Abstract
Purpose
Proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors treatment induce large reductions in low-density lipoprotein cholesterol (LDLc) and major cardiovascular events. Clinical trials might have been underpowered to test the effect of PSCK9 inhibitors treatment on myocardial infarction and stroke, two of the most relevant cardiovascular events, since all analyzed a combined endpoint.
Methods
we performed a meta-analysis, with currently available studies involving PCSK9 inhibitors and event rate adjudication, with the aim of assessing treatment effects on myocardial infarction and stroke.
Results
We included 81,700 patients, 41,979 treated with a PSCK9 inhibitors: 17,244 with evolocumab; 13,720 with bococizumab and 11,015 with alirocumab. A total of 1,319 cases of myocardial infarctions were registered in the treatment group vs. 1,608 in controls, resulting in 19.0% reduction associated with PCSK9 treatment (RR: 0.81, 95% CI 0.76–0.87). Similarly, PCSK9 inhibitors treatment resulted in a 25% reduction of stroke (RR: 0.75, 95% CI 0.65–0.85) when all studies were analyzed together and the statistically significant heterogeneity was not observed in the analysis restricted to end-point based clinical trials. PCSK9 inhibitors treatment had no effect on mortality (RR: 0.95, 95% CI 0.86–1.04).
Conclusions
PCSK9 inhibitors reduce the incidence of myocardial infarction by 19% and stroke by 25%.
Keywords: PCSK9, LDLc, Acute coronary syndrome, Stroke, Meta-analysis
Introduction
Low density lipoprotein cholesterol (LDLc) is the leading effector of atherosclerosis, the underlying cause of coronary heart disease and myocardial infarction [1]. LDLc reduction has been clearly associated to lower incidence of CHD and, therefore, is considered a primary target in cardiovascular prevention [2, 3]. The proprotein convertase subtilisin/kexin 9 (PCSK9) is a circulating protease that binds to the low-density lipoprotein (LDL) receptor leading to its intracellular degradation what reduces the activity of the receptor and promotes higher levels of serum LDLc [4, 3]. Thereafter, monoclonal antibodies that inhibit PCSK9 were developed and clinical trials have demonstrated that PCSK9 inhibitors treatment reduce LDLc [5, 6] and, also, major cardiovascular events (MACE) [7–10]. A recent metanalysis verified the effect of alirocumab or evolocumab on MACE and mortality[10].
Primary and secondary end-point of clinical trials testing the effect of PCSK9 inhibitors were based on composite events (including cardiovascular death, myocardial infarction, stroke and revascularization) [7–9]. Non-fatal MACE have a high incidence in stable patients with chronic coronary heart disease [11, 12] but also after a myocardial infarction [13–15]; interestingly, non-fatal MACE preclude incidence of death in the post-myocardial infarction setting [15]. Since the specific effect of PCSK9 inhibitors on myocardial infarction or stroke was not analyzed in clinical trials [7–9] or metanalyses [10],we performed a meta-analysis to assess in detail the effect of PCSK9 inhibitors treatment on these events.
Methods
We performed a systematic search (using PUBMED, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar), without language restriction, for randomized trials using the Medical Subject Headings terms “PCSK9”, “PCSK9 inhibitor”, “Evolocumab”, “Alirocumab” or “Bococizumab”. Since the objective of our study was assessing the incidence of myocardial infarction and stroke, we excluded trials with < 3 months of follow-up because event rate was very low, or none, and major cardiovascular events were not defined by protocol or were not adjudicated and validate by a specific board. This confirmed that 6 randomized clinical trials were the evidence supporting the mid-term effect of PCSK9 inhibitors on cardiovascular events and, thereafter, we performed a metanalysis including the following 6 clinical trials: the OSLER I-II trials [5], the ODYSSEY Long Term [6], the FOURIER trial [7], the GLAGOV trial [16], the SPIRE I-II [8] and the ODYSSEY Outcomes trial [9]. The primary outcomes for the metanalysis were myocardial infarction and stroke incidence. We performed the same analysis with all 6 studies and also with only the 3 pivotal endpoint-based randomized clinical trials (EBCT) [7–9]. Information on sample size, treatment type and outcomes were collected from the published papers. For the eligible studies, two authors independently abstracted data into a standardized form; discrepancies in data extraction and quality assessment were resolved by discussion or consensus with a third author.
