Abstract
Background
The present study designed to evaluate the Serum levels of CMPF, MASP1 and UA in pre-diabetic subjects was compared to normal subjects.
Methods
This research is a case-control study. We studied 44 pre-diabetics with 44 normal subjects and were evaluated serum concentration of CMPF, Masp1 and U.Ain both groups andfurthermore serum concentration FPG, BUN, Cr, Cho, TG, HDLc, LDLc, AST, ALT, ALP, HbA1c was examined and correlation between of CMPF, Masp1 and U.Aand other parameters was statistically analyzed.
Results
Serum levels of MASP1, CMPF, fasting plasma glucose (P < 0.001)and uric acid (P < 0.002) were significantly increased in pre-diabetic subjects. In this study, a significant difference was found between MASP1 and CMPF in pre-diabetic subjects compared to normal subjects (P=0.005, r=0.291). There was also a significant difference between serum levels of MASP1 with HbA1C (P=0.01, r=0.269).
Conclusion
Serum levels of CMPF, MASP1 and uric acid were increased in pre-diabetic subjects. These parameters can be used as a biomarker for the diagnosis of pre-diabetes.
Keywords: Diabetes mellitus, Prediabetes, MASP1, CMPF, Uric acid, HbA1C, FBS
Introduction
Diabetes, the correct term being diabetes mellitus (DM), it is a common disease of this century that occurs worldwide but it is more common in developed countries [1].Prediabetes mellitus (PDM) is defined as an abnormal blood glucose condition.Prediabetes is a condition between normal and diabetes [2].The American Diabetes Association (ADA) recommends the use of the word pre-diabetes in 2015 in the form of fasting plasma glucose (FPS) 100–125 mg/dl, 2-h plasma glucose (2-HPG) 140–199 mg/dl (IGT) and glycosylated hemoglobin (HbA1c) defined 5.7–6.4%. Evidences propose a relationship between prediabetes and complications of diabetes like early nephropathy, early retinopathy and risk of microvascular disease and small fiber neuropathy [3].
The number of people with pre-diabetes is growing dramatically, reaching 482 million by 2040 [2].Given that the incidence of type 2 diabetes has been reported in 5–10% of pre-diabetes patients annually and also in 5–10% of patients with pre-diabetes can return to Normal Glucose Tolerance Test (NGTT) [4]; type 2 diabetes can be prevented in pre-diabetics with impaired glucose tolerance [5]. Therefore, pre-diabetic people should be identified and included in the Diabetes Prevention Program (DPP). This program includes healthy eating, exercising at least 150 min a week, and weight loss, which can prevent or delay diabetes by 5–7% [6].Thus finding of new markers for early recognition of subjects with pre-diabetes is very important.
One of the factors for recognition prediabetes is MASP1, also referred to as MBL (Mannan Binding Lectin Serine Protease 1.
Increase of MASP1 was observed in complement system and has a significant role in developing of diabetes type 2 [7]. In diabetic patients with nephropathy MASP1increased in comparison diabetic patients with normal function of kidneys [8].
MBL is synthesized by hepatocytes and belongs to the type C lectin family [9]. MBL causes inflammation and activation of complement [10].An increase in MASP1 has been observed in the complement system and plays an important role in type 2 diabetes and the development of diabetes-induced vascular complications [11].
Another factor is investigated in this study is CMPF (3-carboxy-4-Methyl-5-propyl-Furanpropanoic acid) which increases the production of Reactive Oxygen Species (ROS) products and associated insulin depletion [12].
Significant increase of CMPF levels have been observed in people with pre-diabetes who are at risk for diabetes.The level of this factor is stable in pre-diabetic subjects. Thus, the level of CMPF as a screening agent may explain why some people remain in pre-diabetes for up to 2 years before having type 2 diabetes [12, 13].
In the normal glucose state, the circulating concentration of CMPF is (20–40 μM) [14]. Plasma CMPF concentration reflects two events:1- as a cause of β-cell dysfunction leading to type 2 diabetes, 2- in a community without type 2 CMPF levels in subjects with normal glucose tolerance test after consumption of lean fish are lower than those of type 2 diabetic patients and have no association with glucose metabolism deficiency [15, 16].
