessment of Brazilian Portuguese BESES.
Subsample 2
The participants randomized to subsample 2 were submitted to the incremental shuttle walking test (ISWT) to measure the functional capacity by the distance covered in meters [35, 36]. The following variables were monitored: blood pressure, heart rate, glycemia pre- and post-test, and rated perceived exertion using the Modified Borg Scale [37]. The ISWT distance and the rate of perception exercise (RPE) corrected by ISWT distance were considered for convergent validity assessment of Brazilian Portuguese BESES.
Subsample 3
The participants randomized to subsample 3 responded to the Exercise Benefits / Barriers Scale (EBBS) questionnaire to assess the perceptions of the benefits and barriers to regular physical activity [38, 39]. This questionnaire is composed of exercise barriers and benefits scale used to identify both the perception of the barriers to becoming being physically active and the exercise-related benefits. The two scales from EBBS can be applied jointly or separately. In this study, we chose to evaluate independently the items related to exercise barriers scale, which has 14 items with the total score ranging from 14 to 56 points, and higher total scores indicate raised exercise barriers [39]. This questionnaire was applied as an interview by an experienced assessor, and the exercise barriers total scores were considered for convergent validity assessment of Brazilian Portuguese BESES.
Subsample 4
The participants randomized to subsample 4 responded to the Brazilian Portuguese BESES in an interval between seven and twenty-one days after the first response to assess the test-retest reliability of this instrument [40]. The participants completed the instrument likewise previously described and in the same place as the first application.
Statistical analysis
The IBM Statistical Package for Social Sciences (SPSS) version 22.0 software was used to store and data analysis. The normal distribution of data was verified using the Shapiro-Wilk test. Variables with normal distribution were expressed as mean and standard deviation while ones with non-normal distribution were expressed as median and interquartile range. The subsamples data were compared by ANOVA one-way or Kruskal-Wallis test when numerical variables were tested and by Chi-Square Test when categorical variables were tested. Data from physically active and sedentary participants were compared using the unpaired t-test. A significance level of 5% was adopted for all statistical tests. The internal consistency was assessed by Cronbach’s α coefficient calculation and considered as significant α > 0.70. The reliability was assessed by intraclass correlation coefficient (ICC) calculation, considering the coefficient < 0.40 as low reliability, from 0.40 to 0.75 as moderate, from 0.75 to 0.90 as substantial and > 0.90 as excellent [28, 29]. Convergent validity was assessed by the calculation of Spearman’s correlation coefficient between BESES total scores and (1) DASI total scores, (2) exercise barriers total scores, (3) ISWT distance (meters), (4) rate of perception exercise corrected by ISWT distance, considering the coefficient < 0.19 as very weak, from 0.20 to 0.39 as weak, from 0.40 to 0.59 as moderate, from 0.60 to 0.79 as strong and > 0.80 as fairly strong [28, 29]. The presence of ceiling and floor effects in the scores of the 18 items of the Brazilian Portuguese BESES was investigated from the analysis of the proportion of occurrence of the highest and lowest scores achieved expressed in a percentage being considered acceptable when values were below 15% [40, 41].
Results
Participants
Two hundred thirty-eight diabetes patients were approached, of which 227 met the inclusion criteria and were enrolled in the study. Of these, two-hundred concluded the study, as described in Fig. 1. One participant dropped-out from subsample 1 after randomization for personal reasons, six participants were excluded from subsample 2 for not performing the ISWT, and twenty participants were excluded from subsample 4 for not attending the retest (Fig. 1). One hundred and twelve female and an eighty-eight male mean age of 56.9 ± 14.3 years composed the full sample of the study. Sociodemographic and clinical characteristics of participants, as well as their comparison between the four subsamples, are presented in Table 1. The sociodemographic and clinical characteristics were no significant differences between the subsamples. On the other hand, physically active participants had BESES total scores higher compared to the sedentary ones with no significant difference in the BESES total scores between the subsamples (Table 2).
Table 1.
