Abstract
Purpose
Detection and modification of various factors such as life style, smoking and so on can significantly improve the glycemic control status. This study aimed to investigate glycemic control status and its relevant factors among patients with Type 2 DM.
Methods
In this cross-sectional study, the glycemic control status of patients with type 2 DM was investigated. In addition, relevant risk factors including demographic, clinical characteristics, self-care management behavior, medication adherence and laboratory data and their relationship with glycemic control status were assessed. Glycemic control status was defined as very good (HbA1c < 7%), good (HbA1c = 7–7.9%), poor (HbA1c = 8–9.9%) or extremely bad (HbA1c ≥ 10%).
Results
The present study included 562 patients (64.4% female). Most of the patients (37%) had poor glycemic control status. Microvascular complications especially diabetic neuropathy were the most common complications in our study. Glycemic control had significant relationship with level of education (p < .01) and occupation (p = .04). Among laboratory parameters, fasting plasma glucose (FPG) and total cholesterol levels were significantly lower in patients with desirable glycemic control (p < .05). The linear regression test showed that HbA1c had significant relationship with FPG (p < .01) and increasing one standard deviation in FPG can increase the level of HbA1c 0.014.
Conclusion
Glycemic control status in our study was very low and FPG was the strongest predictor of glycemic control status. Some other factors were also associated such as education level, occupation, type of treatment, diastolic blood pressure, the lipid profile and aspartate transaminase.
Keywords: Diabetes mellitus, Glycated hemoglobin, Risk factor, Blood glucose, Self-monitoring
Introduction
Diabetes mellitus (DM) is the most common chronic metabolic disease in the world that has become one of the main problems of the health system. The prevalence of DM is about 8.8% worldwide and 10.8% in Middle East and North Africa according to International Diabetes Federation (IDF). The IDF’s latest report predicted a 54% increase in the prevalence of DM until 2030. The national survey estimated the prevalence of DM at 11.4% among Iranian adults, which showed 35% increase compared to the survey in 2005. It is estimated that DM will involve about 9.2 million of Iranians by the year 2030. Prevalence of DM, especially type 2 DM (T2DM), is nowadays aggressively increasing due to sedentary lifestyles and consumption of unhealthy foods [1–5].
DM causes various macro- and microvascular complications, which leads to major disability and seven to 10 years decrease of life span in the patients. Studies reported four to seven years gap between onset of T2DM until diagnosis. This asymptomatic phase causes delay in treatment which can lead to a 77% increase in the morbidity [6–10]. Obesity, sedentary life style, smoking, and lack of medication adherence were the most common predictive factors of DM complications. Detection and modification of these factors can significantly increase patient’s quality of life.
According to the American Diabetic Association (ADA), glycemic control status is one of the most important indicative factors of T2DM management, which requires the coordinated efforts between patients, their family and the medical team. Glycemic control status can be assessed using hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG). A study in 2010 found that 1 % decrease of HbA1c could decrease the microvascular complications up to 35% and the mortality rates up to 7 %. Some studies found that glycemic control is associated with factors such as gender, duration of DM, body mass index (BMI), FPG and lipid profile [1, 11]. Identification and control of effective factors on inappropriate control of diabetes can be effective for prevention of complications.
This study aimed to investigate glycemic control status in T2DM and to determine factors related to glycemic status of patients.
Materials and methods
Study design
The present cross-sectional study was conducted in diabetes and endocrinology clinic of teaching hospitals affiliated to Shahid Beheshti University of Medical Sciences during 2017 and 2018. Implementation protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. The study was conducted in accordance with the Declaration of Helsinki (seventh revision 2013). Written informed consent was obtained from all participants prior to their participation in the study. This study did not impose additional costs to the patients or on the health system. It also did not interfere with the process of standard diagnosis and treatment prescribed by physician.
Study population
All patients aged 18 years or higher coming to outpatient diabetes and endocrinology clinics were assessed based on ADA and those who had the criteria of T2DM, entered the study. The exclusion criteria were as following: age of less than 18 years, pregnancy, history of type 1 DM, gestational diabetes mellitus, malignancy, participation in an interventional study or hospitalization in past 3 months. Considering a prevalence of 50% for poor glycemic control in T2DM patients and with a confidence level of 95%, at least 384 sample size was considered [1]. Sampling was done in a six-month period and with convenience method.
