Abstract
The study aimed to investigate the effects of 8-weeks AGEs restricted diet on glycemic control as well as lipid profile, inflammatory and oxidative stress biomarkers and IR in overweight patients with Mets. In this randomized, controlled clinical trial 40 clients were randomly assigned to take either a low AGE (L-AGE) or a regular AGE (Reg-AGE) diet. Also, both groups were advised to follow an energy-restricted diet. At baseline and after 8-weeks of intervention, anthropometric parameters, dietary intake, plasma concentrations of malondialdehyde, carboxymethyllysine, TNF-α, hs-CRP and levels of serum glucose, lipid and insulin were assessed. AGEs restriction resulted in significant changes in mean differences levels of CML (p < 0.004), FBG (p < 0.01), HOMA-IR (p < 0.04), TNF-α (p < 0.01) and MDA (p < 0.02) in comparison to Reg-AGE. Moreover, weight (p < 0.0001) and WC (p < 0.001) significantly declined in the intervention group. Our results indicate that dAGEs restriction plus a low-calorie diet is superior to a low-calorie diet in amelioration of central obesity and IR at least partially through reduction of OS and inflammation in Mets subjects.
Keywords: Metabolic syndrome, Obesity, Advanced glycation end products, Inflammation, Insulin resistance, Fasting blood glucose
Introduction
In recent decades, prevalence of metabolic syndrome (Mets) has become the main growing medical problem in the world which is characterized as a cluster of hyperglycemia, dyslipidemia, hypertension and abdominal obesity that when occurring together lead to conflicting outcomes including developing type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), cancer, infertility, dementia, chronic oxidative stress (OS) and inflammation [1, 2]. Interest in these diseases is easily justified when about 20-25% of the world’s adult population has the Mets [2], and synergistic effects of its issues increase the risk of mortality more than each of them separately, all 1.6-fold [3]. The control of Mets through lifestyle changes (energy balance diet and physical activity) [4], and control of its risk factors are the first lines of treatment, although drug therapy can be added to them [5, 6]. One of the most important ways to alleviate the risk factors connected to Mets is dietary pattern [7]. It was shown that modern Western diet which could drive the pathophysiology of Mets [8], is characterized by highly heated processed food consumption that are rich not only in fat, meat, sugar and salt but also contain potentially pathogenic compounds known as advanced glycation end-products (AGEs) [9, 10]. AGEs are a group of compounds formed by the Maillard reaction. According to several researches, animal-origin foods, as well as foods rich in protein and fat had the highest amount of dietary AGEs (dAGEs), while carbohydrate-rich foods such as fruits, vegetables, milk and whole grains had the lowest but this could increase depending on the processing. Additionally, industrialized methods of food processing effectively altered diets. Foods that are grilled, roasted, broiled or fried would produce higher dAGEs than foods that are boiled, poached, stewed or steamed [10–14]. These processes enhance food flavor, color and appearance, and thus increase food consumption [15]. In several studies, it has been demonstrated a direct relationship between high intake of dAGEs and higher serum AGEs, enhancement of obesity, OS, chronic inflammation, insulin resistance and endothelial dysfunction and thereby increase the risk of development of T2DM, Mets and their complications [16–19]. Only an Iranian adult cross-sectional study has shown the association of dAGEs and risk factors for Mets subjects [20]. Furthermore a recent randomized trial showed that a low-dAGEs (L-dAGEs) intake ameliorates insulin resistance levels in obese individuals with the Mets after 1 year of intervention [21]. Although AGEs have been implicated in many diseases, including diabetes and cardiovascular disease, the impact of restricting dietary AGEs has not been well characterized in Mets adults, especially Iranian. We hypothesized that restriction of dietary intake of AGEs would affect serum CML and have an impact on Mets parameters and inflammation. One of the most important aim of this study was to answer the question, the low calorie diet can lower the AGEs intake or not? To address these hypotheses, we conducted a controlled dietary intervention to evaluate the possible superiority of L-dAGEs plus a low calorie diet to a low calorie diet alone in alleviation of Mets features and risk factors in Iranian Mets adults that compared the effects of diets that were normal vs. low in AGEs plus low calorie diet for both
Methods and material
Study population
This study was a randomized control trial study and involved non-smoking men and women, aged between 18 to 70 years who were diagnosed with the Mets and had BMI over 25 kg/m2. The Mets was diagnosed according to the new International Diabetes Federation definition (IDF) [2] as having: central obesity (waist circumference (WC) ≥102 cm in men and ≥88 cm in women); in conjunction with two of the following risk factors: BP ≥130/85 mmHg, or use of BP-lowering medication; fasting blood glucose (FBG)> 100 mg/dl (>5·55 mmol/l), triacylglycerol (TAG) >150 mg/dl (>1·69 mmol/l) or specific treatment for this lipid abnormality, such as fibrates or nicotinic acid; HDL-cholesterol <50 mg/dl(<1·29 mmol/l) in women or <40 mg/dl (<1·03 mmol/l) in men, or specific treatment for this lipid abnormality. The participants were excluded from the study if they had gestation or lactation, any physical activity changes habits through the study, renal failure and having hypo- or hyperthyroidism or cancer, inflammation and rheumatic disease, infection, using of any multivitamin-mineral and vitamin B6 supplements, glucocorticoids, NSAIDs (Nonsteroidal anti-inflammatory) drugs, insulin treatment and hormone replacement therapy as taking estrogen or progesterone, experiencing cognitive problems or chronic diseases that impaired their compliance.