Third main variable investigated in this study is uric acid a heterocyclic compound that combines carbon, nitrogen and hydrogen. This organic compound in humans is derived from the final oxidation of purine nucleotides (adenine, guanine).Production of uric acid is a complex process produced by various factors in the liver; it is excreted through the kidneys and is a constituent of urine [17, 18].For the first time in 1923, the relationship between hyperuricemia and hyperglycemia was expressed [19]. Elevated serum uric acid levels are associated with pre-diabetic status [20]. For each mg/dl increase in serum uric acid level, the risk of type 2 diabetes increases by 20% [21].
Objectives
In this study, MASP1, CMPF and Uric Acid (U.A) were determined as possible biomarkers for early diagnosis of pre-diabetes.Lipid profile, FPS (Fasting Plasma Sugar), Blood Urea Nitrogen (BUN), creatinine (Cr) and liver enzymes (AST, ALT) serum levels in pre-diabetic subjects and normal individuals were determined for early diagnosis of pre-diabetes subjects. Relationship between MASP1, CMPF and U.A serum levels and FPS, HbA1Cwere also evaluated.
Main outcomes of this study were prevention of diabetes and regulation of FPS. Secondary outcome of this study was prevention of nephropathy.
Methods
Study population
This study is a case-control study that was performed on people referring to Shooshtar Medical Diagnostic Laboratories in Khuzestan Province from 08/23/2017 to 11/21/2018. We studied 44 pre-diabetic subjects with 44 normal subjects. The median age of the participants was 34 years, ranging from 18 to 58 years. There were 50 males and 38 females.
Laboratory measurements
The FBS(by GOD-PAP method), BUN (by Urease-GLDH method), Cr (by JAFFE method), U.A(by Toos method), Chol (by CHOD-PAP method), TG (by GPO-PAP method), HDL-c (by enzymatic method), LDL-c (by enzymatic method), SGOT (by IFCC method), SGPT (by IFCC method), and ALP (by DGKC method) tests were performed using Pars Azmoon kits (Iranian manufacturer) and BT4500 auto analyzer. Fasting plasma glucose (FPG), HbA1c, and FBS, Bun, Cr, cholesterol, LDL-c, HDL-c and triglyceride and liver enzymes were measured to evaluate the status of healthy and pre-diabetic subjects. Data on variables such as age, weight, height, cigarette and alcohol consumption, specific drug use, as well as disease risk were collected by questionnaire and written consent.
MASP1 and CMPF tests were performed using Zell bio (Germany) kits by ELISA (sandwich) method.
First, according to the kits brochure, dilute the standards and prepare the ×30 elution solution, and then add 50 μl of diluted standards to the six primary wells and add 40 μl of the samples to the plate wells.
Next, we added 50 μl of Streptavidin-HRP complex solution to six standard wells.We then added 10 μl of antibody conjugated to biotin to the wells of the samples. Then we added 50 μl of Streptavidin-HRP complex solution to the wells of the samples, Streptavidin being able to bind to biotin. We then covered the plate with glue and placed it in the incubator at 37 °C for one hour. After one hour, remove the plate from the incubator and remove the sealant (adhesive) and then flip the well out of the plate by inverting the plate.
Then we spread some napkins on the worktop, rolled the napkins with plates and upside down to completely remove the contents of the wells and create no bubbles. Next, we added 350 μl of X1 wash solution into each well, shaking or stirring the plate for 30 s, then inverting the plate to remove the contents.
Then we applied the plate upside down with a few strokes to the scattered napkins on the table to completely remove the contents and not bubble. We repeated this wash five times. Next, we added 50 μl of chromogenic solution or dye A to each well and then 50 μl of chromogenic B solution.
Then we put the plate on the plate and incubate it at 37 °C for 10 min. In this case, the enzyme reaction is induced by the HRP enzyme. Then we removed the plate from the incubator and separated the flood plate. After adding color solutions, A and B and subsequently enzymatic reactions, the color of the wells became blue.
These dyes also contain H2O2. Blue is due to the reaction between HRP and its substrate (H2O2) and ultimately the effect of free oxygen produced on the chromogenic material. Next, we added 50 μl of stop solution to each well to stop the enzymatic reaction, in which case the blue of the well changed rapidly to yellow. Overall, color intensity is directly related to the concentration of the desired factor present in the serum sample. After adding maximum stop solution, the wells should read up to 10 min (OD). In this experiment, we measured the absorption of wells at 450 nm using Elisa Reader (stat fax −2100 made inUSA).