- α
0.05
- d
0.05
- p
0.5
- Z1-a/2
1.96
Data gathering
All patients underwent a face-to-face interview and all data were included in a two-part questionnaire. The two parts included demographic data and data related to DM. Demographic data included age, gender, occupation, education, marital status, family’s monthly income, number of family members, living place in the past year and insurance status. Family’s monthly income was categorized in three levels based on self-reporting including low, medium and high income with less than ten, ten to fifty and more than fifty million Iranian Rial, respectively.
Data related to diabetes disease included duration of disease, type of treatment, family history of DM, health care access during the past year, history of concomitant diseases such as hypertension and dyslipidemia, history of smoking (at least one yarn daily in the past year), being on a diet (at least 3 days a week), attending diabetes education classes, regular physical activity (at least 30 min a day for 3 days a week), access to glucometer device, patient’s awareness about glucometer principles which is checking of fasting and 2 h post prandial (2HPP) blood glucose, Self-Monitoring of Blood Glucose (SMBG), patient’s awareness of ADA 2017 criteria, number of laboratory glycemic tests in the past year, awareness of patients about HbA1c status in the past year, patient’s satisfaction of current treatment, and history of DM complications. Hypertension was defined as systolic blood pressure more than 139 mmHg or diastolic blood pressure more than 89 mmHg or history of antihypertensive drugs usage. Dyslipidemia was defined as total cholesterol level greater than 200 mg/dl or triglyceride level greater than 150 mg/dl [6].
Medication adherence was determined by Morisky eight-item Medication Adherence Scale (MMAS-8) Questionnaire and Interpretation Key. MMAS-8 is self-reporting questionnaire with binary scoring in the first seven questions. The 8th is a 5-point Liker scoring item but it also contributes a score between zero and one in 0.25-point increments. The score of eight questions shows the Morisky adherence score. The patient’s adherence to treatment is divided into three categories of low adherence (score above two), moderate adherence (score one or two) and high adherence (score 0) [12, 13].
Reliable SMBG was defined as measuring the blood glucose with glucometer at least once a day for 5 days a week. ADA 2017 criteria for glycemic target for nonpregnant adults is fasting blood glucose between 80 to 130 mg/dl, 2HPP lower than 180 mg/dl and HbA1c lower than 7% [14]. DM complications which were included in the questionnaire were proteinuria and renal failure (based on laboratory data), retinopathy (based on the report of fundoscopy done by an expert in the past year), neuropathy (based on history and physical examination), diabetic foot ulcer (history of ulcer that required treatment), cardiovascular and cerebrovascular diseases (based on presence of related symptoms and result of investigations conducted by cardiologist over past year), peripheral vascular diseases (based on examination of peripheral pulses).
Weight, height, waist circumference and systolic (SBP) and diastolic blood pressure (DBP) were measured in the second step after completing the questionnaire.
Finally, Enzymatic Glucose Oxidase method was used to measure serum level of fasting plasma glucose, HbA1c, Triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), blood urea nitrogen (BUN), Creatinine (Cr), thyroid stimulating hormone (TSH), aspartate transaminase (AST) and alanine transaminase (ALT) with coefficient of variation (CV) of less than 5% in all patients.
At the end, glycemic control status was divided into four classes including very good (HbA1c < 7%), good (HbA1c = 7–7.9%), poor (HbA1c = 8–9.9%) and extremely bad (HbA1c ≥ 10%). Very good and good glycemic control status were defined as desirable. Poor and extremely bad glycemic control status were defined as undesirable.
Statistical analysis
All data were analyzed with SPSS (Version 22.0, SPSS Inc., Chicago, IL, USA). Quantitative variables were described using the mean ± standard deviation of the data, and qualitative variables were described using the frequency and percentage of the data. Student’s paired t-test, One Way ANOVA and Chi-square test were applied to analyze the variables. Multivariate linear regression was applied to show the independent effect of variables related to change in HbA1c level. P < 0.05 was considered statistically significant.