Study design
The 8 weeks trial protocol was described carefully to all eligible volunteer patients and written informed consent was obtained from them. The compatibility of the study protocol and ethical standards approved by Ethics Committee. The study was registered in www.clinicaltrials.gov (NCT03147339). A questionnaire about medical history, present illnesses, and cooking methods was filled out by interviewing by an expert dietitian. Participants were stratified by sex and BMI, and then were randomized to receive calorie restriction plus L-AGE diet or calorie restricted diet along with Reg-AGE diet (Fig.1). Both groups diet had the same content of macronutrients (55-60% carbohydrates, 15-20% proteins, and 25-30% fats) and they were given a written and oral instruction of a dietary plan with weight loss recommendation. L-AGE participants prepared their own food at home after being individually instructed on how to reduce d-AGEs intake (<15000 kU/day), which limited d-AGEs intake by ~40–50%: [10, 22] modifying the time, method and temperature of cooking; specifically avoid using dry heat methods like: oven and deep frying, broiling, roasting and grilling; encourage in cooking with higher amount of water content as in stewing, poaching, steaming and boiling; consume bread without a crust and finally exposure of meet to acidic solutions (marinades) of lemon juice and vinegar before cooking [10] (Table 1). All the L-AGE recommendation, cooking limits new AGEs formation in foods, especially animal food products was done according to Uribarri et.al practical guide and it has been previously indicated that replacing to these recommended methods of cooking reduces new AGE formation in foods, especially animal food product [10]. The control group remained consuming their habitual content of AGEs in meals.
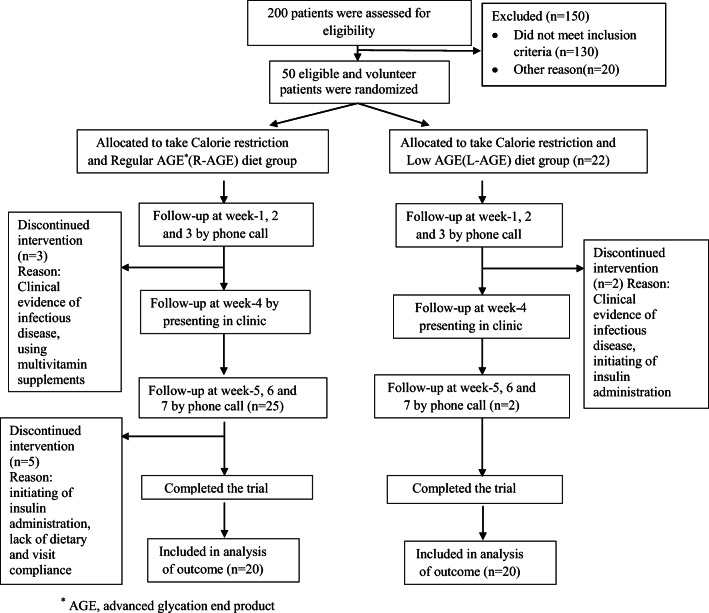
Fig. 1.
Flow diagram of the study participants. * AGE, advanced glycation end product
Table 1.
