Abstract
Background & objective(s)
The current evidence base regarding the impact of propolis consumption on lipid profile in humans is equivocal. Thus, we sought to investigate the impact of propolis consumption on anthropometric indices and lipid profile in this meta-analysis.
Methods
Cochrane Library, Scopus, PubMed, and Web of Science electronic databases were searched up to December 2019. A random-effects model used to pool effect size. The leave-one-out method used to conduct sensitivity analysis.
Results
Five RCTs were included in this study. Our findings indicated a significant decrease in triglyceride (TG) (WMD: −3.91 mg/dl, 95% CI: −4.22, −3.60), and a significant increase in high-density lipoprotein (HDL) (WMD: 3.41 mg/dl, 95% CI: 0.05, 6.76), however, for body mass index, weight, cholesterol (CL), and low-density lipoprotein (LDL), no significant alterations were evident.
Conclusion
This study revealed that consumption of propolis is associated with a reduction in TG levels, in addition to an increase in HDL levels. Nevertheless, additional research is required to further delineate the relationship between propolis consumption and lipid profile.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00604-2) contains supplementary material, which is available to authorized users.
Keywords: Propolis, Weight, Body mass index, Lipid profile
Introduction
Propolis is a naturally occurring, adhesive material, gathered by Apis mellifera L. (Honeybees), from plant sources [1]. It is used by honeybees to cement the honeycombs, in combination with wax and salivary gland enzymes. In humans, propolis has been used in traditional medicine because of its free radical scavenging properties and antimicrobial effects [2]. Even in contemporary medicine, propolis remains a popular over-the-counter (OTC) medication, because of its potential therapeutic applications in, among other illnesses, acne vulgaris, diabetic foot ulcers, and as a cough remedy [3].
Propolis is solid at room temperature; however, when it is heated, it becomes gelatinous with adhesive and flexible properties. It has a lipophilic nature and its color varies from yellow and green to dark brown, depending on the type of material used by the honeybees [4, 5]. It is used extensively in food and beverages for the prevention and improvement of some health conditions [6]. Indeed, the continued use of propolis has garnered scientific interest in its chemical composition and potential clinical applications [7]. Approximately 300 constituents are found in propolis, where the major components are flavonoids, aromatic aldehyde, alcohols, beta-steroids, and terpenes. With regard to the flavonoids; flavanols, flavones, flavanones, dihydroflavonols, and chalcones are the most abundant [8], and possess free radical scavenging potential, especially in reactive nitrogen species, hydrogen peroxide, and reactive oxygen species [9, 10]. Moreover, flavonoids can contribute to the inhibition of the Xanthine oxidase reaction, chelating the metal ions involved in the development of free radicals [11], thus, preventing peroxidation of lipids and protecting the cell membrane. Propolis also comprises another powerful antioxidant, caffeic acid phenyl ester, which prevents the formation of free radicals [12]. Overall, propolis appears to prevent the peroxidation of lipids, regulates carbohydrate and lipoprotein metabolism, regulates gene expression, suppresses the activity of cytokines, attenuates endothelial dysfunction, and prevents aggregation of cytokines [13–16].
However, despite an increase in the number of studies evaluating the effect of propolis on weight, body mass index, and lipid profile, to the best of the authors knowledge, no study has attempted to summarize the effect of propolis on the aforementioned parameters. Thus, we sought to investigate the effect of propolis consumption on anthropometric indices and lipid profile.
Material and methods
The present study was conducted according PRISMA statement [17]. Searches were conducted in Cochrane Library, Web of Science, PubMed, and Scopus, up to December 2019. Our search strategy in detailed in supplemental Table 1. The search was conducted by two of the authors. Disagreements in study selection were resolved through panel discussions method.
Eligibility criteria
Clinical studies that investigated propolis supplementation and its’ resultant effect on weight, BMI, cholesterol, triglyceride, LDL, and HDL were included in this study, if the following criteria were met; followed a RCT study design, conducted an intervention using propolis, and, sufficient baseline and post-intervention data were available. Furthermore, we adopted the following exclusion criteria, where non-interventional studies, studies with no placebo group, and studies that did not have sufficient data at baseline and post-intervention, were excluded.
