Abstract
Purpose
Hypertriglyceridemia (HG) is an independent risk factor with more prevalence than hypercholesterolemia and its attributes to cardiovascular disease (CVD) and pancreatitis. Hence, it becomes imperative to search for new triglyceride (TG) lowering agents. Tinospora cordifolia (TC) is a well-known Ayurvedic drug and a rich source of protoberberine alkaloids hence can contribute to TG lowering without side effects. Hence, to explore the therapeutic efficacy of T. cordifolia and its effects on biochemistry and metabolome in the patients of hyper-triglyceridemia, clinical trials were conducted.
Methods
Patients (n = 24) with hypertriglyceridemia were randomized into two groups to receive T. cordifolia extract (TCE) (3.0 g/per day) and metformin (850 mg/day) for 14 days having >300 mg/dl triglyceride level and cholesterol in the range of 130–230 mg/dl. Lipid profiles of blood samples were analyzed. Urine samples were subjected to HPLC-QTOF-MS to quantify oxidative damage and abnormal metabolic regulation.
Results
Intervention with TCE reduced the triglyceride, LDL, and VLDL levels to 380.45 ± 17.44, 133.25 ± 3.18, and 31.85 ± 5.88 mg/dL and increased the HDL to 47.50 ± 9.05 mg/dL significantly (p < 0.05) in the HG patients after 14 days treatment. TCE dosage potently suppressed the inflammatory and oxidative stress marker’s i.e. levels of isoprostanes significantly (p < 0.01). Qualitative metabolomics approach i.e. PCA and PLS-DA showed significant alterations (p < 0.05) in the levels of 40 metabolites in the urine samples from different groups.
Conclusion
TCE administration depleted the levels of markers of HG i.e. VLDL, TG, and LDL significantly. Metabolomics studies established that the anti-HG activity of TCE was due to its antioxidative potential and modulation of the biopterin, butanoate, amino acid, and vitamin metabolism.
Clinical trials registry
India (CTRI) registration no. CTRI- 2016-08-007187.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00657-3) contains supplementary material, which is available to authorized users.
Keywords: Tinospora cordifolia, Hyperlipidemia, Hyper-triglyceridemia, Metabolomics, HPLC-QTOF-MS
Introduction
Tinospora cordifolia (Wild.) Miers ex Hook. f. & Thoms., a climber shrub of Menispermaceae family is being widely used as a drug in Ayurveda, a 5000-year-old traditional medicinal system of India [1]. Ayurveda describes T. cordifolia as a Rasayana drug that means blood purifier or which alleviates myriads of clinical manifestations. The plant has various curative properties including against skin disease, urinary dysregulation, inflammation, anemia, diabetes, jaundice, allergic responses, rheumatism, antioxidant, heart disease, leprosy, etc. [2–4]. This pharmacokinetic potential of T. cordifolia is due to its diverse chemical constituents such as alkaloids, sesquiterpenoids, diterpenoid, steroids, phenolic, glycan, flavonoids, tannins, coumarins, and xanthones, hence also known as ‘Amrita’ in ancient literature [5–8]. T. cordifolia is reported to enhance the production of inflammatory cytokines such as IL-1 β, IL-6, TNF-α to reduce the metastatic progression of B16-F10 melanoma [1]. It also restores the antioxidant pool to attenuate asthma, insulin resistance, obesity, hyperlipidemia, etc. to sustain the life potency of an individual [9–11]. Furthermore, the non-toxic nature of TC in acute toxicity has also been reported [1]. This indigenous agent suppresses the hepatotoxicity, normalizes the AST, ALT, SOD, GPx, catalase level to inhibit the formation of the necrotic lesion [12]. A recent study reported that TC extract declined the dopamine and H2O2 clearance in Parkinson’s disease [13]. Moreover, the plant and its polyherbal formulation didn’t exhibit any moderate and acute toxicity in the individuals [14].
Hypertriglyceridemia (HG) is a metabolic syndrome characterized by abnormal plasma triglycerides level reflecting the dysregulation of lipid metabolism and other metabolic complications such as pancreatitis, CVD, resistance to insulin, pro-inflammatory, etc. Progressive HG strongly condemns the atherogenic factor with an increased risk of mortality and morbidity. Therefore the management of elevated VLDL and suppressed HDL is still controversial in HG individuals [15, 16]. The disease is most prevalent in developing countries due to the change in lifestyle in the last decades [17]. Existing treatments for HG are less robust and difficult to treat the onset of preliminary complications. Therapeutic intervention by fibrates (metformin) and omega-3 fatty acids containing fish oils was found to reduce both fasting and postprandial serum triglyceride concentrations significantly while other drugs seemed to be less effective against HG [18]. Therefore, our prime objective was to assess the potential of T. cordifolia extract to improve the lipid quality in a randomized controlled clinical trial of patients with hypertriglyceridemia. Furthermore, the metabolomics studies were conducted to explore its mechanism of action and to ensure the safety and efficacy of TC extract.