Sample of daily dAGEs intake from a participant on the L-AGE and Reg-AGE diets
| Meal | Regular AGE diet | Low AGE diet | ||||
|---|---|---|---|---|---|---|
| Item | Portion | AGEs | Item | Portion | AGEs | |
| Breakfast |
Fried eggs Bread, whole wheat, slice Cheddar cheese Skimmed milk Honey Peanut, roasted |
1 2 slice 10 g 240 mL 15 mL 30 g |
1237 82 552 2 1 1934 |
Boiled eggs Bread, whole wheat, slice Cheddar cheese Skimmed milk Honey Pistachios, salted |
1 2 slice 10 mL 240 mL 15 mL 30 g |
75 82 552 20 1 114 |
| Lunch |
Chicken breast, roasted without Skin Rice, white, quick cooking Green salad Caesar dressing Olive oil |
90 g 60 g 1 cup 15 mL 10 mL |
5975 6 0 111 1190 |
Boiled chicken breast without Skin Rice, white, quick cooking Green salad Caesar dressing Olive oil |
84 g 60 g 1 cup 15 mL 10 mL |
1000 6 0 111 1190 |
| Dinner |
Broiled salmon vegetables grilled(broccoli, carrots, celery) Grilled potato with tomatoes Bread, whole wheat, slice Margarine |
90 1 cup 100 g 2 slice 5 mL |
3012 226 220 82 900 |
Salmon, fillet, boiled vegetables raw(broccoli, carrots, celery) mashed potatoes with tomatoes and Bread, whole wheat, slice Margarine |
90 g 1 cup 100 g 2 slice 5 mL |
975 40 17 82 900 |
| Snack |
Muffin, bran Banana Cantaloupe Fresh Plums Black tea Date Pancake |
1 1 medium ¼ small 2 medium 240 mL 2 30 |
102 9 20 50 5 36 292 |
Muffin, bran Banana Cantaloupe Fresh Plums Black tea Date Pancake |
1 1 medium ¼ small 2 medium 240 mL 2 30 |
102 9 20 50 5 36 292 |
| Total AGEs (kU/day) | 16,044 | 5700 | ||||
Arbitrary AGE units are expressed in AGE kilo units based on data from reference11
Dietary assessment
In order to estimate usual dietary intake, each subject was interviewed by a dietitian and completed three 24-hour dietary recalls in three different days of the week, at the baseline and the end of the study appointments. During the interview, subjects were asked for the time and amount of food, while particular emphasis was taken to record the cooking methods. AGEs contents estimated from a published food database of ∼560 listed AGEs values and expressed as AGE equivalents per day by Uribarri et.al [10]. In order to distinguish the accurate portion sizes, the patients were interviewed to report their intake based on household measures. Nutrient intakes were calculated by using nutrition analysis software Nutritionist IV, which was modified using the national composition food tables [23]. For quantification of total AGEs intake, data from the each three recall questionnaires were entered into an Excel database (designed for this purpose, Microsoft, 2011) and the amount of dAGEs was calculated by multiplying the gram or milliliters of food consumed by the AGEs (KU) amount in 100 g of food. Finally, total dAGEs was determined by summing the intakes from each food. Some foods were not found in the database, they were calculated with averages from similar foods available. According to previous studies, high dAGEs consumption was set as an intake higher or equal than 15,000 kU/AGEs/day [10]. At least two researchers made all decisions and all final analyses were reviewed for any mistakes by an experienced dietitian.
Follow-up
Participants received a personal interview with an expert dietitian via phone call (every week) and monthly at the clinic to evaluate participant's compliance to the study protocol. Patients were excluded from the analysis if they could not follow the diet or changed their medications.
Anthropometric and blood pressure measurements
Height was measured in a standing position without shoes, using a stadiometer with a minimum measurement of 5 millimeters. Weight was measured and recorded with a digital scale wearing light clothing with an accuracy of up to 100 g. WC was measured to the nearest 0·5 cm approximately between the lower margin of the last rib and top of the iliac crest at the level of navel with a tape measure. Each subject’s right arm was used for blood pressure measurement, twice with an interval of 30 s. According to standard protocol, the subject was required to be in a relaxed sitting position [24]. Body mass index (BMI) was calculated according to the formula (weight [Kg]/height [m]2).
Biochemical measurement
Venous blood samples were collected after 12-14 hours of overnight fasting, in sitting position. Blood serum was separated and stored at -80°C for further analysis. Serum lipid profiles levels (TG, TC, HDL-C, LDL-C) and FBG were assessed by the enzymatic colorimetric method, using commercial kits (Pars Azmoon). Plasma level of carboxymethyl lysine was measured by a commercially available enzyme-linked immunosorbent assay (ELISA) [26] kit (ZellBio GmbH, Germany). Moreover, OS and inflammation factors were assessed as Malondialdehyde (MDA) concentration (μmol/L) by colorimetrical assay by a commercial kit according to manufacturer's protocol (MDA assay kit; Zellbio, Germany), TNF_α by ELISA method (Diaclone, Commercial ELISA kit, France), hs-CRP by ELISA method (Zellbio, Germany). Fasting plasma level of insulin was analyzed Monobind ELISA kit (Monobind, California, USA). Finally, the homeostasis model assessment for insulin resistance (HOMA-IR) was conducted according to their suggested formula as follows: (HOMA-IR= (Glucose (mg/dL) × insulin (μIU/ml))/405) or (glucose (mmol/l) × insulin (μIU/ml))/22·5 [25].
Statistical analysis
The estimated number for each group was 18 at a power (1-β) of 90% and α=1.05 for a two-arm parallel study with two-tailed testing to detect a difference of 0.18 μg/mL in serum CML (sCML) concentration with a standard deviation of 0.2 μg/mL, obtained from Poulsen et al. [26]. Allowing for ∼20% dropout, a sample size of 44 were proposed (22 in each group, to maximize power). Due to a higher percent drop out in the L-AGE group, the total recruitment was increased to 50. Quantitative parameters were given as mean ± standard deviation. Moreover, they were compared between the groups by independent t-test and changes within each group were tested by paired t-test. To eliminate the effect of confounding factors, covariance analysis was used. Also, repeated-measures ANOVA was used to compare within subjects’ dietary intake values of pre and post-trial in each group. Correlation analyses were also examined by the Pearson correlation coefficient. Statistical analyses were performed using the 21st version of SPSS Software.