Statistical analysis
The quantities variables were described using mean and standard deviation (SD) or interquartile range (IQR). The normality of quantities variables was assess using Shapiro-Wilk test. Independent sample t-test and Mann-Whitney test were applied to compare the FPG, BUN, Cr, U.A, Chol, TG, HDL-c, LDL-c, SGOT (AST), SGPT (ALT), and ALP in normal and pre-diabetes groups. Pearson correlation test was applied to assess the relationship between quantitative. Scoter plot was drowning to show relationship between some variables. P value <0.05 was considered statistically significant in all tests.
Results
The present study was performed on 88 subjects including 44 (50%) normal subjects and 44 (50%) subjects with pre-diabetes. The results of Shapiro-Wilk test showed that except of BUN (p = .021) for other variables normality was established (p > 0.05).
The mean age for normal subjects and pre-diabetes were 33.57 ± 7.44 and 38.81 ± 8.39, respectively (p = .057). The mean BMI for pre-diabetes subjects significantly was more than normal subjects (p = 0.024). Also, the results of independent sample t-test indicated that FPG (p < 0.001), U.A(p = 0.002), Chol (p < 0.001), TG(p = 0.028), HDL-c(p = 0.04), LDL-c(p < 0.001), ALP(p < 0.001), CMPF(p < 0.001), HbA1C(p = 0.004) and MASP1(p < 0.001) significantly were higher in pre-diabetic subjects (Table 1).
Table 1.
Comparison the levels of the studied parameters in normal and pre-diabetes groups
| Variables | Normal | Pre-diabetes | p value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age, year | 33.0 ± 7.0 | 38.81 ± 8.39 | .057† |
| BMI, | 25.9 ± 5.1 | 28.3 ± 4.5 | 0.024† |
| FBS | 88.4 ± 6.3 l | 114.7 ± 4.7 | < 0.001† |
| BUN | 11.3(5.10)* | 12.0(4.08)* | 0.260‡ |
| Cr | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.340† |
| U.A | 4.6 ± 0.8 | 5.3 ± 1.2 | 0.002† |
| Chol | 156.9 ± 34.2 | 183.6 ± 31.5 | < .001† |
| TG | 115.1 ± 62.8 | 143.6 ± 57.1 | 0.028† |
| HDL-c | 43.8 ± 8.9 | 47.9 ± 9.8 | 0.045† |
| LDL-c | 83.3 ± 20.8 | 117.7 ± 25.3 | < 0.001† |
| AST | 23.5 ± 7.2 | 24.0 ± 8.0 | 0.795† |
| ALT | 24.4 ± 8.6 | 27.6 ± 16.3 | 0.262† |
| ALP | 175.3 ± 41.8 | 213.2 ± 48.7 | < 0.001† |
| HbA1C | 4.3 ± 0.3% | 4.6 ± 0.6% | 0.004† |
| CMPF | 60.91 ± 12.75 | 12.61 ± 71.29 | < 0.001† |
| MASP1 | 3.13 ± 0.94 | 4.75 ± 1.67 | < 0.001† |
*Median (IQR), †Independent sample t-test, ‡Mann-Whitney test
There was also a significant relationship between CMPF and MASP1 (r = 0.291, p < 0.01) and the results showed that CMPF (r = 0.428, p < 0.001), MASP1(P < 0.001,r = 0.454), U.A (r = 0.301, p = 0.004) increased with fasting blood sugar, respectively. The results also showed that with increasing fasting plasma sugar, factors such as Chol (r = 0.424, p = 0.002), TG (r = 0.261, p = 0.014), LDL-c (r = 0.597, p < 0.001), HDL-c (r = 0.200, p = 0.062), ALP (r = 0.375, p = 0.001), and HbA1c (r = 0349, p = 0.001) were increased, respectively. But other indices, such as BUN (r = 0.176, p = 0.101), Cr (r = 0.001, p = 0.99), AST (r = 0.046, p = 0.795), ALT (r = 0.110, p = 0.262) did not increase significantly.