Data extraction and quality assessment
Two authors (J.R and A.S) evaluated the titles/abstracts, independently. Full-texts of the eligible articles were examined to extract the required information. In any instance of disagreement, consensus was achieved through panel discussion with the author team. The Cochrane Collaboration tool [18] was used to assess risk of bias.
Statistical analysis
The SD change of the mean difference was calculated using the following formula: SD2 = [(SD baseline 2 + SD final 2) – (2 × R × SD baseline × SD final)]. In order to calculate the pooled effect size, a random-effects model was employed. The I2 test was used to evaluate heterogeneity, whilst the Begg’s and Egger’s tests were used to assess publication bias. STATA 11 software (StataCorp, College Station, Texas, USA) used to conduct all statistical analyses.
Results
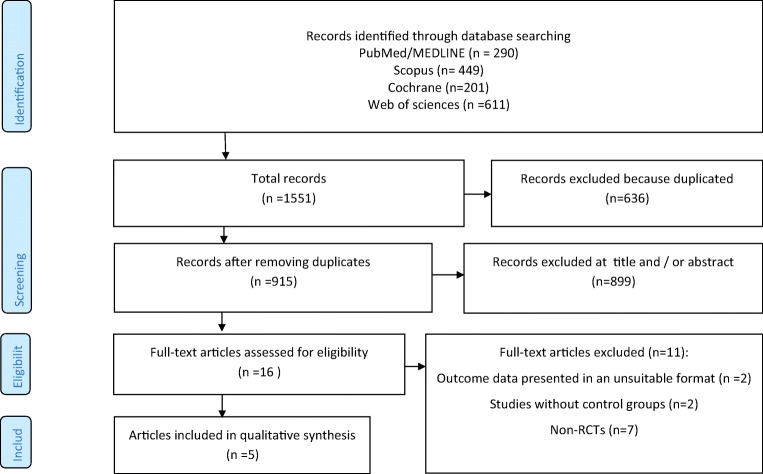
As depicted in Fig. 1, following the primary systematic search, and removal of duplicated studies, 915 papers were identified for title and abstract screening. Accordingly, 899 papers were excluded due to irrelevant title and abstracts, resulting in 16 papers included for full text evaluation. Of the 16 studies, 10 studies did not meet our inclusion criteria, so they were excluded; subsequently, five papers were included in the present study [19–23].
Fig. 1.
Flow chart of included studies
Study characteristics
The characteristics of the included studies are detailed in Table 1, where all were conducted on both genders and published between 2017 and 2019. The included studies were conducted in Iran [19, 20, 22], Chile [21], and Japan [23], respectively, and utilized a mean propolis dose of 906 mg per day, with a mean age of 53.77 y. Four studies were conducted on diabetic patients, and one on ‘at risk’ adults. The mean intervention duration was 10 weeks, whilst the quality of included studies was good (Fig. 2).
Table 1.