Results
From 50 eligible patients who were recruited in this study, 40 individuals completed the trial (Fig. 1). Participants in both groups were of a similar age, gender, anthropometric and metabolic characteristics at study initiation (all P=NS) (Table 2, 3). None of the patients who completed the study had any serious adverse events of L-AGE diet indicating tolerance to treatment. Ten of the enrolled subjects dropped out of the study due to clinical evidence of an infectious disease, initiating of insulin administration, using multivitamin supplements and lack of dietary and/or visit compliance through the intervention. Out of 40 patients having Mets, 33 persons (82.5%) were diabetes and used hypoglycemic treatment, 18 persons (45%) used drug treatment for hypertension and 30 persons (75%) used drug treatments for dyslipidemia (Table 2). The AGEs intake in the intervention group significantly decreased (47%) as compared to controls. Post-trial mean of calorie intake declined in both groups, while there were not any significant differences between two groups (Table 4).
Table 2.
Baseline characteristics of participants in both study groups (Numbers and percentage; mean values and standard deviations)
| Baseline Variables | Reg-AGE group (n = 20) | L-AGE group (n = 20) | P* | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 11 | 60 | 10 | 50 | NS |
| Female | 9 | 40 | 10 | 50 | |
| Oral Hypoglycemic Drugs | |||||
|
Biguanides Sulfonylureas Alpha glucosidase inhibitors None |
16 7 5 4 |
80 35 25 20 |
17 11 3 3 |
85 55 15 15 |
NS NS NS NS |
| Anti-hypertensive Drugs | |||||
|
Angiotensin II receptor antagonists ACE inhibitors Diuretics Beta blockers None |
5 2 2 5 10 |
25 10 10 25 50 |
3 3 2 6 12 |
15 15 10 30 60 |
NS NS NS NS NS |
| Lipid Lowering Drugs | |||||
|
Statins None |
15 5 |
75 25 |
15 5 |
75 25 |
NS NS |
| Age(years) | |||||
|
Mean SD |
54.4 8.9 |
53 8.2 |
NS | ||
AGE, advanced glycation end product; ACE inhibitors, angiotensin-converting enzyme inhibitors
*P-values are for comparison of the variables between the two groups (all by χ2 test, except for age, which was analyzed by independent t test)
Table 3.
Participants baseline biochemical and anthropometric variables (Mean values and standard deviations)
| Metabolic variable | L-AGEsІ (Mean ± SD) | Reg-AGEs (Mean ± SD) | Pa |
|---|---|---|---|
| Weight (kg) |
86.2 ± 10.0 33.2 ± 3.9 129.6 ± 20.2 90.3 ± 12.3 109.4 ± 7.8 0.95 ± 0.04 8.6 ± 3.12 19.7 ± 14.6 7.0 ± 4.8 2.08 ± 0.78 4.3 ± 1.33 1.18 ± 0.19 2.27 ± 0.71 0.79 ± 0.62 5.8 ± 1.2 19.9 ± 17.0 5.9 ± 2.7 |
81.1 ± 11.11 31.3 ± 4.1 129.2 ± 22.1 86.7 ± 9.2 107.6 ± 8.1 0.97 ± 0.07 7.4 ± 2.07 13.3 ± 8.4 4.6 ± 3.8 2.03 ± 0.83 3.8 ± 0.75 1.11 ± 0.21 2.10 ± 0.38 1.1 ± 1.02 6.2 ± 1.7 16.9 ± 8.7 5.4 ± 2.8 |
NS |
| BMI (Kg/m2) | NS | ||
| SBP (mmHg) | NS | ||
| DBP (mmHg) | NS | ||
| WC (cm) | NS | ||
| WHR | NS | ||
| FBG (mmol/l)II | NS | ||
| Insulin (μIU/mL) II | NS | ||
| Homa_IR | NS | ||
| TG (mmol/l) II | NS | ||
| T-Col(mmol/l) II | NS | ||
| HDL-C (mmol/l) II | NS | ||
| LDL-C (mmol/L) II | NS | ||
| sCML (μg/mL) | NS | ||
| Malondialdehyde (μmol/L) | NS | ||
| TNF_α (pg/mL) | NS | ||
| hs-CRP(mg/L) | NS |
AGE, advanced glycation end product; BMI, body mass index; WC, Waist Circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; FBG, fasting blood sugar; HDL-C, high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; sCML, carboxymethyl lysine; HOMA-IR, Homeostatic model assessment of insulin resistance; HbA1c, glycated Hb; TC, total cholesterol; TG, triglyceride; hs-CRP, high-sensitivity C-reactive protein; TNF_α, tumor necrosis factor
aP values (Paired t-tests)
І Total n for L-AGE group and Reg-AGE group were 20 for both
II To convert insulin in μIU/mL to pmol/l, multiply by 6·945. To convert TAG, cholesterol and glucose in mmol/l to mg/dl, divide by 0·0113, 0·0259 and 0·0555, respectively
Table 4.