Sociodemographic and clinical data
| Variables | Full sample | Subsample 1 | Subsample 2 | Subsample 3 | Subsample 4 | P a |
|---|---|---|---|---|---|---|
| Sex - Female/Male (%) | 56.0/44.0 | 44.0/56.0 | 58.0/42.0 | 60.0/40.0 | 62.0/38.0 | 0.255 |
| Age (years) |
58.5 (48.0–67.8) b |
54.4 ± 15.7 c | 56.9 ± 13.5 c | 58.6 ± 13.6 c | 57.4 ± 14.3 c | 0.517 |
| Education level (%) | 0.242 | |||||
| Illiterate | 1.5 | 2.0 | – | 4.0 | – | |
| Literate non-school | 1.0 | – | 2.0 | 2.0 | – | |
| Elementary school inconcluded | 32.2 | 24.0 | 28.0 | 38.0 | 38.8 | |
| Elementary school concluded | 7.5 | 4.0 | 4.0 | 10.0 | 12.2 | |
| High school inconcluded | 4.0 | 2.0 | 6.0 | 6.0 | 2.0 | |
| High school concluded | 27.6 | 36.0 | 38.0 | 22.0 | 14.3 | |
| Undergraduate unconcluded | 4.5 | 4.0 | 4.0 | 6.1 | ||
| Undergraduate concluded | 12.1 | 20.0 | 12.0 | 6.0 | 10.2 | |
| Postgraduate | 9.5 | 8.0 | 6.0 | 8.0 |
16.3 (n = 49) |
|
| Family income d (%) | 0.197 | |||||
| ≤ 1 | 11.6 | 4.0 | 14.0 | 10.0 | 18.4 | |
| > 1 up to 2 | 22.6 | 22.0 | 24.0 | 22.0 | 22.4 | |
| > 2 up to 3 | 26.1 | 32.0 | 22.0 | 26.0 | 24.5 | |
| > 3 up to 4 | 17.6 | 32.0 | 12.0 | 14.0 | 12.2 | |
| > 4 up to 6 | 8.5 | – | 10.0 | 12.0 | 12.2 | |
| > 6 up to 9 | 3.5 | 4.0 | 4.0 | 6.0 | – | |
| > 9 up to 12 | 4.0 | – | 8.0 | 4.0 | 4.1 | |
| > 12 up to 24 | 5.5 | 4.0 | 6.0 | 6.0 | 6.1 | |
| > 24 | 0.5 | 2.0 | – | – |
– (n = 49) |
|
| Diabetes type (%) | 0.142 | |||||
| Type 1 | 19.5 | 30.0 | 20.0 | 14.0 | 14.0 | |
| Type 2 | 80.5 | 70.0 | 80.0 | 86.0 | 86.0 | |
| Diabetes time (years) |
10.0 (5.0–19.0) b |
13.0 (5.8–20.0) b |
10.0 (4.0–19.5) b (n = 49) |
10.0 (5.8–16.0) b |
10.0 (4.0–19.0) b |
0.567 |
| Glycated hemoglobin (%) | 7.3 (6.3–8.5) b |
7.6 (6.4–8.5) b (n = 45) |
7.3 (6.3–8.1) b (n = 37) |
7.4 (6.7–8.5) b (n = 44) |
7.1 (6.0–8.9) b (n = 37) |
0.873 |
| Fasting glucose (mg/dL) |
122.0 (101.0–62.3)b |
132.0 (104.8–4.5)b (n = 46) |
122.0 (105.2–168.0)b (n = 43) |
120.5 (100.3–152.8)b (n = 48) |
120.0 (93.0–159.0)b (n = 37) |
0.839 |
| Body mass index (kg/m2) |
122.0 (101.0–62.3)b |
28.4 ± 5.9 c |
28.3 (25.6–32.4)b |
29.6 ± 5.1 c |
29.6 (26.0–35.1)b |
0.263 |
| (n = 46) | (n = 49) | (n = 47) | ||||
| Oral antidiabetics (%) | 80.5 | 68.0 | 84.0 | 86.0 | 84.0 | 0.081 |
| Insulin therapy (%) | 42.5 | 58.0 | 34.0 | 38.0 | 40.0 | 0.074 |
aP value from the comparison between subsamples; b Median (interquartile range); c Mean ± standard deviation; d Family income was reported as the number of Brazilian minimum salaries monthly paid in reais
Table 2.