Statistical analyses
We performed an intention-to-treat meta-analysis in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [17]. Relative risk reductions and percent incidences were used. Myocardial infarction and stroke incidence were reported in the 6 trials. The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic and potential sources of heterogeneity between trials were tested by meta-regression analyses. A random effects model was selected when the significant heterogeneity was observed; otherwise, a fixed-effect model was applied. The identification of potential sources of heterogeneity between trials was tested by meta-regression analyses. The Egger test was used to assess the small-study effects [18]. All analyses were performed using STATA 14.3 (StataCorp. 2009. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
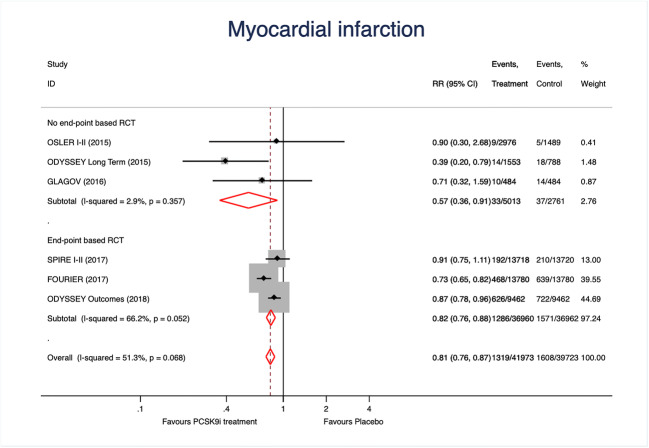
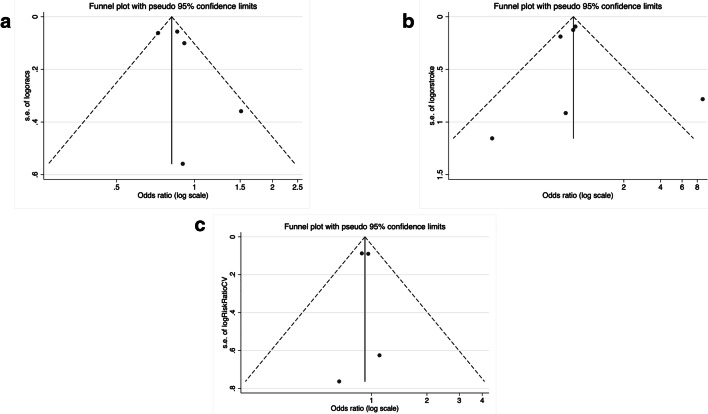
We included 81,7000 patients, 41,979 treated with a PSCK9 inhibitors: 17,244 with evolocumab; 13,720 with bococizumab and 11,015 with alirocumab (Table 1). A total of 1,319 cases of myocardial infarction were registered in the treatment group vs. 1,608 in controls, resulting in 19.0% reduction associated with PCSK9 treatment (Fig. 1). Results were similar in both types of studies although a non-significant heterogeneity was detected (p = 0.052). Metaregression analyses did not demonstrate the implication of the study (p = 0.45), study drugs (p = 0.26), age (p = 0.89), hypertension (p = 0.81) or diabetes (p = 0.81) on such result. The funnel plot (Fig. 2A) and the Egger test (p = 0.38) excluded relevant bias or small-study effects.
Table 1.
Clinical features and number of myocardial infarction (MI) and strokes reported in each clinical trial
| Trial | Study Drug | Number of PCSK treated | Number of controls | Age | DM (%) | HT (%) | Smoking (%) | Number of MI | Number of strokes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treated | Controls | Treated | Controls | ||||||||
| OSLER I-II | Evolocumab | 2976 | 1489 | 58.1 | 13.0 | 52 | 15.5 | 9 | 5 | 1 | 3 |
| ODYSSEY Long Term | Alirocumab | 1553 | 788 | 60.4 | 34.5 | - | 20.5 | 14 | 18 | 9 | 2 |
| Glagov | Evolocumab | 484 | 484 | 59.8 | 20.5 | 83 | 23.3 | 1 | 14 | 2 | 3 |
| FOURIER | Evolocumab | 13784 | 13780 | 62.5 | 36.5 | 80.0 | 28.3 | 468 | 639 | 207 | 262 |
| SPIRE I-II | Bococizumab | 13720 | 13718 | 63.3 | 47.5 | 80.8 | 24.5 | 192 | 210 | 45 | 75 |
| ODYSSEY | Alirocumab | 9462 | 9462 | 58.5 | 29.0 | 65.0 | 24.0 | 626 | 722 | 111 | 152 |
| Total: | 41,979 | 39,721 | 60.5 | 32.1 | 69.5 | 22.6 | 1,319 | 1,608 | 375 | 497 | |
DM; diabetes mellitus; HT: hypertension
Fig. 1.