Changes of the calorie and AGE intake over the 8 weeks of intervention estimated from 3-day food records at baseline and end of study (Mean values and standard deviations)
| L-AGE (n = 20) | Reg-AGE (n = 20) | P* | P† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 8 weeks | Baseline | After 8 weeks | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Energy (Kcal/d) | 2042.1 | 266.1 | 1673.1 | 230 | 1920.7 | 192.6 | 1667.7 | 230.8 | NS | NS |
| dAGEs (as CML, kU/d) | 12,772 | 3369 | 6022 | 1191 | 12,917 | 3658 | 13,875 | 2931 | NS | <0.0001 |
AGE, advanced glycation end product; dAGEs, dietary AGEs; CML, carboxymethyl lysine; kU, kilo unit
*P values are for comparison between the two groups at baseline (independent t test)
† P values are for comparison between the two groups after 8 weeks (independent t test)
Serum FBG and fasting insulin levels were significantly lower in L-AGE group by 18.3% vs. 39.6% in the control group at the end of the trial. Moreover, a remarkable reduction was seen in HOMA-IR (P=0.03) index in L-AGE group. Statistically significant improvements in levels of TG (P = 0.05) were observed post intervention (Table 5) in L-AGE group. In L-AGE group T-col, LDL-c and HDL-C demonstrated a trend toward reduction, although the decrease failed to attain statistical significance.
Table 5.
Changes of biochemical and anthropometric after 8 weeks of intervention (95% confidence intervals)
| Variable |
Change from baseline (%), L-AGEsІ |
Change from baseline (%), Reg-AGEs |
Pa | Pb | Pc | Pd | Pe | 95% CIe |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | −3.5(4.1) ±1.1 | −0.8(1.1) ± 1.1 | 0.0001 | NS | 0.0001 | 0.0001 | – | −3.4,-1.9 |
| BMI (Kg/m2) | −1.3(3.9) ±0.5 | −0.3(1) ±0.44 | 0.0001 | NS | 0.0001 | 0.0001 | – | −1.2,-0.6 |
| SBP (mmHg) | −2.1(1.6) ±9.6 | 2.9(2.2) ±9.3 | 0.001 | NS | NS | 0.38 | NS | −10.3,1.8 |
| DBP (mmHg) | −3.0(3.3) ±6.0 | −1.7(2) ±8.2 | 0.001 | NS | NS | 0.984 | NS | −6.8,4.3 |
| WC (cm) | −4.7(4.3) ±2.0 | −1.5(1.4) ±1.5 | 0.001 | NS | 0.0004 | 0.0001 | NS | −4.3,-1.9 |
| WHR | 0.002(2.1) ±0.02 | −0.0009(−) ± 0.06 | NS | NS | NS | 0.725 | NS | −0.01,0.01 |
| FBG (mmol/l)II | −1.55(18.3) ±2.2 | −0.13(1.4) ±1.08 | 0.005 | NS | 0.01 | 0.038 | NS | −24.7,-0.32 |
| Insulin (μIU/mL) II | −7.7(39.6) ± 17.6 | 3.7(28.6) ±17.9 | NS | NS | 0.04 | 0.259 | NS | −22.95, −0.14 |
| Homa_IR | −3.1(45.7) ±6.1 | 1.1(26.1) ±6.9 | 0.03 | NS | 0.04 | 0.261 | NS | −8.47, −0.05 |
| TG (mmol/l) II | −0.29(14.4) ±0.64 | −0.02(1) ±0.43 | 0.05 | NS | NS | 0.90 | NS | −0.60, 0.05 |
| T-Col(mmol/l) II | −0.19(3.7) ±0.95 | 0.21(5.5) ±0.62 | NS | NS | NS | 0.454 | NS | −0.88, 0.15 |
| HDL-C (mmol/l) II | −1.2(2.6) ±4.3 | 1.35(4.2) ±4.04 | NS | NS | NS | 0.126 | NS | −5.2, 0.1 |
| LDL-C (mmol/L) II | −0.10(0.9) ±0.7 | 0.13(11) ± 0.47 | NS | NS | NS | 0.624 | NS | −0.5, 0.27 |
| sCML (μg/mL) | −0.1(13.9) ±0.36 | 0.29(27.3) ± 0.44 | NS | 0.004 | 0.004 | 0.001 | 0.003 | −0.65, −0.13 |
|
Malondialdehyde (μmol/L) |
−1.1(17.2) ±2.0 | 0.6(6.5) ± 2.3 | NS | 0.007 | 0.02 | 0.018 | 0.06 | −3.19,-0.25 |
| TNF_α (pg/mL) | −2.3(12.1) ±5.8 | 1.6(9.5) ± 4.07 | NS | NS | 0.01 | 0.008 | NS | −7.18, −0.70 |
| hs-CRP(mg/L) | −0.8(14.1) ±1.9 | −0.1(3.7) ± 1.7 | NS | NS | NS | 0.291 | NS | −1.89, 0.47 |
AGE, advanced glycation end product; BMI, body mass index; WC, Waist Circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; FBG, fasting blood sugar; HDL-C, high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; sCML, carboxymethyl lysine; HOMA-IR, Homeostatic model assessment of insulin resistance; HbA1c, glycated Hb; TC, total cholesterol; TG, triglyceride; hs-CRP, high-sensitivity C-reactive protein; TNF_α, tumor necrosis factor
All values are means (%) ± SDs. Differences between variables at the beginning and end of each test diet are shown
Pavalues are for comparison between means of baseline and after 8-weeks variables of L-AGE group (Paired t test)
pbvalues are for comparison between the two groups after the intervention (Independent t test)
pcvalues are for comparison of changes of each variable between the two groups (Independent t test)
pdvalues are for comparison between two groups after intervention after adjusting for baseline values (analysis of covariance)
ePvalues are for elimination of weight differences confounder (analysis of covariance)
eCohen’s d effect sizes and their 95% CI are displayed for changes of variables during the study
І Total n for L-AGE group and Reg-AGE group were 20 for both
II To convert insulin in μIU/mL to pmol/l, multiply by 6·945. To convert TAG, cholesterol and glucose in mmol/l to mg/dl, divide by 0·0113, 0·0259 and 0·0555, respectively
Total CML intake in the L-AGE diet group greatly decreased by 13.9% compared to controls (-0.1 ± 0.36) vs. 0.29 ± 0.44) μg/mL, (P=0.004). Additionally, changes of serum CML positively correlated with changes in d- AGEs (r = 0.424, P = 0.006).