BESES total scores and practicing physical exercise data
| Percentage of participants physically | BESES total score | BESES total score from participants physically active (n = 110) | BESES total score from participants not physically active (n = 90) | |
|---|---|---|---|---|
| Full Sample | 55.0 | 52.8 ± 21.2 a | 56.8 ± 21.4 a- | 47.9 ± 20.0 a- |
| Subsample 1 | 56.0 | 54.7 ± 2.8 a | – | – |
| Subsample 2 | 56.0 | 53.1 ± 3.0 a | – | – |
| Subsample 3 | 48.0 | 48.0 ± 3.1 a | – | – |
| Subsample 4 | 60.0 | 55.2 ± 3.1 a | – | – |
| P b | 0.674 | 0.313 | P c = 0.003* | – |
BESES, Bandura’s Exercise Self-Efficacy Scale; a Mean ± standard deviation; b P value from the comparison between subsamples; c P value from the comparison between participants physically active and not physically active or sedentary; *statistical significance from Unpaired t-test
Psychometric validation
The psychometric properties analysis of the Brazilian Portuguese BESES is summarized in Table 3. Considering that Cronbach’s α coefficient was higher than 0.70, and the ICC calculated was between 0.75 and 0.90, the instrument showed significant internal consistency and substantial reliability, respectively. The BESES total scores were negatively correlated with the exercise barriers total scores as well as with the rate of perception exercise corrected by distance covered in the ISWT with weak and moderate correlation, respectively. The BESES total scores were not correlated with the perceived functional capacity score, neither functional capacity measured by the distance covered in the ISWT (Table 3). Ceiling or floor effect was not found using a cutoff proportion of 15%. The minimum score of Brazilian Portuguese BESES was noticed only in 0.5% of the total sample, and no participant reached the maximum score. The instrument completion took from 0.88 to 14.83 min, with a median of 3.34 min and an interquartile range of 2.32 min.
Table 3.
Results of psychometric properties analysis of the Brazilian Portuguese BESES in diabetes patients
| Internal consistency | ||
| Cronbach’s alpha coefficient | 0.92 | |
| Test-retest reproducibility | ||
| Intraclass correlation coefficient | 0.83 | |
| Convergent validity | ||
| Spearman’s Correlation Coefficient | P | |
| BESES total scores and RPE/ISWT distance | - 0.426 | 0.002* |
| BESES total scores and exercise barriers total scores | - 0.333 | 0.018* |
| BESES total scores and DASI total scores | 0.105 | 0.469 |
| BESES total scores and the ISWT distance | 0.054 | 0.709 |
BESES, Bandura’s Exercise Self-Efficacy Scale; RPE, rated perceived exertion; DASI, Duke Activity Status Index; ISWT, incremental shuttle walking test; *statistical significance from Spearman’s correlation test
Discussion
This study aimed to evaluate the psychometric properties of the Brazilian Portuguese BESES in diabetes patients, and the results showed that this instrument has adequate measurement properties to assess confidence in the ability to pursue a physical exercise routine in people living with diabetes.
Cronbach’s alpha coefficient found in the present study (α = 0.92) indicated that the Brazilian Portuguese BESES presents adequate internal consistency [29]. It was similar to that reported by Everett et al. [18] (α = 0.95) in a study using the original English version of this scale in cardiac rehabilitation patients. In another study developed in Korea [22], the same instrument was validated in a sample of patients with chronic diseases, including diabetes, and it also achieved a Cronbach’s alpha coefficient similar (α = 0.94). Additionally, a study developed aiming the validation of the Iranian version of BESES in diabetes patients [24] found a Cronbach’s alpha coefficient identical to the present study (α = 0.92). Besides that, in the study of translation, cross-cultural adaptation, and validation of original BESES to Brazilian Portuguese in metabolic syndrome patients [27], the Cronbach’s alpha coefficient was 0.97. The high internal consistency indicates that all items from BESES adequately measure the exercise self-efficacy construct.
The result from the test-retest reproducibility analysis of the Brazilian Portuguese BESES was considered substantial (ICC = 0.83) [28], and it was a little higher than that from Shin et al. [22] who obtained an ICC = 0.77 [22]. This small difference can be related to the fact that the retest in the Shin et al. [22] study was performed in a sample of only 14 participants. In contrast, in the current study, the instrument was reapplied in 50 participants as recommended for test-retest reproducibility analysis [28]. Both studies reveal a substantial test-retest reproducibility of the instrument. So, it reinforces the good reliability of BESES in assessing the exercise self-efficacy in patients with diabetes and other chronic diseases, such as cardiovascular, respiratory, renal, gastrointestinal, and musculoskeletal disorders.