Forest plots showing the pooled relative risk (RR) with 95% confidence intervals for myocardial infarction incidence
Fig. 2.
Funnel plots for myocardial infarction (A), stroke (B) and all-cause mortality (C)
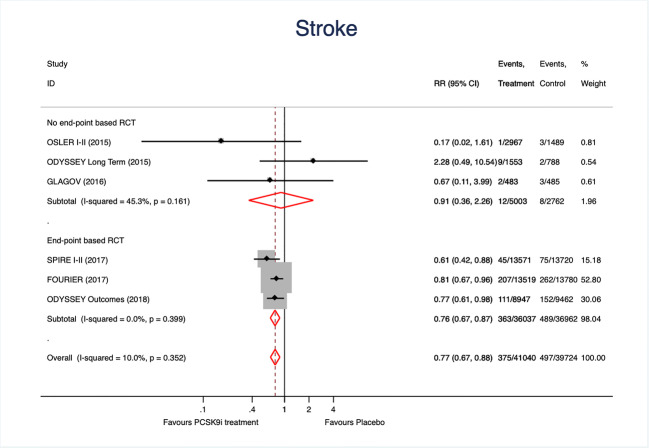
A total of 872 strokes were registered. PCSK9 inhibitors treatment resulted in a 25% reduction of stroke when all studies were analyzed together (Fig. 3). The effect was were mainly driven by the results of EBCT and no significant heterogeneity was observed. Once again, the funnel plot (Fig. 2B) and the Egger test (p = 0.74) excluded relevant bias or small-study effects.
Fig. 3.
Forest plots showing the pooled relative risk (RR) with 95% confidence intervals for stroke incidence
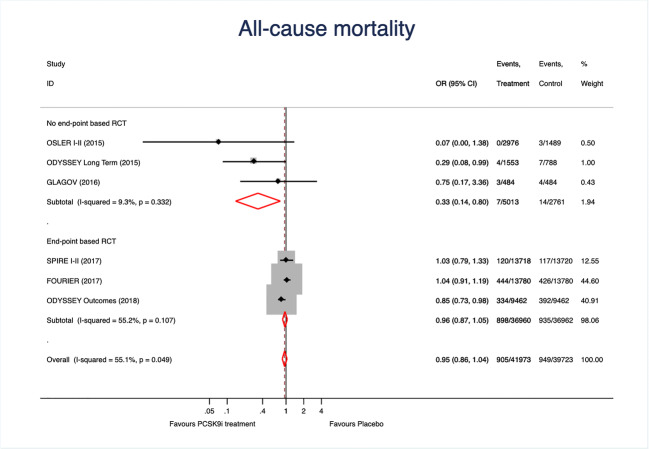
We finally tested the effect of PCSK9 inhibitors on mortality. As shown in Fig. 4, treatment effect was neutral for mortality and significant heterogeneicity was observed in EBCT; the funnel plot (Fig. 2C) and the Egger test (p = 0.22) excluded relevant bias or small-study effects. The study drug (p < 0.001) and the type of study (p < 0.001) were the main sources of heterogenicity.
Fig. 4.
Forest plots showing the pooled relative risk (RR) with 95% confidence intervals for mortality
Discussion
This meta-analysis supports the benefit of PCSK9 inhibitors in cardiovascular prevention by a significant and large reductions on myocardial infarction and stroke incidence. PCSK9 inhibitors decrease LDLc by > 50% with a very specific mechanism that make them very safe and effective on LDLc and MACE incidence prevention [7–9]. Moreover, the results of our meta-analysis add compelling evidence of the significant reductions on myocardial infarction and stroke.