Results
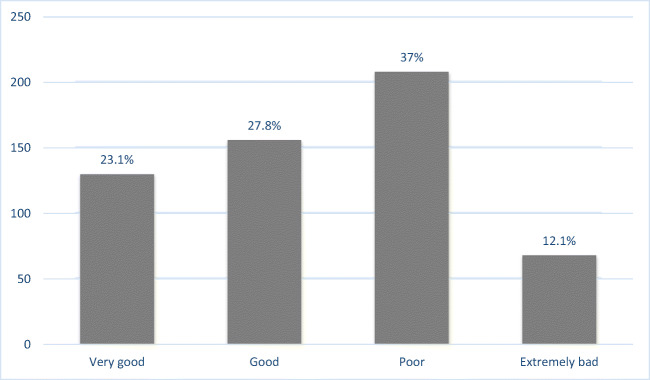
The present study included 562 patients of whom 64.4% were female. The mean age was 56.06 ± 10.38 years (ranging from 26 to 86). Demographic data for qualitative variables are shown in Table 1. Majority of patients were married, housewife, living in urban area, with middle school education, low income and had health insurance coverage. Frequencies of very good, good, poor and extremely bad glycemic control were 130 (23.1%), 156 (27.8%), 208 (37%) and 68 (12.1%), respectively. (Fig. 1) Glycemic control status had significant relationship with level of education (p < .01) and occupation (p = .04). But glycemic control status had no significant relationship with gender, marital status, level of income, insurance status and living place (p > .05).
Table 1.
Demographic Data for Qualitative Variables
| Variables | Frequency (percent) | |
|---|---|---|
| Gender | Male | 200 (35.6) |
| Female | 362 (64.4) | |
| Occupation | Merchant | 120 (21.4) |
| Employed | 46 (8.2) | |
| Retired | 80 (14.2) | |
| Housewife | 310 (55.2) | |
| Unemployed | 4 (0.7) | |
| Income | Low | 290 (51.6) |
| Intermediate | 251 (44.7) | |
| High | 1 (0.1) | |
| Education | Illiterate | 84 (15) |
| Middle School | 264 (47) | |
| Diploma | 166 (29.5) | |
| Bachelor | 40 (7.1) | |
| Master and Higher | 8 (1.4) | |
| Marital Status | Single | 28 (5) |
| Married | 472 (84) | |
| Widowed | 18 (3.2) | |
| Divorced | 44 (7.8) | |
| Living Place | Urban | 508 (90.4) |
| Rural | 52 (9.3) | |
| Insurance Coverage | Yes | 510 (90.7) |
| No | 46 (8.2) | |
Fig. 1.
Glycemic Control Status among the Studied Population
Anthropometric measurements and demographic data for quantitative variables are shown in Table 2. Age, weight, BMI, systolic blood pressure, waist circumstance, duration of DM and number of family members had no significant difference in different groups of glycemic control status (p > .05).
Table 2.
Anthropometric Measurements and Demographic Data for Quantitative Variables
| Variables | Mean ± SD |
|---|---|
| Age | 56 ± 1.3 |
| Weight (kg) | 74.7 ± 12.9 |
| BMI (kg/m2) | 28.1 ± 4.6 |
| SBP (mmHg) | 126.9 ± 18.5 |
| Waist Circumference (cm) | 98.5 ± 12 |
| Duration of DM (year) | 8.9 ± 7.1 |
| Number of Family Members | 3.5 ± 1.5 |
cm: centimeter; kg: kilogram; BMI: body mass index; kg/m2: kilogram per square meter; SBP: systolic blood pressure; DM: diabetes mellitus; SD: standard deviation
Investigation of treatment type showed that 374 (66.5%), 88 (15.7%) and 60 (10.7%) patients were treated with oral medications, insulin or the combination of both, respectively and 28 patients (5%) did not receive any of these treatments. Very good glycemic control status was significantly higher in patients taking oral medications (74 patients, 56.9%) (p < .01).
Four hundred thirty patients (76.5%) reported positive family history of DM among whom 342 (79.5%) had history in first-degree relatives while 56 (13%) had history in second-degree relatives. Positive family history of diabetes had no significant relationship with glycemic control status (p > .05).