The association of BESES total scores with the measures used to assess the validity of this instrument was weak to moderate. However, the negative correlation between BESES total scores and exercise barriers total scores (ρ = − 0.333, P = 0.018) suggests that the more exercise barriers perceived by diabetes patients, the lower their confidence level in maintaining an active lifestyle. Similarly, the BESES total scores showed a significant and negative correlation with the rate of perception exercise corrected by distance covered in the walking test (ρ = −0.426; P = 0.002), suggesting that the higher the perceived effort during the exercise the lower their confidence level to perform physical exercising regularly. These findings support the understanding that people living with diabetes who are more symptomatic of effort and who refer more barriers to physical exercising have less confidence to maintain a regular exercise routine. Besides that, the result comparing BESES total scores between physically active and sedentary participants evidenced higher exercise self-efficacy in diabetes patients who maintain a regular exercising routine compared to those who do not. Additionally, as no floor or ceiling effect was detected in the instrument, this finding indicates that the Brazilian Portuguese BESES can discriminate differences in exercise self-efficacy among diabetes patients.
To our knowledge, there is not another exercise self-efficacy scale that has been validated in Brazil for this population that could have been used in the validation analysis of Brazilian Portuguese BESES in diabetes patients. A strong point in the study procedures was the random distribution of the subsamples.
On the other hand, the BESES total scores were not correlated to perceived and measured functional capacity. This finding corroborates the results from Boff [27], in which there was no correlation between cardiorespiratory fitness assessed by maximal oxygen uptake (VO2 max) and the BESES total scores when these two variables were analyzed before the inclusion of participants in a lifestyle counseling program for people with cardiovascular risks. However, these variables were significantly correlated after a three-month intervention period since positive correlation was observed between the variation in the BESES total scores and VO2 max, indicating that the Brazilian Portuguese BESES was responsive to the lifestyle counseling program. Also, higher scores of exercise self-efficacy at baseline implied higher values of VO2 max post-intervention, suggesting that from BESES total scores is possible to predict the improvement of cardiorespiratory fitness [27]. Indeed, BESES could be utilized as a screening tool to identify those with higher self-efficacy who are more likely to achieve physical activity benefits. Moreover, it could also be used to identify those who may benefit from adjunctive interventions to increase self-efficacy to attain more benefits of rehabilitation programs.
The cross-sectional design of the present study did not permit to evaluate the psychometric responsiveness property of the instrument, i.e., changes over time and after interventions, and it is considered a limitation. However, this study is an earlier component of a multi-center randomized controlled study that will investigate the effects of an exercise and lifestyle education program on adults living with diabetes in Brazil, from this clinical trials will be provided information on the responsiveness of Brazilian Portuguese BESES.
In conclusion, the Brazilian Portuguese version of BESES presented adequate psychometric properties in assessing the confidence to pursue a physical exercise routine in diabetes patients. BESES may be used to screen Brazilians with diabetes for the achievement of the best benefits from more comprehensive physical activity programs.
Electronic supplementary material
(DOCX 32 kb)
Acknowledgments
The authors acknowledge the contribution of researcher Raquel Boff for providing the Brazilian Portuguese version of BESES. We would like to thank the researchers Dr. Paul Oh (Canada, University Health Network – UHN), Gabriela L. M. Ghisi (UHN), Raquel R. Britto (Brazil, Federal University of Minas Gerais – UFMG), and Danielle A. G. Pereira (UFMG); the masters’ students Ana Paula D. B. Batalha, and Carolina M. M. Félix; and the undergraduate students Gabriel L. L. Almeida, Laís J. T. Silva, Larissa B. Carvalho and Marcela G. de Carvalho from Federal University of Juiz de Fora. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Finance Code 001.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carla Cristina da Silva Machado, Email: carlamachado.fst@hotmail.com.
Carla Malaguti, Email: carlamalaguti@gmail.com.
Patrícia Fernandes Trevizan, Email: patricia_trevizan@yahoo.com.br.
Danielle Guedes Andrade Ezequiel, Email: daniezequiel@hotmail.com.
Mariana Balbi Seixas, Email: mariana.balbi@ufjf.edu.br.
Lilian Pinto da Silva, Email: lilian.pinto@ufjf.edu.br.