Characteristics of included studies
| Author | Country | Type of studies | Year | Participants (n) | Age (year) | Dose (mg/day) | Length of intervention (weeks) | Type | Population (health status) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Zakerkish et al. | Iran | RCT (double-blind, parallel) | 2019 | 94 Propolis (n = 50) Placebo (n = 44) | 55.15 | 1000 | 12 | Iranian propolis | T2DM | Weight, BMI, TC, TG, LDL, HDL |
| Mujica et al. | Chile | RCT (double-blind, parallel) | 2017 | 67 Propolis (n = 35) Placebo (n = 32) | 46.4 | Unstated | 12 | Beepolis (from Maule region of Chile) | Having at least one of the following altered parameters: fasting glycemia, lipids profile, blood pressure, or diabetes mellitus, cardiovascular disease, and/or overweight | Weight, BMI, TC, TG, LDL, HDL |
| Afsharpour et al. | Iran | RCT (double-blind, parallel) | 2017 | 60 Propolis (n = 30) Placebo (n = 30) | 50.43 | 1500 | 8 | Iranian propolis | T2DM | Weight, BMI |
| Fukuda et al. | Japan | RCT (double-blind, parallel) | 2015 | 80 Propolis (n = 41) Placebo (n = 39) | 63.31 | 226.8 | 8 | Brazilian green propolis | T2DM | TC, TG, LDL, HDL |
| Samadi et al. | Iran | RCT (double-blind, parallel) | 2017 | 57 Propolis group (n = 30) Placebo group (n = 27) | 53.56 | 900 | 12 | Propolis (unstated) | T2DM | Weight, BMI, TC, TG, LDL, HDL |
RCT randomized clinical trial, T2DM type 2 diabetes mellitus, BMI body mass index, TC total cholesterol, TG triglycerides, LDL low-density lipoproteins, HDL high-density lipoproteins