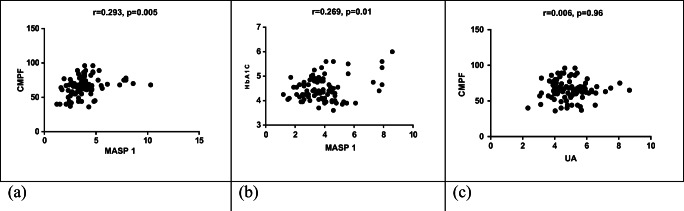
A significant relationship was observed between MASP1 and CMPF levels. As CMPF increases, so does MASP1 (r = 0.291, P < 0.01, Fig. 1a). There was a significant relationship between MASP1 and HbA1C (r = 0.269, p = 0.01, Fig. 1b). But there was no significant relationship between CMPF and uric acid (r = 0.001, p > 0.05, Fig. 1c).
Fig. 1.
Correlation between CMPF and MASP1 (a), HbA1C and MASP1 (b) and CMPF and uric acid (c)
Discussion
Given the importance of identifying people with pre-diabetes, many studies have been conducted around the world to identify these factors. In our study, uric acid, MASP1 and CMPF were used to identify pre-diabetic subjects.MASP1 has a potent role in complement activation by removing C2 complement [10].An increase in MASP1 has been observed in the complement system and plays an important role in type 2 diabetes and the development of diabetes-induced vascular complications [7]. In the present study, this factor (MASP1) was significantly correlated with FPG (P˂0.001,r = 0.454, Table 1).
A significant relationship was observed between MASP1 and CMPF levels (r = 0.269, P = 0.005, Figure 1a). Also a significant relationship was observed between MASP1 and HbA1C levels(r = 0.269, P = 0.01,Fig. 1b).
The results of the Kroghs study showed that MASP1,FPG HbA1C, profile lipid (except TG) levels and BMI, were significantly different between the normal and diabetic groups (P < 0.001).But the levels of TG was not significantly different between the normal and diabetic groups(p = 0.011) [11].Also in Kroghs study, there was a significant correlation between MASP1 and HbA1C, which was similar to the results of our study(P < 0.001).In Von Toerne study, factors included FPG, HDLc, TG, and BMI were investigated, and the levels of these factors were significantly different between normal, prediabetes and diabetic groups (P < 0.001) [22].The results of Von Toerne study were also in line with the present study. Jenny’s results showed that MASP1was significantly correlated with HbA1c(P < 0.001) but no significant relationship was found between age and BMI in non diabetic adults and diabetic type I [7]. HbA1C levels, is in line with our study and the results in terms of age, contradicts our findings.
Other factor to consider is CMPF which significantly increases in people with pre-diabetes who are exposed to type 2 diabetes [23].In the present study, CMPF level was significantly correlated with FPG(P < 0.001,r = 0.428, Table 1) and MASP1 (P < 0.01, r = 0.291) levels.
In this regard, Zhang’s study showed a significant relationship between CMPF, HbA1C and FPG in pregnant women with normal glucose tolerance and gestational diabetes mellitus(p= 0.001) [24].A study by Liu showed that there was a significant difference in CMPF level between normal, pre-diabetes and diabetes groups. Also, a significant correlation was observed between FPG and CMPF in three mentioned groups(p˂0.001) [12].In our study, there was a significant difference in serum CMPF levels and FPG between prediabetes and normal individuals(P < 0.001).
In the Yi study, CMPF and age, lipid profile (included TG, Total chol, HDL-c, LDL-c), HbA1C and FPG were investigated between three groups included normal, prediabetes and type 2 diabetes. The results of this study showed that CMPF, HbA1C,Total chol, TG, Age and FPG were significantly different in 3 mentioned groups (p˂0.001) [25]. In our study, there was a significant difference in serum CMPF, FPG, total chol, and LDL-c levels between prediabetes and normal individuals.
Another factor investigated in this study was uric acid, arises in humans from the final oxidation of purine nucleotides (adenine, guanine), which have many enzymes involved in the conversion of these two bases into uric acid [17, 18].Elevated serum uric acid level is associated with pre-diabetic status [20]. For each mg/dl increase in serum uric acid level, the risk of type 2 diabetes increases by 20% [21].
In our study, there was a significant relationship between serum uric acid level and fasting plasma sugar(p=0.004, r=0.301).Also, uric acid level significantly increases in people with prediabetes compared to normal people, (p < 0.002). In our study, there was no significant relationship between CMPF and uric acid (r = 0.001, p > 0.05) (Fig. 1c).