One hundred thirty-six patients (24.2%) had adequate physical activity but there was no significant relationship between physical activity and glycemic control status (p > .05). One hundred sixteen patients (20.6%) attended the diabetes training classes but there was no significant relationship between training and glycemic control status (p > .05).
Annual plasma glucose (PG) checking was conducted thrice in 376 patients (66.9%), twice in 138 patients (24.6%) and once in 48 patients (8.5%). Number of PG checking had no significant relationship with glycemic control status (p > .05).
In addition, 282 patients (50.2%) were aware of their HbA1c level in past year, but awareness of HbA1c had no significant relationship with glycemic control status (p > .05).
In our study, 130 patients (23.1%) were without concomitant diseases, 104 (18.5%) had hypertension, 126 (22.4%) had dyslipidemia and 200 patients (35.6%) had both. Presence of concomitant diseases had no significant relationship with glycemic control status (p > .05).
Five hundred two patients (89.3%) were nonsmoker, 54 patients (9.6%) smoked less than thirty yarn a day and two patients (0.3%) smoked at least thirty yarn a day but smoking had no significant relationship with glycemic control status (p > .05).
Two hundred ten (37.4%), 190 (33.8%), 58 (10.3%), 80 (14.2%) and 24 (4.3%) patients were satisfied, relatively satisfied, indifferent, relatively dissatisfied and dissatisfied with current treatment method, respectively. Seventy-six patients (36.2%) of satisfied group had very good glycemic control and the relationship was statistically significant (p < .01).
The most common microvascular complications were neuropathy (38.1%), retinopathy (21.4%), proteinuria (14.6%) and nephropathy (3.2%). Moreover, 22.7% of patients had no microvascular complication. The most common macrovascular complications were cardiovascular disease (11.7%), diabetic foot ulcer (7.5%), cerebrovascular disease (1.4%) and peripheral vessels disease (0.7%). However, 78.7% of patients had no macrovascular complication. Presence of complications had no significant relationship with glycemic control status (p > .05). The data related to DM is included in Table 3. The mean score of MMAS-8 in our study was high which shows low adherence to treatment. However, there was no significant relationship between medical adherence and glycemic control status.
Table 3.
Variables Related to Diabetes Mellitus and their Relation with Glycemic Control Status
| Variable | Total (percent) | Glycemic Control Status Frequency (percentage) | p value* | ||||
|---|---|---|---|---|---|---|---|
| Desirable | Undesirable | ||||||
| Very Good | Good | Poor | extremely Bad | ||||
| Physicians Access | GP | 276 (49.1) | 44(15.9) | 98(35.5) | 88(31.9) | 46(16.7) | 0.00 |
| Internist | 78 (13.9) | 6(7.7) | 30(38.5) | 20(25.6) | 22(28.2) | 0.44 | |
| Other Specialists | 48 (8.5) | 8(16.7) | 20(41.6) | 8(16.7) | 12(25) | 0.30 | |
| Endocrinologist | 146 (26) | 8(5.5) | 54(37) | 40(27.4) | 44(30.1) | 0.01 | |
| Nutritional Consultant | 178 (31.7) | 8(4.5) | 48(27) | 62(34.8) | 60(33.7) | 0.00 | |
| Diet Adherence | 160 (28.5) | 4(2.5) | 46(28.8) | 58(36.2) | 52(32.5) | 0.00 | |
| Awareness of ADA Criteria | 182 (32.4) | 10(5.5) | 56(30.7) | 68(37.4) | 48(26.4) | 0.00 | |
| Access to Glucometer | 442 (78.6) | 42(9.5) | 164(37.1) | 140(31.7) | 96(21.7) | 0.00 | |
| Awareness of Glucometer Principles | 404 (71.9) | 28(6.9) | 160(39.6) | 124(30.7) | 92(22.8) | 0.00 | |
| SMBG | 294 (52.3) | 20(6.8) | 114(38.8) | 92(31.3) | 68(23.1) | 0.001 | |
| Medication Adherence | 6.8 ± 1.3 | 7.1 ± 1.5 | 6.6 ± 1.3 | 6.8 ± 1.2 | 6.7 ± 1.3 | 0.05 | |
| DBP | 78.9 ± 10.5 | 82.1 ± 12.6 | 79.5 ± 9.5 | 77.9 ± 11 | 77.5 ± 10.3 | 0.01 | |
| Laboratory Data | FPG | 171.9 ± 67.4 | 255.4 ± 74.1 | 186.8 ± 65.5 | 155.3 ± 43.9 | 124.3 ± 32.6 | 0.00 |