A significant reduction was seen in serum TNF_α and MDA in L-AGE group compared to control after the trial (P<0.01). Although there was more reduction of hs-CRP in the intervention group, no statistical significant discrepancy was seen.
Both diets were associated with weight loss but it was about fourfold in L-AGE group compared to Reg-AGE group. Likewise, BMI and WC were markedly declined in intervention group (P<0.0001) compared to control.
To eliminate the cofounder effects of weight loss changes on CML and biochemical factors, an adjustment was done and there were still significant differences in CML (P=0.003) between the 2 groups. No other correlations were seen between biochemical markers and other anthropometric and metabolic variables.
Discussion
Present study showed that the daily consumption of calorie-AGEs restricted diet for 8 weeks resulted in lower serum CML level and improved central obesity, FBG, insulin sensitivity, serum levels of triglycerides, OS and inflammatory markers (MDA, TNF_α) than did a calorie restricted, regular AGEs diet, in subjects with Mets.
Given the rising prevalence of Mets globally, it is important to determine modifiable risk factors for it. It is known that AGEs production is higher in Mets patients due to higher FBG and dyslipidemia [16]. The L-AGE diet used in our trial-implemented 47% less AGEs from the regular diet of Mets patients. Our finding of marked differences in CML showed subjects’ compliance to the dietary L-AGE protocol and a direct correlation between dAGEs and levels of CML in human serum which is consistent with previously published studies in healthy [9], overweight and obese people with Mets [16, 21, 22, 27] and patients with diabetes [15, 28].
However, the mean CML concentration increased in the control group at the end of the study and by analyzing the cooking method of this group, we found that due to the calorie intake restriction and no advice to lower AGE intake, they used grilling, broiling and roasting methods to enhance the taste of foods, which is a characteristic of dietary AGEs [10].
In this study, although low energy diet was matched in both groups, weight, BMI, and WC reduction were about triple in L-AGE participants as much as the control, which was much more than the reported by Vlassara et al. in same subjects and diets for 1 year [21]. As pro-oxidant AGEs serve as ‘appetite-enhancing’ agents that simultaneously spur over-nutrition and obesity [15, 16], thus, it has postulated that AGE-restricted diet attenuate peripheral fat mass and obesity by lowering leptin level [29], and pro-inflammatory and OS markers, and nuclear receptor PPARγ (peroxisome proliferator-activated receptor-γ) expression [30].