References
- 1.Sociedade Brasileira de Diabetes. Diretrizes Sociedade Brasileira de Diabetes 2019–2020. https://www.diabetes.org.br/profissionais/images/DIRETRIZES-COMPLETA-2019-2020.pdf. Accessed 03 Mar 2020.
- 2.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. Ninth. Brussels, Belgium: International Diabetes Federation; 2019. https://diabetesatlas.org/. Accessed 03 March 2020.
- 4.Vigitel Brazil 2018: Surveillance of risk and protective factors for chronic diseases by telephone survey: estimates of frequency and Sociodemographic distribution of risk and protective factors for chronic diseases in the capitals of the 26 Brazilian Sta. Ministério da Saúde; 2019. https://portalarquivos2.saude.gov.br/images/pdf/2019/julho/25/vigitel-brasil-2018.pdf. Accessed March 3, 2020.
- 5.Herdy AH, López-Jiménez F, Terzic CP, Milani M, Stein R, Carvalho T, et al. South American guidelines for cardiovascular disease prevention and rehabilitation. Arq Bras Cardiol. 2014;103. 10.5935/abc.2014S003. [DOI] [PubMed]
- 6.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, American College of Sports Medicine, American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010; 10.2337/dc10-9990 [DOI] [PMC free article] [PubMed]
- 7.American Diabetes Association. 5. Facilitating behavior change and wellbeing to improve health outcomes: Standards of Medical Care in Diabetes 2020. Diabetes Care 2020; 10.2337/dc20-S005. [DOI] [PubMed]
- 8.Bazargan-Hejazi S, Arroyo JS, Hsia S, Brojeni NR, Pan D. A racial comparison of differences between self-reported and objectively measured physical activity among US adults with Diabetes Ethn Dis. 2017; 10.18865/ed.27.4.403 [DOI] [PMC free article] [PubMed]
- 9.Oliveira APN, Maia EG, Silva FM, Martins APB, Claro RM. Needed improvements in diabetes prevention and Management in Brazil. Prev Chronic Dis. 2018;15. 10.5888/pcd15.180269. [DOI] [PMC free article] [PubMed]
- 10.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int. 2009;24:416–427. doi: 10.1093/heapro/dap031. [DOI] [PubMed] [Google Scholar]
- 11.Pitanga FJG, Almeida MCC, Queiroz CO, Aquino EML, Matos SMA. Physical activity in Brazil: lessons from ELSA-Brasil. Narrative review Sao Paulo Med J. 2017;135:391–395. doi: 10.1590/1516-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Extra 3 - Alharbi M, Gallagher R, Neubeck L, et al. Exercise barriers and the relationship to self-efficacy for exercise over 12 months of a lifestyle-change program for people with heart disease and/or diabetes. Eur J Cardiovasc Nurs. 2017; 10.1177/1474515116666475 [DOI] [PubMed]
- 13.Azami G, Soh KL, Sazlina SG, Salmiah MS, Aazami S. Behavioral interventions to improve self-management in Iranian adults with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord. 2018;17:365–380. doi: 10.1007/s40200-018-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 15.Barros MB, Iaochite RT. Autoeficácia para a prática de atividade física por indivíduos adultos. Motricidade. 2012;8. 10.6063/motricidade.8(2).710.
- 16.Lee LL, Avis M, Arthur A. The role of self-efficacy in older people’s decisions to initiate and maintain regular walking as exercise—Findings from a qualitative study Prev Med. 2007;45:62–65. doi: 10.1016/j.ypmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Heijden MMP, Pouwer F, Romeijnders AC, Pop VJ. Testing the effectiveness of a self-efficacy based exercise intervention for inactive people with type 2 diabetes mellitus: design of a controlled clinical trial. BMC Public Health. 2012;12. 10.1186/1471-2458-12-331. [DOI] [PMC free article] [PubMed]
- 18.Dutton GR, Tan F, Provost BC, Sorenson JL, Allen B, Smith D. Relationship between self-efficacy and physical activity among patients with type 2 diabetes. J Behav Med. 2009;32:270–277. doi: 10.1007/s10865-009-9200-0. [DOI] [PubMed] [Google Scholar]
- 19.Bandura A. Guide for constructing self-efficacy scales. Self-efficacy Beliefs of Adolescents 2006. https://www.uky.edu/~eushe2/Bandura/BanduraGuide2006.pdf. Accessed 29 October 2019.