Fig. 2.
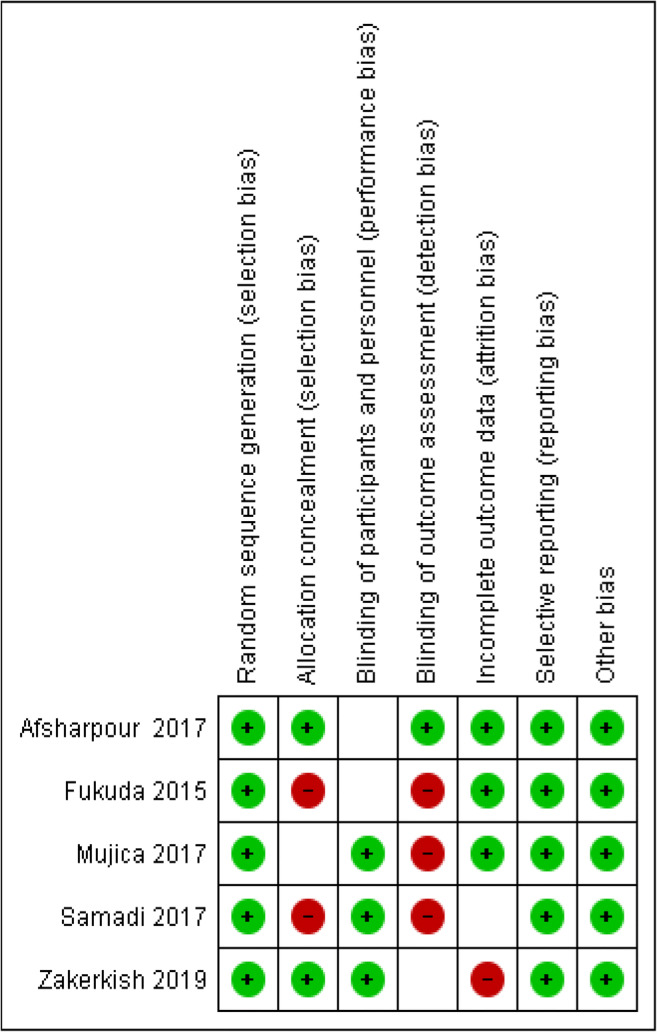
Cochrane risk of bias assessment
Meta-analysis results
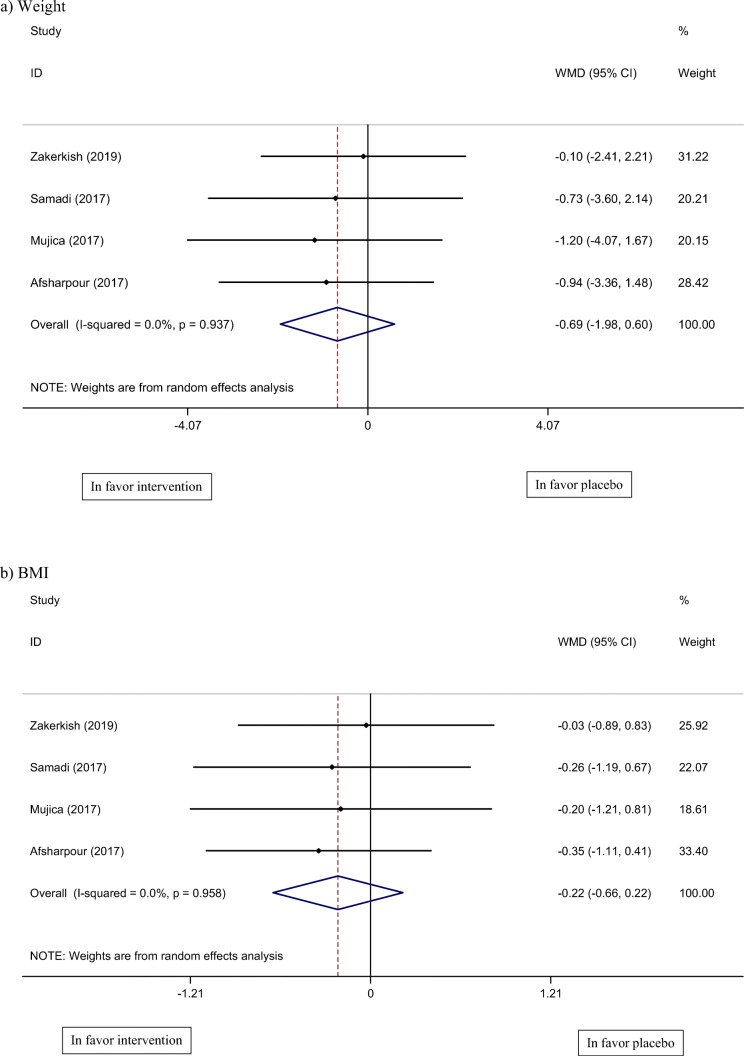
Four studies, including 278 participants, reported weight and BMI as anthropometric outcome measures. According to Fig. 3, combined results of these four studies did not show any significant change in the propolis group, vs. control group, in weight (WMD: −0.69 kg, 95% CI: −1.98, 0.60, I2 = 0%) and BMI (WMD: −0.22 kg/m2, 95% CI: −0.66, 0.22, I2 = 0%) [19–22]. There was no significant heterogeneity evident in the pooled results of weight and BMI.
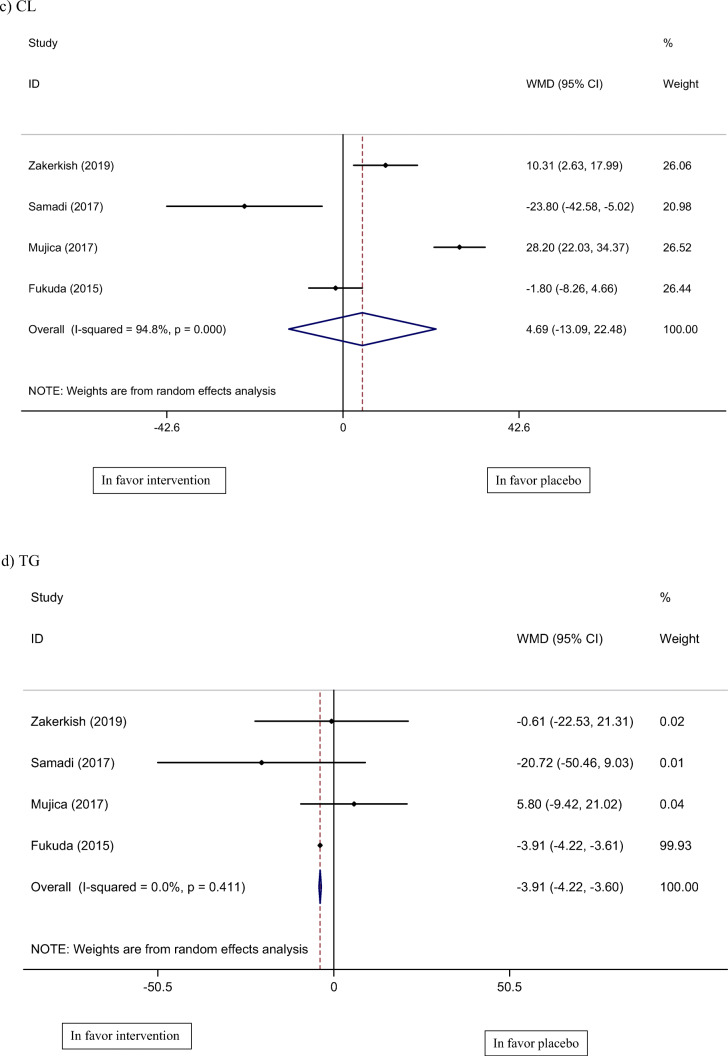
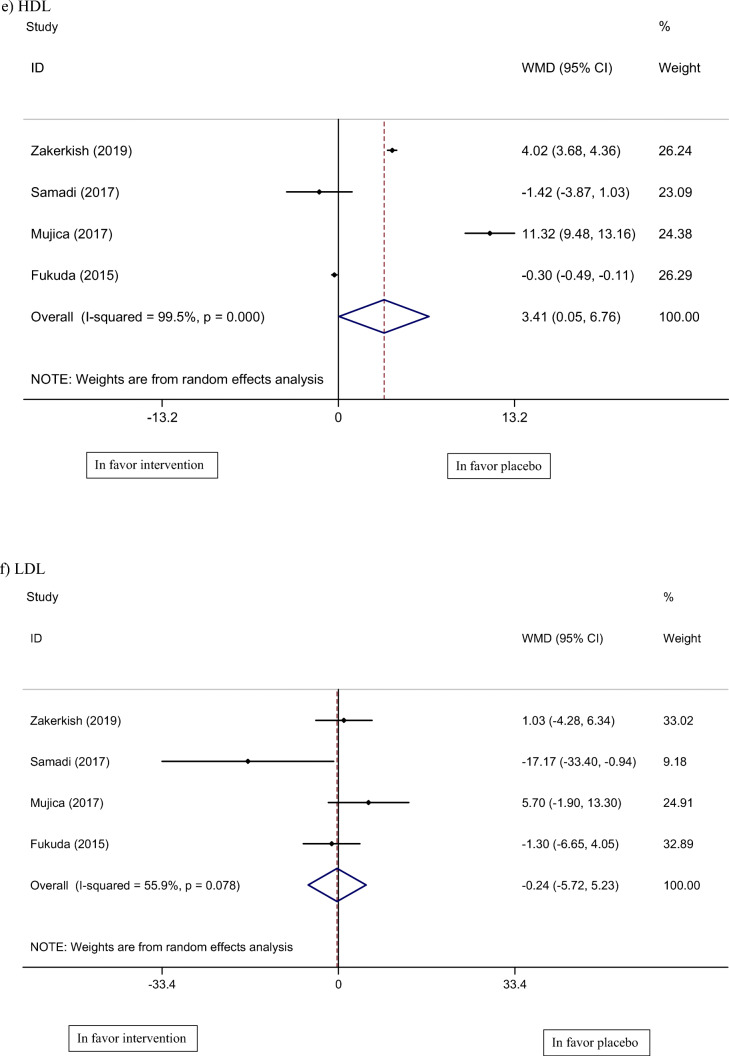
Fig. 3.