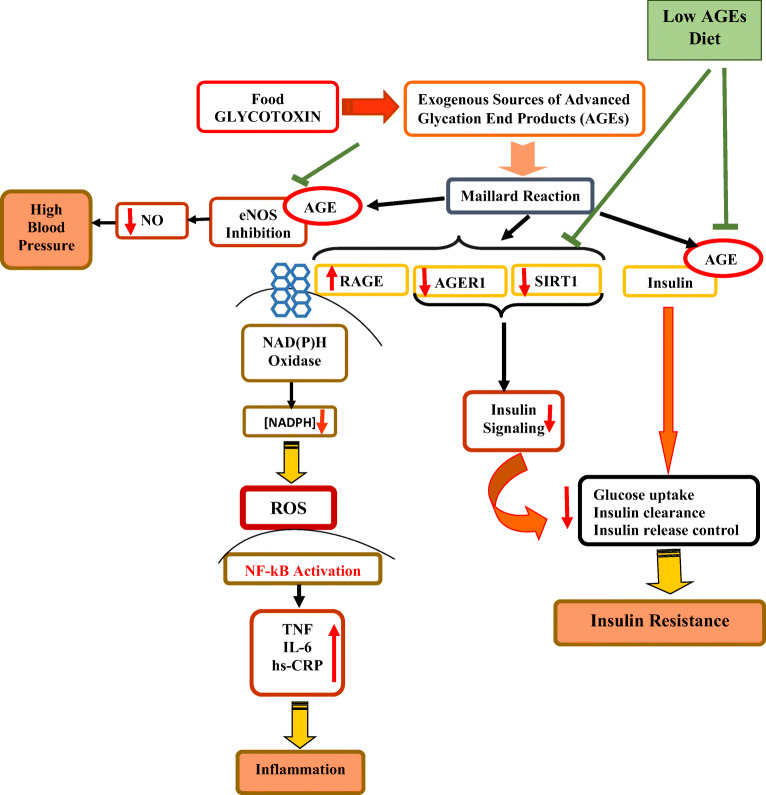
High serum AGE levels induced by high dAGEs consumption are associated with insulin resistance and promote inflammation and IR. [5, 22, 31]. We reported a significant decrease in FBG and HOMA-IR, thereby confirming the previous reports of amelioration of insulin resistance by AGEs restricted diet, in obese patients with diabetes [28] and Mets [21]. In the present study after adjustment for weight changes, still CML serum concentration showed significant difference between two groups. It could be suggested that L-dAGEs plus a low calorie diet might improve central clinical features of Mets (central obesity and IR), and decrease its adverse outcomes (T2DM and CVD) in short time. The possible mechanism might be through the activation of AGER1, disruption of RAGE signaling [32] and expression of SIRT1, a major part in insulin signaling and secretion, insulin resistance, inflammation [17, 28, 29]. Increased SIRT1 levels via NAD+/NADH inhibits NFκB (Nuclear Factor κB) p65 hyper acetylation and inflammatory events in monocytes and AGE-induced impaired signaling via the insulin receptor and IRS1 (Insulin receptor substrate 1) in adipocytes [29]. (Fig. 2)
Fig. 2.
AGE’s effects on insulin resistance, inflammation, oxidative stress and blood pressure. AGEs, advanced glycation end products; NO, Nitric oxide; eNOS, endothelial nitric oxide synthase; SIRT1, Sirtuin 1; AGER1, AGE receptor 1; RAGE, Receptor for AGEs; NADPH, Dihydronicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; NF-κB, Nuclear factor-κB; TNF- α, tumor necrosis factor-α; IL-6, interleukin 6; hs-CRP, high-sensitivity C-reactive protein
The marked reductions of MDA (17.2%) and TNF_α (12.1%), due to L-AGE diet confirm the view that dAGEs intake is significantly correlated with OS and inflammation process [17]. The aforementioned studies in prediabetes [33], obese subjects with Mets [21] and T2DM [28, 34], demonstrated an attenuation of inflammatory markers and MDA [35] after intake of L-dAGEs compared to high dAGEs. Our findings are not concurrent with previous studies conducted in obese and healthy overweight adults [26, 36, 37]. As mentioned in Baye et al. study [37], L-AGEs diets can be more useful for subjects who had higher baseline values in inflammatory markers such as patients with Mets who are susceptible of chronic inflammation [4]. Thus, we did not find a significant reduction in some inflammatory markers due to normal level of them before the intervention.
Low calorie-AGEs diet in our study was capable of mildly reducing T-C, LDL-C, HDL-C and significantly decreased TG compared with the baseline. The aforementioned studies have shown similar results of L-AGEs diet. In a 24-week randomized dietary intervention in 62 prediabetes subjects, L-dAGEs resulted in significant reduction of lipid profile levels compared to R-dAGEs [33]. Furthermore, low diet-derived oxidant AGEs can abate abdominal obesity and lipid profiles by changing the form of fats to less processed oils [10]. A recent study showed that one month restriction of dietary AGE made a significant microbiota differences in peritoneal dialysis patients' population, which might decrease cardiovascular events in this population [38].
Finally, this study had some limitations; first, we did not have access to a body composition analyzer to assess alterations in body fat and lean body mass percentage. Second, we used the published food CML–AGEs database for ∼560 commonly consumed food items to evaluate the dAGEs intake of each patient; several specific food items of the study participants were not available in the database and we estimated dAGEs content from similar food. In conclusion, our findings again confirm that even short- term restriction of dAGE intake plus a low calorie diet can significantly decrease circulating AGE levels and is superior to a low calorie diet in amelioration of central obesity and insulin resistance at least partially through reduction of OS and inflammation in Mets subjects. Further studies with longer duration are recommended.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
ClinicalTrials.gov ID NCT03147339
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39(2):99–110. doi: 10.1016/j.diabet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 4.Kelli H, Kassas I, Lattouf O. Cardio metabolic syndrome: a global epidemic. J Diabetes Metab. 2015;6(513):2. [Google Scholar]
- 5.Lam DW LD, De Groot LJ, Chrousos G, Dungan K, et al., editors. Metabolic Syndrome. Endotext [Internet] South Dartmouth (MA): MDTextcom, Inc; 2000-. 2015.