Materials and methods
Chemicals
Solvents used for LC-MS such as acetonitrile, methanol, formic acid, and pantothenic acid, amino acids, acyl carnitine standards were purchased from Sigma (St. Louis, MO, USA). Internal and external calibrants, ESI tuning solution were purchased from Agilent Technologies. Diamet-metformin tablets (850 mg) were purchased from the local market (Bal Pharma Limited, India). Standards of isoprostanes (IsoPs) were purchased from Cayman Chemicals.
Preparation of extract
T. cordifolia was grown as a cultivar in the botanical garden of NRIBAS, Kothrud, Pune, India, identified and collected. The voucher specimen (No.207) was deposited in the Institutional herbarium. The stems were washed with distilled water properly, ground to paste, and extracted overnight with water (1:5 w/v) at room temperature in aseptic conditions. The extract was filtered through charcoal, cotton, and finally centrifuged at 5000 rpm. Charcoal filtration improves the concentration of some active metabolites in the TCE extracts i.e. magnoflorine, palmitate etc. due to adsorption of some unwanted compounds (Figure S1 A-D). The fresh extract was given to the volunteers to avoid the degradation of phytoconstituents under the guidance of an Ayurvedic physician. Extracts were subjected to LC-MS analysis for quality control purposes as discussed previously [19].
Ethics statement
The randomized trial was approved by the Human Ethics Committee of the PDDYP Ayurveda College, Pune, India with letter No. RRI/2011/HEC/2023 dated 18-02-2011. While doing actual experimentation no deviations were made from the approved study protocol. This clinical trial was registered with the Clinical Trials Registry – India (CTRI) wide registration No. CTRI- 2016-08-007187.
Study protocol
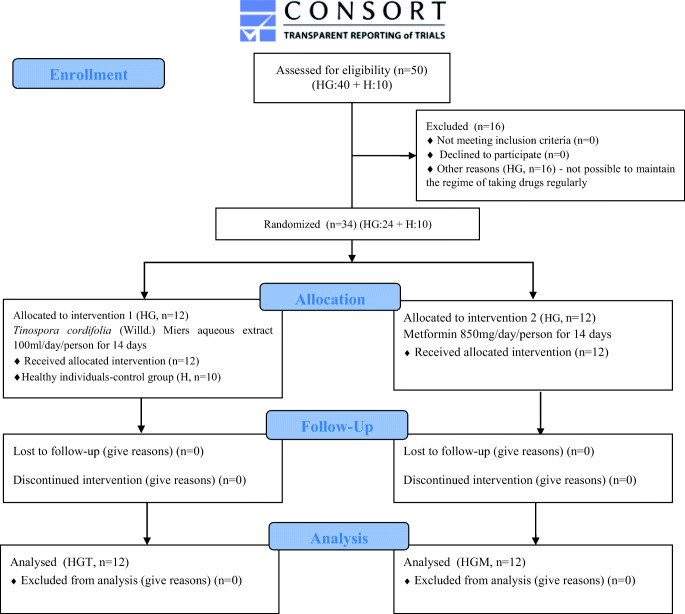
A phase of the randomized trial was conducted with patients having triglyceride >300 mg/dL and cholesterol in the range of 130–230 mg/dL. Healthy males served as a control group (n = 10) and HG (n = 24) patients who completed the above criteria were selected for the present study from the local areas of Pune, Maharashtra, India. The NRIBAS, Pune was the only place for sample collection. The sample size was calculated using Gpower analysis 3.1. The initial study was conducted in small scale blocks randomized using the Container method for patients and the control group to achieve an equal number of allocation ratios and to reduce the variability among the treatment. An Ayurvedic physician assigned random allocation using numbered containers and concealed the sequence until intervention was dispensed. The present trial had 14 days of treatment period followed by 1 week of post and pre-treatment observation. The clinical status of individual patients was assessed at baseline and followed for all. By contrast, patients having mass index >30 kg/m2, blood pressure > 160/90 mmHg with the present or prior history of cardiovascular diseases, diabetes mellitus, respiratory, gastrointestinal, hepatic, renal, endocrine, or reproductive disorders or use of anti-hypertensive agents were excluded from the study. Exceptionally, HG patients (n = 16) who were unable to maintain the regime of taking drugs regularly were also excluded from the study. Patients received a daily dose of 100 ml (~3.0 g solid extracts) TCE extract and 850 mg Diamet-metformin before breakfast orally. No adjustments to the dose were made during the study. On day 0 and 14th, 12 h fasting blood and first-pass urine samples were collected in the morning. Urine samples were collected in tubes that had sodium azide (2.5 mM), centrifuged, filtered (0.2 μm filters), and were stored at −80 °C till further analysis. Plasma fractions were separated from collected blood samples and subjected to blood biochemical auto-analyzer (Sinnowa, China) and blood cell counter (Erma, China). In this study, we also gave TCE treatment to a healthy group for 14 days (HT) to study any changes in metabolism and biochemical level (Fig. 1). The primary outcomes were characterized through changes in basal lipid profiling of patients, including HDL, VLDL, LDL, TG, etc. Baseline lipid profiling was measured after and before the administration of the first dose of study TCE. The secondary outcomes were measured to study the change in elevated oxidative stress and comparing the urinary metabolite profiles of control and treated groups.