| TC | 184.5 ± 45.3 | 201.9 ± 56 | 189.2 ± 45 | 181.6 ± 41.3 | 171.5 ± 40.5 | 0.00 | |
| TG | 179.9 ± 89.1 | 210 ± 144.7 | 175.1 ± 59.7 | 187.5 ± 95.4 | 163 ± 80.2 | 0.00 | |
| AST | 25.9 ± 16.1 | 24.7 ± 10.7 | 23.8 ± 8.7 | 26 ± 8.2 | 29.3 ± 28.1 | 0.04 | |
*p value refers to the relation of each variable with glycemic control status
ADA: American Diabetic Association; SMBG: Self-Monitoring of Blood Glucose; DBP: Diastolic blood pressure; GP: General practitioner; FPG: Fasting plasma glucose; TC: Total cholesterol; TG: Triglycerides; AST: Aspartate transaminase
Our study showed that the history of nutritional counseling, adherence to diet, awareness of ADA criteria, access to glucometer, awareness of glucometer principles, access to physicians, type of treatment, satisfaction of treatment and using SMBG had significant relationship with glycemic control status (p < .05).
In this study, 14 patients did not visit a physician to control their glycemic status of whom 57.1% had undesirable glycemic control. The number of patients visiting general practitioners, internists, endocrinologists and other specialists were 276, 78, 146 and 48, respectively. There was significantly more variation on glycemic control status of patients visiting general practitioners (p < .01). The same is true with the patients visiting endocrinologists (p = .01). On the other hand, general practitioners and endocrinologists had the most efficient role for a very good glycemic control (p < .01). In patients with good (p = .02) and extremely bad (p = .01) glycemic control, general practitioners also had the most effective role. However, different physicians had no significant role in poor glycemic control status (p > .05). (Table 3).
The results also showed that DBP was significantly lower in patients with desirable glycemic control (p = .01).
Laboratory parameters like AST, TG, TC and FPG level had significant relationship with glycemic control status. FPG and TC levels were significantly lower in patients with desirable glycemic control. On the other hand, TG and AST levels were significantly higher in patients with very good glycemic control status (p < .05).
However, the mean of HDL-c, LDL-c, BUN, Cr, ALT and TSH level had no significant relationship with the glycemic control status (p > .05).
In this study, linear regression test was used to determine the best predictor of HbA1c level. The result showed that HbA1c had significant relationship with FPG (p < .01) and increasing one standard deviation in FPG can increase 0.014% in the level of HbA1c.
Discussion
This study investigated the glycemic control status, complications and relevant factors among T2DM patients. The results indicated that most of the patients had poor glycemic control status. The most common complication in our study was microvascular type especially diabetic neuropathy. The results also showed that various variables had significant role in glycemic control including education level, occupation, access to physicians, history of nutritional counselling and adherence to diet, awareness of ADA criteria, access to glucometer and awareness of glucometer principles, using SMBG, type of treatment, satisfaction of the treatment method, DBP and the level of FPG, TG, TC and AST.
A review conducted by Noshad et al. (2015) on monitoring the glycemic control status of patients with DM showed that very good glycemic control in the diabetes clinics of Tehran with 865 and in Mashhad with 752 patients were 56% and 25%, respectively [10]. Another study conducted by Amirkhizi et al. (2018) among 348 patients with T2DM in Iran found that the prevalence of very good glycemic control was 45% [15]. Our study showed a 23.1% prevalence for very good glycemic control. The difference between the results of our study and the aforementioned studies may be due to selection bias and also the difference in the definition of glycemic control status.