PCSK9 inhibitors are one of the last advances in lipid-lowering drugs and have emerged very fast from phase-I trials to EBCT. The OSLER [5] and the ODYSSEY Long Term [6] were not designed to assess the effect of the study drugs on MACE but the positive results on MACE were very well received. The EBCT involving PCSK9 inhibitors were stopped when the incidence of primary and secondary endpoints were accomplished [7, 9] or when the evidence of neutralizing antibodies against bococizumab were clearly outlined [8]. These issues have two clear consequences: follow-up was shorter than most EBCT involving lipid-lowering treatments and none of the studies were statistically powered to demonstrate reductions in specific endpoints. Therefore, a meta-analysis might be pertinent and clinically relevant since a clear tendency to better outcomes was noted in most studies. A previous metanalysis [10] summarized the results of clinical trials only with evolocumab or alirocumab and our study highlights a clear and consistent protective effect of PCSK9 inhibitors on myocardial infarction and stroke. Casula, et a l[10] also included studies with very short-term follow-up, such as 8 or 12 weeks, were no events were recorded or MACE were codified as side effects with no clear methodology. We decided to include all patients treated with any PCSK9 inhibitor for > 3 months in order to provide the most reliable evidence related to PCSK9 inhibitors.
Evolocumab and alirocumab are currently being used worldwide [19–22]; bococizumab will not be available in clinical practice since its efficacy was clearly impaired by neutralizing antibodies [8] but we decide to include the results of the SPIRE program in order to assess the effect of the whole PCSK9 inhibitors; moreover, more molecules could be developed in the future. Treatment of hypercholesterolemia has been focused on targeting LDL production and intestinal reabsorption, with statins and ezetimibe respectively [2, 3]. After wide and well-supported evidence, statins are the cornerstone of LDLc reduction in primary and secondary prevention [2, 3, 23]. Nonetheless, monotherapy with statins is usually not enough for treatment target achievement [24, 25, 23] and the addition of ezetimibe has demonstrated to be effective for reduction of LDLc and cardiovascular events prevention [26]. Nonetheless, ezetimibe combined with statins is not widely used in high-risk patients. For example, the EUROASPIRE V highlighted, a part from the low rates LDLc control, that less than 10% of patients with coronary heart disease were receiving combined treatment with statin and ezetimibe [27]. In fact, despite > 75% of the patients in the FOURIER [7] and ODISSEY Outcomes [9] trials were receiving high-doses of statins less than 7% sere also on ezetimibe. PCSK9 inhibitors are a completely new, and complementary, strategy for LDLc reduction that can be used as monotherapy or combined with any other lipid-lowering drugs[28]. Combination of statin plus ezetimibe received a recommendation class I (level of evidence B) for the first time in the 2019 ESC/EAS guidelines[3], but for patients not achieving their treatment goal in secondary prevention, the addition of a PCSK9 inhibitor has recommendation class I (level of evidence A). Since treatment targets were also lowered to LDLc < 45 m/dl in patients with high-cardiovascular risk, or established cardiovascular disease, PCSK9 inhibitors seem to be most accurate combination strategy in this setting.
We performed a meta-analysis with currently available studies that constitute the cornerstone in the knowledge related to PCSK9 inhibitors effects. There is scarce evidence in the actual effect and safety of this therapy in real-world patients [19–21] as well as long-term safety or cost-efficiency according to current fees [2]. A recent study in a wide population area of Spain estimated that the overall prevalence of people suitable for PCSK9 treatment, under current indications, was < 5%; nonetheless, when in the subset of patients with coronary heart disease or familial hypercholesterolemia were analyzed it increased up to 20% and 75% respectively [29]. These estimations have also been verified in a large registry of patients with chronic stable coronary heart disease in Spain [30] and a greater population with atherosclerotic cardiovascular disease [31]. Despite these estimations, we believe that efforts should be focused on defining which patients would benefit mostly from PCSK9 inhibitors treatment, the so-call priority patients. Based on currently available evidence, patients that would obtain the most benefit from PCSK9 inhibitors treatment are those at higher cardiovascular risk, such as patients with recent myocardial infarction (< 2 years) [9], diabetes [32], multivessel disease [33] or peripheral arterial disease [34].
Our metanalysis also showed that PCSK9 inhibitors treatment did not reduce mortality. A recent metanalysis highlighted that mortality benefit can be expected only in patients with baseline LDLc > 100 mg/dl [35]. PCSK9 inhibitors are currently reimbursed in Spain, and most European countries, only for patients with LDLc > 100 mg/dl despite optimal lipid-lowering treatment and it has been recently demonstrated the effect on LDLc reduction, under such indications, is the same as clinical trials [36]. It should also be noted that inclusion criteria of the EBCT involving PCSK9 inhibitors was baseline LDLc > 70 mg/dl [7–9]. Moreover, EBCT tested the effect of PCSK9 inhibitors on top of statin-treated patients and, therefore, patients in the control group were all receiving statins and most of them on high dose s[7–9]. A recent subanalysis of the ODYSSEY Outcomes trial demonstrated that occurrence of non-fatal cardiovascular events have a great impact on prognosis and they increase 2-fold the incidence of mortality afterwards [37]; alirocumab reduced significantly major cardiovascular event rates and, thus, a relevant effect on mortality could have been observed in a longer follow-up reflecting that the analysis of long-term mortality should be analyzed with much complex methodology, taking into account intermediate events. Moreover, subsequent subanalyses of the ODYSSEY Outcomes trial [15] and the FOURIER trial[38] highlighted that PCSK9 inhibitors reduced total MACES incidence. Therefore, the long-term effect of PCSK9 inhibitors treatment, that has not been evaluated yet, will probably provide reductions on mortality as well. Meanwhile, we believe that our metanalysis provides clinically relevant information on myocardial infarction and stroke prevention by PCSK9 inhibitors.