Meta-analysis of effect of propolis consumption on: a Weight b BMI c CL d TG e HDL f LDL
Four studies, with a total of 298 participants, reported lipid profile as an outcome measure (CL, TG, HDL, and LDL) [19–21, 23]. Pooled results of these studies show no significant change in CL (WMD: 4.69 mg/dl, 95% CI: −1.09, 22.48, I2 = 94%) and LDL (WMD: −0.24 mg/dl, 95% CI: −5.72, 5.23, I2 = 55%) levels, however, TG (WMD: −3.91 mg/dl, 95% CI: −4.22, −3.60, I2 = 0%) and HDL (WMD: 3.41 mg/dl, 95% CI: 0.05, 6.76, I2 = 99%) were significantly lower and higher, respectively, compared to the control group.
Risk of bias
A funnel plot of included studies did not show any asymmetry between included studies (Supplemental Fig. 1). Furthermore, the Begg’s and Egger’s tests were non-significant for weight (p = 0.17, p = 0.33), BMI (p = 0.68, p = 0.62), CL (p = 0.49, p = 0.45), TG (p = 0.49, p = 0.85), HDL (p = 0.99, p = 0.48), and LDL (p = 0.34, p = 0.44), respectively.
Discussion
In the present study, we pooled results from five trials concerning the effect of propolis consumption on anthropometric indices and lipid profile. According to results consumption of propolis did not yield any significant effect on weight, BMI, CL, and LDL. However, the pooled estimates demonstrated that consumption of propolis was associated with a significant decrease in TG levels, whilst HDL levels were significantly increased.
Propolis, colloquially known as bee glue, has long been reported to have various beneficial health effects. Indeed, these effects are attributed to anti-inflammatory [24], antioxidant [25], anti-microbial [26], and immunomodulatory properties [27]. Propolis is known to possess over 300 different compounds, and the main biologically active ingredients are flavonoids, polyphenols, vitamins, amino acids, and caffeic acid phenethyl ester, respectively [28]. These compounds combat oxidative stress by eliminating the release of reactive oxygen species, such as free radicals, hydrogen peroxide, and nitric oxide [29].
In vivo and in vitro studies have demonstrated the potential cardioprotective properties of propolis via inhibition of LDL peroxidation [30], limitation of inflammatory cytokines [31], regulation of glucose metabolism [32], prevention of atherosclerotic plaque formation [33], as well as improvement of endothelial function and decreases in aggravation of thrombocytes [34]. These effects can be particularly beneficial for patients with existing metabolic abnormalities, such as those with type 2 diabetes mellitus. However, despite a large number of experimental data, there is a lack of human studies to have evaluated the clinical efficacy of propolis.
In this study, individuals receiving propolis showed statistically significant reductions in the levels of TG, in addition to increased levels of HDL, compared to the control group. These outcomes may be promising in the management of individuals with altered lipid profile, conceivably representing an alternative to many pharmaceutical options. In particular, Hesadi et al. and Samadi et al., respectively, reported significantly lower LDL levels by the end of the trial [21]. Samadi and colleagues also demonstrated additional benefits through reductions in total cholesterol levels, while, Zakerkish et al. showed propolis could elicit an increased concentration of HDL [20].
A putative mechanism for the beneficial effect of propolis on lipid profile may be that ATP-binding cassette transporters, which are associated with HDL formation and peripheral tissue efflux, are expressed to a greater extent in the liver proteins following supplementation [20]. Furthermore, Mujica et al., demonstrated, in a RCT, that 90 days consumption of propolis significantly increased HDL-C levels [22], and Zakerkish et al. demonstrated that, in T2DM patients, supplementation with Iranian propolis yielded increases in HDL-C. Indeed, it is accepted that HDL-C represents an significant lipoparticle which is capable of providing protection against various cardiovascular diseases, prevention of LDL oxidation, and neutralization of atherogenic effects within the arterial walls [20].