Other studies in Jordan, Kuwait, Bosnia, Nepal, Lebanon, Malaysia and Italy reported 23 to 43% of the prevalence of very good glycemic control, which was roughly the same as our study. Most studies assumed the diversity of glycemic control status to be due to the difference in sample size, duration of DM, and the degree of medication adherence [3, 4, 9, 16–19].
Extremely bad glycemic control status in our study was similar to the study of Adham et al. (2010). They reported 16.1% prevalence of extremely bad glycemic control among 1000 patients with T2DM [1]. In our study, the level of FPG was the most important predictor of HbA1c level where increasing of FPG was accompanied by increasing of HbA1c. Similar to our results, some studies showed that the level of FPG was related to the glycemic control status [3, 9, 19, 20]. However, other studies showed significant relationship between the level of lipids and glycemic control status [3, 8, 9, 16, 17, 20].
Duration of DM in our study was 8.9 ± 7.1 years. The duration was lower in patients with very good glycemic control but the relationship was not statistically significant. However, in many studies, glycemic control status had significant relationship with duration of DM [1, 2, 16–18, 20]. This difference in results may be due to the patients’ wrong reports on the time of DM diagnosis.
Similar to our study, some studies also found significant relationship between glycemic control status and level of education [21], diet and medication adherence, blood glucose monitoring at home and physical activity [16, 17].
In our study, most of the patients had at least one of the risk factors such as hypertension or dyslipidemia. In various studies the prevalence of hypertension had wide range from 20% in newly diagnosed patients to 50% in others [6, 22, 23]. On the other hand, some studies reported the prevalence of hypertension and dyslipidemia ranged from 60 to 80% [1, 4, 16, 17, 24].
In many studies, microvascular complication especially neuropathy was the most common complication in patients with DM, which was consistent to our results [8, 22]. The prevalence of complications had wide range in different studies. In a study on Asian patients with DM conducted by Dhillon et al. (2019), prevalence of neuropathy, retinopathy and cardiovascular complications in T2DM were 61%, 35% and 12.7%, respectively [11]. Sobhani et al. (2014) by reviewing 21 studies in Iran, reported the prevalence of peripheral neuropathy between 15 to 85% [25].While, Harzallah et al. (2006) studied 370 patients with newly diagnosed DM in Tunisia and showed that the most common complication was nephropathy (29%) and then neuropathy (25.1%) [23].
Noshad estimated prevalence of diabetic retinopathy from 30 to 40% and diabetic nephropathy from 16 to 87% [10]. In the study of Adham, one third of patients had diabetic retinopathy [1]. Javadi et al. (2009) reported that among 634 examined patients with DM, 37% had diabetic retinopathy [7]. Prevalence of diabetic foot ulcers in our study was 7 %. Prevalence of ulcer was 4 % in investigation by Alavi et al. (2007) on 247 patients with DM [26]. Many studies interrelated the wide range of complications’ prevalence to the differences of sample size, type of diabetes, duration of diabetes and the diagnostic criteria used for complications [10, 11].
Our results showed that patients who had very good glycemic control were mostly visited by general practitioners and endocrinologists. Azizi et al. (2009) investigated the role of various physicians in management of diabetic patients and showed that general practitioners had higher role than endocrinologists. They concluded that this may be due to inadequate number of endocrinologists in Iran. They also estimated that population of Iran may reach to 90 million by 2021 and 790 endocrinologists is needed while only 370 will be available [27].
Noshad referred the lower glycemic control of patients visited by specialists and endocrinologists to overcrowded clinic that leads to decrease of time spent for examination and face-to-face patient-physician communication [10].
Our study investigated the effect of various factors among DM patients and showed that some of those can predict the glycemic control status.
Limitations
In our study, we did not use the level of FPG or 2HPP as a criteria of glycemic control status and did not investigate more details of T2DM complications such as the information about their severity and time of diagnosis. Although we investigated the medication adherence, attending the diabetic training classes and awareness of ADA criteria, but we did not consider psychologic status of patients and awareness of family members as two effective factors on glycemic control status. Investigation of family history, history of nutritional counseling, diet adherence and physical activity status were based on patient’s self-report, which could increase the bias of the results. This study was conducted in referral and specialized university-hospitals. This may lead to selection bias.