Our study might be limited by the fact that it only included randomized clinical trials and, therefore, patients’ characteristics, medication adherence and other clinical features might not be fully representative of daily clinical practice. Nonetheless, there is scarce evidence related to PCSK9 inhibitors treatment on real-world patients [19, 20, 36] and we believe that our results can help the generalization of these therapies in very-high risk patients in whom LDLc control remains a treatment challenge [39, 27, 2, 3].
In conclusion, this meta-analysis demonstrates that PCSK9 inhibitors treatment reduces myocardial infarction by 19% and stroke by 25%. We believe that these results provide solid evidence for treating patients at high-risk, with non-controlled LDLc, with PCSK9 inhibitors.
Acknowledgements
Investigators received the support of the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) Spain, the National Network for Biomedical Research in Cardiovascular Disease.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest related to the results of this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2020;41(1):111–88. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, Jr, DePalma SM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(14):1785–822. doi: 10.1016/j.jacc.2017.07.745. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–26. doi: 10.1056/NEJMoa1614062. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 10.Casula M, Olmastroni E, Boccalari MT, Tragni E, Pirillo A, Catapano AL. Cardiovascular events with PCSK9 inhibitors: an updated meta-analysis of randomised controlled trials. Pharmacol Res. 2019;143:143–50. doi: 10.1016/j.phrs.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 12.Cordero A, Lopez-Palop R, Carrillo P, Frutos A, Bertomeu-Martinez V. Addition of antiangina drugs and recurrent cardiovascular events associated with incomplete revascularization in acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2018;71(3):217–9. doi: 10.1016/j.rec.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Manero M, Cordero A, Kreidieh O, Garcia-Acuna JM, Seijas J, Agra-Bermejo RM, et al. Proposal of a novel clinical score to predict heart failure incidence in long-term survivors of acute coronary syndromes. Int J Cardiol. 2017;243:211–5. doi: 10.1016/j.ijcard.2017.07.084. [DOI] [PubMed] [Google Scholar]
- 14.Agra Bermejo R, Cordero A, Garcia-Acuna JM, Gomez Otero I, Varela Roman A, Martinez A, et al. Determinants and prognostic impact of heart failure and left ventricular ejection fraction in acute coronary syndrome settings. Rev Esp Cardiol (Engl Ed) 2018;71(10):820–8. doi: 10.1016/j.rec.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, et al. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019 doi: 10.1016/j.jacc.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–84. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 19.Kohli M, Patel K, MacMahon Z, Ramachandran R, Crook MA, Reynolds TM, et al. Pro-protein subtilisin kexin-9 (PCSK9) inhibition in practice: lipid clinic experience in 2 contrasting UK centres. Int J Clin Pract. 2017;71(11):e13032. doi: 10.1111/ijcp.13032. [DOI] [PubMed] [Google Scholar]
- 20.Knickelbine T, Jia L, White SK, Garberich RF, Oberembt SJ, Wills S, et al. A systematic approach for successful PCSK9 inhibitor prescribing in clinical practice. J Clin Lipidol. 2019;13(2):265–71. doi: 10.1016/j.jacl.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Cordero A, Fácila L, Rodríguez-Mañero M, Gómez-Martínez MJ, Bertomeu-Martínez V, González-Juanatey JR. Initial real-world experience with PCSK-9 inhibitors in current indications for reimbursement in Spain. Revista Española de Cardiología (English Edition). 2019. 10.1016/j.rec.2019.03.008. [DOI] [PubMed]
- 22.Trankle C, Wohlford G, Buckley LF, Kadariya D, Ravindra K, Markley R, et al. Alirocumab in Acute Myocardial Infarction: Results from the Virginia Commonwealth University Alirocumab Response Trial (VCU-AlirocRT). Journal of Cardiovascular Pharmacology. 2019. [DOI] [PMC free article] [PubMed]