In Bhole study, there was a significant difference in serum uric acid and FPG, age, total Chol, TG, Cr and BMI between normal subjects and patients with type 2 diabetes(P˂0.001) [21].A cross-sectional study of Liu aimed to investigate the relationship between serum uric acid level and the prevalence of pre-diabetes in different age groups [26]. In this study, there was a significant difference in serum uric acid level with age, BMI,TG,HDLc,LDLc, BUN,in different pre-diabetic age groups (p˂0.001).In this study, they found a significant association between increasing serum uric acid levels and a higher risk of prediabetes, especially in older females with age ≥48 years than younger females(p=0.001). In our study, there was a significant difference in serum uric acid (p = 0.002), FPG, total Chol, and LDLc levels between prediabetes and normal individuals (p˂0.001).
Based on the searches in various database, it is the first time that MASP1 and CMPF were determined in Iranian prediabetes subjects. The limitations of this study were to recognize and gathering samples of prediabetes subjects, and expensive prices of kits and high costs of the tests.
Conclusion
Considering the significant correlation between serum levels of uric acid, MASP1 and CMPF in pre-diabetic individuals in comparison to normal individuals, these parameters can be suitable biomarkers for the diagnosis of pre-diabetes, but it requires more research with a larger community and wider geographical areas.
Acknowledgments
Authors wish to thank the patients that participated in the current study.
Funding
The study was financially supported by Hormozgan University of Medical Sciences (grant number: 960326).
Compliance with ethical standards
Conflict of interests
The authors declared no conflict of interest.
Ethical considerations
The study was approved by the Ethical Committee of Hormozgan University of Medical Sciences, Bandar Abbas, Iran (Code: IR.HUMS.REC.1397.048). All patients signed consent form before participation in the study.
Financial disclosure
Authors declared no financial disclosure related to the material in the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad REAR, Lim B, Kardivelu A, Hussain R, Ahmad WAW, Ismail IS, et al. Prediabetes–The New Target for Primary Prevention of Cardiovascular Metabolic Dysfunction.
- 2.Dorcely B, Katz K, Jagannathan R, Chiang SS, Oluwadare B, Goldberg IJ, et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes, metabolic syndrome and obesity: targets and therapy. 2017;10:345. [DOI] [PMC free article] [PubMed]
- 3.Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6(2):296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numata Y, Ohya T, Nitta Y, Yoshinaka Y, Shogakiuchi A, Toyota A. Repetition of Prediabetes enhances the risk of developing diabetes. Journal of Diabetes Research. 2019;2019:1–6. doi: 10.1155/2019/4916546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Döring A, Meisinger C, Gieger C, Prehn C, Roemisch-Margl W, Carstensen M, Xie L, Yamanaka-Okumura H, Xing G, Ceglarek U, Thiery J, Giani G, Lickert H, Lin X, Li Y, Boeing H, Joost HG, de Angelis MH, Rathmann W, Suhre K, Prokisch H, Peters A, Meitinger T, Roden M, Wichmann HE, Pischon T, Adamski J, Illig T. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8(1):615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen SC (2019) Prediabetes identification and diabetes prevention program referral
- 7.Jenny L, Ajjan R, King R, Thiel S, Schroeder V. Plasma levels of mannan-binding lectin-associated serine proteases MASP-1 and MASP-2 are elevated in type 1 diabetes and correlate with glycaemic control. Clinical & Experimental Immunology. 2015;180(2):227–232. doi: 10.1111/cei.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelgaard E, Østergaard JA, Thiel S, Hansen TK. Diabetes is associated with increased autoreactivity of mannan-binding lectin. Journal of diabetes research. 2017;2017:1–12. doi: 10.1155/2017/6368780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan L-Z, Tong Q, Xu J. Elevated serum levels of mannose-binding lectin and diabetic nephropathy in type 2 diabetes. PLoS One. 2015;10(3):e0119699. doi: 10.1371/journal.pone.0119699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitved L, Holmskov U, Koch C, Teisner B, Hansen S, Skjødt K. The homologue of mannose-binding lectin in the carp family Cyprinidae is expressed at high level in spleen, and the deduced primary structure predicts affinity for galactose. Immunogenetics. 2000;51(11):955–964. doi: 10.1007/s002510000232. [DOI] [PubMed] [Google Scholar]