It is better to carry out a large sample size in different populations and also to include other effective variables such as patients’ psychological status and family members’ awareness in future studies which can increase the external validity of the results.
Conclusion
In the present study, most of the patients with T2DM had poor glycemic control status. Given the importance of glycemic status in occurrence of diabetes complications, it is important to know the associated factors. According to the results of our study, factors including level of education, occupation, access to physicians, history of nutritional counseling or diet adherence, awareness of ADA criteria, access to glucometer, awareness of glucometer principles, using SMBG, type of treatment, satisfaction with current treatment, DBP and level of FPG, TG, TC are associated with glycemic control status. Our results also showed that the level of AST and FPG can be a useful predictor of glycemic control status.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sepideh Babaniamansour, Email: sepideh_babania@yahoo.com.
Ehsan Aliniagerdroudbari, Email: eag1368@yahoo.com.
Mahtab Niroomand, Email: mahtabniroomand@yahoo.com.
References
- 1.Adham M, Froelicher ES, Batieha A, Ajlouni K. Glycaemic control and its associated factors in type 2 diabetic patients in Amman, Jordan. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2010;16(7):732–739. [PubMed] [Google Scholar]
- 2.Madmoli M, Madmoli Y, Khodadadi M, Samsamipour M. Some factors affecting quality of life in patients with diabetes: a systematic review. Annals of Microbiology and Infectious Diseases. 2019;2(1):26–30. [Google Scholar]
- 3.Abdullah A, Alkandari A, Longenecker JC, Devarajan S, Alkhatib A, Al-Wotayan R, et al. Glycemic control in Kuwaiti diabetes patients treated with glucose-lowering medication. Primary care diabetes. 2020;14:311–316. doi: 10.1016/j.pcd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Mroueh L, Ayoub D, El-Hajj M, Awada S, Rachidi S, Zein S, et al. Evaluation of medication adherence among Lebanese diabetic patients. Pharmacy practice. 2018;16(4):1291. doi: 10.18549/PharmPract.2018.04.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, Saadat M, Esfahani EN, Ganji M, Noshad S, Khajeh E, Ghajar A, Heidari B, Afarideh M, Mechanick JI, Ismail-Beigi F. Diabetes in Iran: Prospective analysis from first Nationwide diabetes report of National Program for prevention and control of diabetes (NPPCD-2016) Sci Rep. 2017;7(1):13461. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heydari I, Radi V, Razmjou S, Amiri A. Chronic complications of diabetes mellitus in newly diagnosed patients. International Journal of Diabetes Mellitus. 2010;2(1):61–63. doi: 10.1016/j.ijdm.2009.08.001. [DOI] [Google Scholar]
- 7.Javadi MA, Katibeh M, Rafati N, Dehghan MH, Zayeri F, Yaseri M, Sehat M, Ahmadieh H. Prevalence of diabetic retinopathy in Tehran province: a population-based study. BMC Ophthalmol. 2009;9(1):12. doi: 10.1186/1471-2415-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Li T, Liu J, Wu X, Wang H, Li X, Xu E, Chen Q, Yan C, Li H, Xu Y, Wei W. Association between glycosylated hemoglobin A1c and bone biochemical markers in type 2 diabetic postmenopausal women: a cross-sectional study. BMC Endocr Disord. 2019;19(1):31. doi: 10.1186/s12902-019-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babic N, Valjevac A, Zaciragic A, Avdagic N, Zukic S, Hasic S. The Triglyceride/HDL Ratio and Triglyceride Glucose Index as Predictors of Glycemic Control in Patients with Diabetes Mellitus Type 2. Medical archives (Sarajevo, Bosnia and Herzegovina) 2019;73(3):163–168. doi: 10.5455/medarh.2019.73.163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noshad S, Afarideh M, Heidari B, Mechanick JI, Esteghamati A. Diabetes Care in Iran: where we stand and where we are headed. Annals of global health. 2015;81(6):839–850. doi: 10.1016/j.aogh.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon H, Nordin RB, Ramadas A. Quality of life and associated factors among primary care asian patients with type 2 diabetes mellitus. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193561. [DOI] [PMC free article] [PubMed]