- 23.Thongtang N, Sitthananun C, Sriussadaporn S, Nitiyanant W. Efficacy of low- and moderate-intensity statins for achieving low- density lipoprotein cholesterol targets in Thai type 2 diabetic patients. J Diabetes Metab Disord. 2017;16(1):6. doi: 10.1186/s40200-017-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galve E, Cordero A, Cequier A, Ruiz E, Gonzalez-Juanatey JR. Degree of lipid control in patients with coronary heart disease and measures adopted by physicians. REPAR Study. Rev Esp Cardiol (Engl Ed) 2016;69(10):931–8. doi: 10.1016/j.rec.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Reiner Z, De Backer G, Fras Z, Kotseva K, Tokgözoglu L, Wood D, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries – Findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243–50. doi: 10.1016/j.atherosclerosis.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 27.De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Ž, Rydén L, et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis. 2019;285:135–46. doi: 10.1016/j.atherosclerosis.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Descamps OS, Fraass U, Dent R, März W, Gouni-Berthold I. Anti-PCSK9 antibodies for hypercholesterolaemia: overview of clinical data and implications for primary care. Int J Clin Pract. 2017;71(8):e12979. doi: 10.1111/ijcp.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamora A, Masana L, Comas-Cufi M, Plana N, Vila A, Garcia-Gil M, et al. Number of patients eligible for PCSK9 inhibitors based on real-world data from 2.5 million patients. Rev Esp Cardiol (Engl Ed) 2018;71(12):1010–7. doi: 10.1016/j.rec.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Cordero A, Fácila L, Galve E, González Juanatey JR. Estimated percentage of patients with stable coronary heart disease candidates for PCSK9 inhibitors. Rev Esp Cardiol. 2019;72(6):518–9. doi: 10.1016/j.recesp.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Cannon CP, Sanchez RJ, Klimchak AC, Khan I, Sasiela WJ, Reynolds MR, et al. Simulation of the impact of statin intolerance on the need for Ezetimibe and/or Proprotein Convertase Subtilisin/Kexin type 9 inhibitor for meeting low-density lipoprotein cholesterol goals in a population with atherosclerotic cardiovascular disease. Am J Cardiol. 2019 doi: 10.1016/j.amjcard.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–50. doi: 10.1016/S2213-8587(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 33.Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation. 2018;138(8):756–66. doi: 10.1161/CIRCULATIONAHA.118.034309. [DOI] [PubMed] [Google Scholar]
- 34.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137(4):338–50. 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed]
- 35.Khan SU, Riaz H, Rahman H, Khan MU, Khan MS, Alkhouli M, et al. Association of baseline LDL-C with total and cardiovascular mortality in patients using proprotein convertase subtilisin-kexin type 9 inhibitors: a systematic review and meta-analysis. J Clin Lipidol. 2019 doi: 10.1016/j.jacl.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordero A, Fácila L, Rodríguez-Mañero M, Gómez-Martínez MJ, Bertomeu-Martínez V, González-Juanatey JR. Initial real-world experience with PCSK-9 inhibitors in current indications for reimbursement in Spain. Rev Esp Cardiol (Engl Ed) 2019;72(11):968–70. doi: 10.1016/j.rec.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, et al. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73(4):387–96. doi: 10.1016/j.jacc.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SA, Pedersen TR, Gaciong ZA, Ceska R, Ezhov MV, Connolly DL, et al. Effect of the PCSK9 inhibitor evolocumab on total cardiovascular events in patients with cardiovascular disease: a prespecified analysis from the FOURIER trial reduction in total cardiovascular events with the PCSK9 inhibitor evolocumab in patients with cardiovascular diseasereduction in total cardiovascular events with the PCSK9 inhibitor evolocumab in patients with cardiovascular disease. JAMA Cardiol. 2019;4(7):613–9. doi: 10.1001/jamacardio.2019.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordero A, Galve E, Bertomeu-Martinez V, Bueno H, Facila L, Alegria E, et al. Trends in risk factors and treatments in patients with stable ischemic heart disease seen at cardiology clinics between 2006 and 2014. Rev Esp Cardiol (Engl Ed) 2016;69(4):401–7. doi: 10.1016/j.rec.2015.08.011. [DOI] [PubMed] [Google Scholar]