Fig. 1.
A CONSORT flow diagram of study design, sample collection, data processing, and data analysis in different study groups
Quantitative analysis of isoprostanes to assess the oxidative burden
The urine samples were also analyzed in selective ion monitoring mode to study molecular ions of six isoprostanes (IsoPs) along with one deuterated isoprostane to maintain quality control (Table 2). HPLC-QTOF-MS conditions for IsoPs analysis were similar to qualitative metabolomics studies except for collision energy. Collision energies for each IsoPs were kept as per suggested earlier [20]. The IsoPs quantification was done by spiking samples with standard IsoPs (from 0.01 to 50 μg/ml). Each IsoPs standard and urine samples were quantified by Agilent MassHunter Quantitative Analysis software (version B.04.00) from the area ratio of the peaks. Concentrations of IsoPs in urine samples were analyzed using a standard curve with the highest correlation coefficient (R2 > 0.95). The limit of detection (LOD/ qualifier) and limit of quantitation (LOQ/quantifier) values were calculated based on the reported formulae, LOD = 2 N/m, where N is the noise value in the signal-to-noise ratio (SNR), m is the slope in peak height v/s concentration plot; LOQ = 5 x LOD.
Table 2.
Table is showing precursor ion, the transition of the parent ion, retention time (RT), calibration range, R2, collision energy, LOD and LOQ for the isoprostanes analyzed in both positive and negative mode of ion polarity
| S. no. | Name | Precursor Ion [M + H]+/ [M-H]− | Transition | RT (min) | Calibration range (μg/ml) | R2 | CE (V) | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8-iso PGA1 | 335.5 | 335.51 → 112.98 | 9.64 | 0.01–50.00 | 0.9742 | 28 | 0.256 | 1.281 |
| 2 | Prostaglandin F2α | 353.4 | 353.48 → 193.26 | 11.92 | 0.01–50.00 | 0.9979 | 30 | 0.007 | 0.039 |
| 3 | 11-epi-Prostaglandin F2α | 355.5 | 355.50 → 314.97 | 26.13 | 0.01–50.00 | 0.9113 | 30 | 0.015 | 0.078 |
| 4 | Prostaglandin F1α | 357.5 | 357.51 → 105.03 | 23.52 | 0.01–50.00 | 0.9997 | 30 | 0.072 | 0.360 |
| 5 | PGF2α –d 4 | 357.5 | 357.52 → 112.98 | 28.41 | 0.01–50.00 | 0.9065 | 30 | 0.700 | 3.502 |
| 6 | 8-iso-16-cyclohexyl-tetranor-PGE2 | 377.5 | 377.50 → 190.94 | 28.10 | 0.01–50.00 | 0.9972 | 32 | 0.00095 | 0.0047 |
PGA1, Prostaglandin A1, PGF2α –d 4 Prostaglandin F2α-d4(Dinoprost-d4), PGE2 Prostaglandin E2
Qualitative metabolomics studies
Urine samples were diluted with acetonitrile: water (80:20 v/v) in a 1:4 ratio, centrifuged at 8000 rpm for 5 min at 15 °C. Samples (20 μl) were injected into the HPLC (Agilent 1290 Infinity Series) fitted with ZORBAX 300SB C-18 (4.6 × 150 mm, 5.0 μm particle size) and interfaced to Agilent 6538 Accurate-Mass Q-TOF-MS to resolve urine samples. The mobile phase had (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile: water (80:20). Initially, mobile phase A was 5% in the isocratic mode for the first 5 min and then increased to 95% in a linear gradient mode up to 40 min. It decreased back to 5% up to 45 min and remained constant for the next 5 min. The flow rate of the mobile phase was 0.3 ml/min. Parameters of Q-TOFMS were optimized for the positive ion polarity mode. It was extended to dynamic mode (1700 m/z, 2GHz) with fragmentor voltage 195 V, ramped collision energy, and 3 spectra s−1 acquisition rate. Continuous internal calibration was maintained during the data collection. MassHunter Qualitative Analysis (B.04.00, Agilent Technologies) software was used for preliminary analysis of raw MS/MS data. Signal to noise ratio was calculated automatically from the initial and last points of spectra and deducted from the spectra. Molecular features having >5000 cycles per second (cps), minimum absolute counts 10, relative height (5%) of largest peak, peak space tolerance, i.e. 0.0025 m/z, and 5.0 ppm were extracted from mass spectra to avoid false molecular features.