- 12.Gatti ME, Jacobson KL, Gazmararian JA, Schmotzer B, Kripalani S. Relationships between beliefs about medications and adherence. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2009;66(7):657–664. doi: 10.2146/ajhp080064. [DOI] [PubMed] [Google Scholar]
- 13.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of clinical hypertension (Greenwich, Conn) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Glycemic Targets: Standards of Medical Care in Diabetes-2019 Diabetes Care. 2019;42(Suppl 1):S61–s70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 15.Jafarian-Amirkhizi A, Sarayani A, Gholami K, Taghizadeh-Ghehi M, Heidari K, Jafarzadeh-Kohneloo A, Morisky DE. Adherence to medications, self-care activity, and HbA1c status among patients with type 2 diabetes living in an urban area of Iran. Journal of diabetes and metabolic disorders. 2018;17(2):165–172. doi: 10.1007/s40200-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complicat. 2010;24(2):84–89. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Pokhrel S, Shrestha S, Timilsina A, Sapkota M, Bhatt MP, Pardhe BD. Self-care adherence and barriers to good Glycaemic control in Nepalese type 2 diabetes mellitus patients: a hospital-based cross-sectional study. J Multidiscip Healthc. 2019;12:817–826. doi: 10.2147/jmdh.s216842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad NS, Islahudin F, Paraidathathu T. Factors associated with good glycemic control among patients with type 2 diabetes mellitus. Journal of Diabetes Investigation. 2014;5(5):563–569. doi: 10.1111/jdi.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palella E, Cimino R, Pullano SA, Fiorillo AS, Gulletta E, Brunetti A et al. Laboratory parameters of hemostasis, adhesion molecules, and inflammation in type 2 diabetes mellitus: correlation with glycemic control. Int J Environ Res Public Health. 2020;17(1). doi:10.3390/ijerph17010300. [DOI] [PMC free article] [PubMed]
- 20.Zhu H-T, Yu M, Hu H, He Q-F, Pan J, Hu R-Y. Factors associated with glycemic control in community-dwelling elderly individuals with type 2 diabetes mellitus in Zhejiang, China: a cross-sectional study. BMC Endocr Disord. 2019;19(1):57. doi: 10.1186/s12902-019-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassahun T, Eshetie T, Gesesew H. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: a cross-sectional survey in Ethiopia. BMC research notes. 2016;9(1):78. doi: 10.1186/s13104-016-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weerasuriya N, Siribaddana S, Dissanayake A, Subasinghe Z, Wariyapola D, Fernando DJ. Long-term complications in newly diagnosed Sri Lankan patients with type 2 diabetes mellitus. QJM : monthly journal of the Association of Physicians. 1998;91(6):439–443. doi: 10.1093/qjmed/91.6.439. [DOI] [PubMed] [Google Scholar]
- 23.Harzallah F, Ncibi N, Alberti H, Ben Brahim A, Smadhi H, Kanoun F, Slimane H. Clinical and metabolic characteristics of newly diagnosed diabetes patients: experience of a university hospital in Tunis. Diabetes Metab. 2006;32(6):632–635. doi: 10.1016/s1262-3636(07)70319-1. [DOI] [PubMed] [Google Scholar]
- 24.Albarakat M, Guzu A. Prevalence of type 2 diabetes and their complications among home health care patients at Al-Kharj military industries corporation hospital. Journal of family medicine and primary care. 2019;8(10):3303–3312. doi: 10.4103/jfmpc.jfmpc_634_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobhani S, Asayesh H, Sharifi F, Djalalinia S, Baradaran HR, Arzaghi SM, Mansourian M, Rezapoor A, Ansari H, Masoud MP, Qorbani M. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. Journal of Diabetes & Metabolic Disorders. 2014;13(1):97. doi: 10.1186/s40200-014-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alavi A, Sanjari M, Haghdoost A, Sibbald R. Diabetic foot ulcers in Kerman, Iran: prospective, descriptive review 123. Wound Repair and Regeneration. 2007;15(2).
- 27.Azizi F. Sub-specialty training of endocrinologyin the Islamic Republic of Iran: estimating the need for endocrinologist training. J Med Educ 2009;12.