This study represents the first meta-analysis to have examined the influence of propolis consumption on anthropometric indices and lipid profile. Accordingly, there are some strengths and limitations that must be recognized. One of the biggest strengths of this study is the methodological approach, where the results from five RCTs were shown to be consistent, as demonstrated by Funnel plots. Moreover, four out of five included studies were double-blinded, two of which were placebo-controlled. Finally, the bias assessment conducted in our study demonstrated minimal bias, allowing us to assert veracity in our results. Notwithstanding the strengths highlighted above; there are several limitations that should be addressed. First, studies evaluated different types of propolis. Indeed, it is not certain to what extent they differ in biological activity and whether there could be any clinical implication to their interchangeable use. Second, discrepancies existed in the doses used per day; however, to date, there is no standard dosage currently advocated, and thus, future investigations should delineate the effects of various doses of propolis. Third, despite the robust methodology of the trials analyzed, the study population was small; it is therefore difficult to correctly estimate the exact impact of propolis in a wider population. Fourth, the intervention period in all studies was limited to 3 months, which might not be sufficient to draw definite conclusions regarding the sustainability and long-term effects of propolis.
Future studies are encouraged to recruit large sample sizes, using crossover study designs, to yield more robust evidence. In addition, investigations should focus on the long-term evaluation, dosage comparison, as well as considering the type of propolis, to provide greater insights into the clinical efficacy of propolis.
Conclusion
In conclusion, our results show that, following propolis consumption, TG levels were significantly decreased, whilst HDL levels were significantly increased. However, further research is required to better elucidate the relationship between propolis consumption and lipid profile markers.
Electronic supplementary material
(DOCX 38 kb)
(PDF 1091 kb)
Acknowledgments
This study is associated with project NO. 1398/10544 From Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. We are indebted to the Student Research Committee and Research & Technology Chancellor in SBMU for their financial support of this study.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Toreti VC, Sato HH, Pastore GM, Park YK. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med. 2013;2013:697390. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016;30(6):894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- 3.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15(7):561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 4.Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, Alencar SM. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evid Based Complement Alternat Med. 2008;5(3):313–316. doi: 10.1093/ecam/nem059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado CS, Mokochinski JB, de Lira TO, de Oliveira Fde C, Cardoso MV, Ferreira RG, et al. Comparative study of chemical composition and biological activity of yellow, green, brown, and red Brazilian propolis. Evid Based Complement Altern Med. 2016;2016:6057650. [DOI] [PMC free article] [PubMed]
- 6.Kuropatnicki AK, Szliszka E, Krol W. Historical aspects of propolis research in modern times. Evid Based Complement Alternat Med. 2013;2013:964149. doi: 10.1155/2013/964149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szliszka E, Kucharska AZ, Sokol-Letowska A, Mertas A, Czuba ZP, Krol W. Chemical composition and anti-inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages. Evid Based Complement Alternat Med. 2013;2013:976415. doi: 10.1155/2013/976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot AC. Propolis: a review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis. 2013;24(6):263–282. doi: 10.1097/DER.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 9.Lopes AA, Ferreira TS, Nesi RT, Lanzetti M, Pires KM, Silva AM, et al. Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke. Bioorg Med Chem. 2013;21(24):7570–7577. doi: 10.1016/j.bmc.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 10.Kurek-Gorecka A, Rzepecka-Stojko A, Gorecki M, Stojko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2013;19(1):78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara R, Ohta Y, Hayashi T, Ikeno T. Evaluation of antilipid peroxidative action of propolis ethanol extract. Phytother Res. 2002;16(4):340–347. doi: 10.1002/ptr.894. [DOI] [PubMed] [Google Scholar]