- 6.Ahima RS. Overview of metabolic syndrome. In: Ahima RS, editor. Metabolic syndrome: a comprehensive textbook. Cham: Springer International Publishing; 2016. pp. 3–12. [Google Scholar]
- 7.Rodríguez-Monforte M, Sánchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56(3):925–947. doi: 10.1007/s00394-016-1305-y. [DOI] [PubMed] [Google Scholar]
- 8.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 9.Birlouez-Aragon I, Saavedra G, Tessier FJ, Galinier A, Ait-Ameur L, Lacoste F, Niamba CN, Alt N, Somoza V, Lecerf JM. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91(5):1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 10.Uribarri J. Advanced Glycation End Products in Foods and a Practical Guide to Their Reductsion in the Diet. Journal of the American Dietetic Association. 2010;110(6):911–16.e12. [DOI] [PMC free article] [PubMed]
- 11.P-c C, C-c H, M-c Y. Analysis of glycative products in sauces and sauce-treated foods. Food Chem. 2009;113(1):262–266. doi: 10.1016/j.foodchem.2008.06.076. [DOI] [Google Scholar]
- 12.Hull GL, Woodside JV, Ames JM, Cuskelly GJ. Nε-(carboxymethyl) lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012;131(1):170–174. doi: 10.1016/j.foodchem.2011.08.055. [DOI] [Google Scholar]
- 13.Scheijen JL, Clevers E, Engelen L, Dagnelie PC, Brouns F, Stehouwer CD, et al. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: presentation of a dietary AGE database. Food Chem. 2016;190:1145–1150. doi: 10.1016/j.foodchem.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, Woodward M, Striker GE, Vlassara H. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. 2014;111(13):4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Current diabetes reports. 2014;14(1):1–10. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, Yee K, Tansman L, Chen X, Mani V, Fayad ZA, Vlassara H. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? The Journal of Clinical Endocrinology & Metabolism. 2015;100(5):1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Puyvelde K, Mets T, Njemini R, Beyer I, Bautmans I. Effect of advanced glycation end product intake on inflammation and aging: a systematic review. Nutr Rev. 2014;72(10):638–650. doi: 10.1111/nure.12141. [DOI] [PubMed] [Google Scholar]
- 18.Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125. doi: 10.3390/nu8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur J Clin Nutr. 2013;67(3):239–248. doi: 10.1038/ejcn.2012.220. [DOI] [PubMed] [Google Scholar]
- 20.Angoorani P, Ejtahed H-S, Mirmiran P, Mirzaei S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr. 2016;67(2):170–176. doi: 10.3109/09637486.2015.1137889. [DOI] [PubMed] [Google Scholar]
- 21.Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA, Nadkarni GN, Striker GE, He JC, Uribarri J. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59(10):2181–2192. doi: 10.1007/s00125-016-4053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. The Journal of Clinical Endocrinology & Metabolism. 2009;94(11):4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 1999;7:213. [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Poulsen M, Bak M, Andersen J, Monošík R, Giraudi-Futin A, Holst J, et al. Effect of dietary advanced glycation end products on postprandial appetite, inflammation, and endothelial activation in healthy overweight individuals. Eur J Nutr. 2014;53(2):661–672. doi: 10.1007/s00394-013-0574-y. [DOI] [PubMed] [Google Scholar]
- 27.Macías-Cervantes MH, Rodríguez-Soto JMD, Uribarri J, Díaz-Cisneros FJ, Cai W, Garay-Sevilla ME. Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition. 2015;31(3):446–451. doi: 10.1016/j.nut.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34(7):1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011;7(9):526–539. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, Ramdas M, Striker GE, Vlassara H. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2014;46(2):301–309. doi: 10.1007/s00726-013-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mark AB, Poulsen MW, Andersen S, Andersen JM, Bak MJ, Ritz C, Holst JJ, Nielsen J, de Courten B, Dragsted LO, Bügel SG. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care. 2014;37(1):88–95. doi: 10.2337/dc13-0842. [DOI] [PubMed] [Google Scholar]
- 32.Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci. 2006;103(37):13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pino A, Currenti W, Urbano F, Mantegna C, Purrazzo G, Piro S, et al. Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. Journal of clinical lipidology. 2016;10(5):1098–1108. doi: 10.1016/j.jacl.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci. 2002;99(24):15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luevano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52(1):22–26. doi: 10.3164/jcbn.12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semba RD, Gebauer SK, Baer DJ, Sun K, Turner R, Silber HA, Talegawkar S, Ferrucci L, Novotny JA. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144(7):1037–1042. doi: 10.3945/jn.113.189480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baye E, De Courten MP, Walker K, Ranasinha S, Earnest A, Forbes JM, et al. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: a randomised crossover trial. Sci Rep. 2017;7(1):4123. doi: 10.1038/s41598-017-04214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil SA, Bryniarski MA, Sun Y, Buck M, Genco RJ, Quigg RJ, He JC, Uribarri J. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One. 2017;12(9):e0184789. doi: 10.1371/journal.pone.0184789. [DOI] [PMC free article] [PubMed] [Google Scholar]