- 20.Schwarzer R, Renner B. Health-specific self-efficacy scales. Freie Universität Berlin.2009. https://userpage.fu-berlin.de/health/healself.pdf. Accessed 29 October 2019.
- 21.Rech CR, Sarabia TT, Fermino RC, Hallal PC, Reis RS. Psychometric properties of a self-efficacy scale for physical activity in Brazilian adults. Rev Panam Salud Publica. 2011;29:259–266. doi: 10.1590/S1020-49892011000400007. [DOI] [PubMed] [Google Scholar]
- 22.Shin Y, Jang H, Pender NJ. Psychometric evaluation of the exercise self-efficacy scale among Korean adults with chronic diseases. Res Nurs Health. 2001;24:68–76. doi: 10.1002/1098-240x(200102)24:1<68::aid-nur1008>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Everett B, Salamonson Y, Davidson PM. Bandura’s exercise self-efficacy scale: validation in an Australian cardiac rehabilitation setting. Int Journal Nurs Stud. 2009;46:824–829. doi: 10.1016/j.ijnurstu.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Noroozi A, Ghofranipour F, Heydarnia AR, Nabipour I, Tahmasebi R, Tavafian SS. The Iranian version of the exercise self-efficacy scale (ESES) factor structure, internal consistency and construct validity. Health Educ J. 2011;70:21–31. doi: 10.1177/0017896910374547. [DOI] [Google Scholar]
- 25.Darawad MW, Hamdan-Mansour AM, Khalil AA, Arabiat D, Samarkandi OA, Alhussami M. Exercise self-efficacy scale: validation of the Arabic version among Jordanians with chronic diseases. Clin Nurs Res. 2018;27:890–906. doi: 10.1177/1054773816683504. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Silva J, Peralta-Ramírez MI, Navarrete NN, Silva-Silva D, Caballo VE. Validez y fiabilidad de la escala de autoeficacia para el ejercicio físico en pacientes con síndrome metabólico. Revista Española de Salud Pública. 2018;92. [PMC free article] [PubMed]
- 27.Boff RM. Evidências psicométricas das escalas de auto-eficácia para regular hábito alimentar e auto-eficácia para regular exercício físico. 2012. http://repositorio.pucrs.br/dspace/handle/10923/5000. Accessed 29 October 2019.
- 28.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Hair JF Jr, Black WC, Babin BJ, Anderson RE, Tatham RL. Análise Multivariada de Dados. 6th ed. Bookman: Porto Alegre; 2009.
- 30.Xue J, Chiu HFK, Liang J, Zhu T, Jiang Y, Chen S. Validation of the six-item screener to screen for cognitive impairment in primary care settings in China. Aging Ment Health. 2017;22:453–457. doi: 10.1080/13607863.2017.1280768. [DOI] [PubMed] [Google Scholar]
- 31.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;40:1–55. [Google Scholar]
- 32.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho-Myrrha, MA, Dias RC, Fernandes AA, Araújo CG, Hlatky MA, Pereira DG, Britto RR. Duke activity status index for cardiovascular diseases: validation of the Portuguese translation. Arq Bras Cardiol 2014; 10.5935/abc.20140031 [DOI] [PMC free article] [PubMed]
- 34.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke activity status index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 35.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47:1019–1024. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteiro DP, Britto RR, Carvalho MLV, Montemezzo D, Parreira VF, Pereira DAG. Shuttle walking test como instrumento de avaliação da capacidade funcional: uma revisão da literatura. Ciência & Saúde. 2014;7(2):92–97. doi: 10.15448/1983-652X.2014.2.16580. [DOI] [Google Scholar]
- 37.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Victor JF, Ximenes LB, Almeida PC. Adaptação transcultural para o Brasil da Exercise Benefits/Barriers Scale (EBBS) para aplicação em idosos: uma avaliação semântica. Cadernos de Saúde Pública. 2008;24:2852–2860. doi: 10.1590/S0102-311X2008001200014. [DOI] [PubMed] [Google Scholar]
- 39.Victor JF, Ximenes LB, Almeida, PC. Confiabilidade e validade da Exercise Benefits/Barriers scale em idosos. Acta Paul Enferm. 2012; 10.1590/S0103-21002012000800008.
- 40.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3. Pearson/Prentice Hall: NJ; 2009. [Google Scholar]
- 41.Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. Fifth ed. USA: Oxford University Press; 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 32 kb)