- 11.Krogh SS, Holt CB, Steffensen R, Funck KL, Høyem P, Laugesen E, Poulsen PL, Thiel S, Hansen TK. Plasma levels of MASP-1, MASP-3 and MAp44 in patients with type 2 diabetes: influence of glycaemic control, body composition and polymorphisms in the MASP1 gene. Clinical & Experimental Immunology. 2017;189(1):103–112. doi: 10.1111/cei.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Prentice KJ, Eversley JA, Hu C, Batchuluun B, Leavey K, Hansen JB, Wei DW, Cox B, Dai FF, Jia W, Wheeler MB. Rapid elevation in CMPF may act as a tipping point in diabetes development. Cell Rep. 2016;14(12):2889–2900. doi: 10.1016/j.celrep.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 13.Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem. 2012;403(7):1841–1850. doi: 10.1007/s00216-012-5929-3. [DOI] [PubMed] [Google Scholar]
- 14.Sassa T, Matsuno H, Niwa M, Kozawa O, Takeda N, Niwa T, Kumada T, Uematsu T. Measurement of furancarboxylic acid, a candidate for uremic toxin, in human serum, hair, and sweat, and analysis of pharmacological actions in vitro. Arch Toxicol. 2000;73(12):649–654. doi: 10.1007/s002040050020. [DOI] [PubMed] [Google Scholar]
- 15.Lankinen MA, Hanhineva K, Kolehmainen M, Lehtonen M, Auriola S, Mykkänen H, Poutanen K, Schwab U, Uusitupa M. CMPF does not associate with impaired glucose metabolism in individuals with features of metabolic syndrome. PLoS One. 2015;10(4):e0124379. doi: 10.1371/journal.pone.0124379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savolainen O, Lind MV, Bergström G, Fagerberg B, Sandberg A-S, Ross A. Biomarkers of food intake and nutrient status are associated with glucose tolerance status and development of type 2 diabetes in older Swedish women. Am J Clin Nutr. 2017;106(5):1302–1310. doi: 10.3945/ajcn.117.152850. [DOI] [PubMed] [Google Scholar]
- 17.Lima WG, Martins-Santos MES, Chaves VE. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 2015;116:17–23. doi: 10.1016/j.biochi.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–345. doi: 10.1146/annurev-physiol-021113-170343. [DOI] [PubMed] [Google Scholar]
- 19.Nan H, Pang Z, Wang S, Gao W, Zhang L, Ren J, et al. Serum uric acid, plasma glucose and diabetes. Diabetes and Vascular Disease Research. 2010;7(1):40–46. doi: 10.1177/1479164109347408. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan E, Pandya BJ, Chung L, Hariri A, Dabbous O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 2012;176(2):108–116. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 21.Bhole V, Choi JWJ, Kim SW, De Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. 2010;123(10):957–961. doi: 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Toerne C, Huth C, de Las Heras Gala T, Kronenberg F, Herder C, Koenig W, et al. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia. 2016;59(9):1882–1892. doi: 10.1007/s00125-016-4024-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH (2017) Molecular mechanism and effects of furan fatty acid metabolite CMPF on adipocytes
- 24.Zhang S, Chen P, Jin H, Yi J, Xie X, Yang M, Gao T, Yang L, Hu C, Zhang X, Yu X. Circulating 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) levels are associated with hyperglycemia and β cell dysfunction in a Chinese population. Sci Rep. 2017;7(1):3114. doi: 10.1038/s41598-017-03271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi J, Jin H, Zhang R, Zhang S, Chen P, Yu X, Zhang X. Increased serum 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) levels are associated with glucose metabolism in Chinese pregnant women. J Endocrinol Investig. 2018;41(6):663–670. doi: 10.1007/s40618-017-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhao Z, Mu Y, Zou X, Zou D, Zhang J, Chen S, Tao L, Guo X. Gender differences in the association between serum uric acid and prediabetes: a six-year longitudinal cohort study. Int J Environ Res Public Health. 2018;15(7):1560. doi: 10.3390/ijerph15071560. [DOI] [PMC free article] [PubMed] [Google Scholar]