- 12.Jin UH, Chung TW, Kang SK, Suh SJ, Kim JK, Chung KH, Gu YH, Suzuki I, Kim CH. Caffeic acid phenyl ester in propolis is a strong inhibitor of matrix metalloproteinase-9 and invasion inhibitor: isolation and identification. Clin Chim Acta. 2005;362(1–2):57–64. doi: 10.1016/j.cccn.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Ichi I, Hori H, Takashima Y, Adachi N, Kataoka R, Okihara K, Hashimoto K, Kojo S. The beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat diet. J Food Sci. 2009;74(5):H127–H131. doi: 10.1111/j.1750-3841.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- 14.Nader MA, el-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33(4):637–643. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 15.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017;2017:1259510. [DOI] [PMC free article] [PubMed]
- 16.Braakhuis A. Evidence on the health benefits of supplemental propolis. Nutrients. 2019;11(11):2705. [DOI] [PMC free article] [PubMed]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N. The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep. 2019;9(1):7289. doi: 10.1038/s41598-019-43838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samadi N, Mozaffari-Khosravi H, Rahmanian M, Askarishahi M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. J Integr Med. 2017;15(2):124–134. doi: 10.1016/S2095-4964(17)60315-7. [DOI] [PubMed] [Google Scholar]
- 21.Mujica V, Orrego R, Perez J, Romero P, Ovalle P, Zuniga-Hernandez J, et al. The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evid Based Complement Alternat Med. 2017;2017:4272940. doi: 10.1155/2017/4272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afsharpour F, Hashemipour S, Khadem-Haghighian H, Koushan Y. Effects of Iranian propolis on glycemic status, inflammatory factors, and liver enzyme levels in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. J Nutr Sci Diet. 2017;3(2):9–14. [Google Scholar]
- 23.Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, et al. Effect of Brazilian green propolis in patients with type 2 diabetes: a double-blind randomized placebo-controlled study. Biomed Rep. 2015;3(3):355–360. doi: 10.3892/br.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado JL, Assunçao AKM, da Silva MCP, Reis ASD, Costa GC, Arruda DDS, et al. Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evid-Based Complement Altern Med. 2012;2012:157652. doi: 10.1155/2012/157652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes AA, Ferreira TS, Nesi RT, Lanzetti M, Pires KMP, Silva AM, Borges RM, Silva AJR, Valença SS, Porto LC. Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke. Bioorg Med Chem. 2013;21(24):7570–7577. doi: 10.1016/j.bmc.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Bueno-Silva B, Alencar SM, Koo H, Ikegaki M, Silva GV, Napimoga MH, et al. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J Agric Food Chem. 2013;61(19):4546–4550. doi: 10.1021/jf305468f. [DOI] [PubMed] [Google Scholar]
- 27.Fan Y, Ma L, Zhang W, Wang J, Chen Y, Gao Y, Feng W, Zhong L, Song X. The design of propolis flavone microemulsion and its effect on enhancing the immunity and antioxidant activity in mice. Int J Biol Macromol. 2014;65:200–207. doi: 10.1016/j.ijbiomac.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Toreti VC, Sato HH, Pastore GM, Park YK. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med. 2013;2013:1–13. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonar ME, Yonar SM, Silici S. Protective effect of propolis against oxidative stress and immunosuppression induced by oxytetracycline in rainbow trout (Oncorhynchus mykiss, W.) Fish Shellfish Immunol. 2011;31(2):318–325. doi: 10.1016/j.fsi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Silva V, Genta G, MaN M, Masner M, Thomson L, Romero N, et al. Antioxidant activity of Uruguayan propolis. In vitro and cellular assays. J Agric Food Chem. 2011;59(12):6430–6437. doi: 10.1021/jf201032y. [DOI] [PubMed] [Google Scholar]
- 31.Araujo MA, Libério SA, Guerra RN, Ribeiro MNS, Nascimento FR. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: a brief review. Rev Bras. 2012;22(1):208–219. [Google Scholar]
- 32.Li Y, Chen M, Xuan H, Hu F. Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med. 2012;31(2):318–25. [DOI] [PMC free article] [PubMed]
- 33.Norata GD, Marchesi P, Passamonti S, Pirillo A, Violi F, Catapano AL. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis. 2007;191(2):265–271. doi: 10.1016/j.atherosclerosis.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 34.Grassi D, Aggio A, Onori L, Croce G, Tiberti S, Ferri C, Ferri L, Desideri G. Tea, flavonoids, and nitric oxide-mediated vascular reactivity. J Nutr. 2008;138(8):1554S–1560S. doi: 10.1093/jn/138.8.1554S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)
(PDF 1091 kb)