Statistical analysis
For the present clinical trial, baseline data were represented as mean ± SD using GraphPad Prism 7 to assess the effect of pre and post-treatment of TCE and metformin in HG patients. The sample size and power analysis were calculated using Gpower software version 3.1. Sample size (n = 10) per group, significance level (α > 0.05), a standardized difference (d = 0.5), and 80% statistical power was used in the study. For the analysis of mass data, raw data were imported in the Mass Profiler Professional software (B.12.02, Agilent Technologies) and the molecular features having mass error < 5.0 ppm and retention time (RT) variation <0.2 min were aligned. Compounds present in less than 75% of samples of a group and having p > 0.05, fold change >2.0, and CV < 15 were removed from data sets and subjected to principal component analysis (PCA). Partial least-squares-discriminant analysis (PLS-DA) was performed to distinguish different groups and explore unique features present across the groups. Also, interlinking between the metabolic pathways was analyzed through Cytoscape 3.8.0.
Results
Characterization of TCE
An optimized solvent system, HPLC-QTOF-MS parameters, and major metabolites present in water extract of T. cordifolia have already been described in previous studies [5, 21]. The total ion chromatogram of TCE showed the highest abundance of magnoflorine and palmatine along with other minor compounds i.e. columbin, tinocordifoliside A, sulfocysteine, and abscisic acid. PLS-DA analysis of mass data of randomly selected TCE samples showed only 4 different molecular features out of 2617 molecular features present across the samples. It was an insignificant difference and 99.7 samples were similar in composition. Multivariate analysis showed 0.987 ± 0.021% (mean ± SD) confidence in the data.
Clinical biochemistry
The baseline characteristic such as BMI, age, TG, VLDL, LDL, HDL, AST, ALT showed significant differences among the treatment groups (Table 1). Metformin treatments to HG patients suppressed glucose production (hepatic gluconeogenesis) and altered the levels of alanine transaminase/ aspartate transaminase (ALT/AST) as compared to non-treated HG. ALT activity was found to be elevated in HG (36.05 ± 6.37 U/L) group as compared to healthy (21.46 ± 2.22 U/L) and it decreased in TCE treated HG patients (24.95 ± 4.01 U/L). However, metformin treatment had significantly increased ALT activity (38.12 ± 5.43 U/L) in the HGM group (p < 0.001). AST activity was found low in HG patients (23.45 ± 4.21 IU/L) compared to the healthy group (31.10 ± 3.62 IU/L) while treatment with TCE and metformin to HG patients (HGT and HGM) decreased the AST activity to 20.55 ± 6.33 and 22.13 ± 6.23 IU/L (p < 0.05) respectively. This decrease in the activity of AST was also observed in the healthy group as well in TCE treated healthy group (HT; 18.96 ± 7.50 IU/L) as compared to the non-treated control group (H; 31.10 ± 3.62 IU/L). In the HG group, the TG level was recorded about 550.00 ± 18.58 mg/dl while on the TCE- and metformin-treated group (HGT and HGM) the level of TG was depleted to 380.45 ± 17.44, and 433.00 ± 13.56 mg/dl respectively. Likewise, levels of LDL were decreased to 133.25 ± 3.18 and 129.12 ± 13.59 mg/dL in HGT and HGM groups as compared to the non-treated HG group (157.91 ± 11.10 mg/dl). The level of HDL was found to be lower (35.50 ± 6.50 mg/dl) in the HG group as compared to H (45.10 ± 2.52 mg/dl) and this level increased in HGT and HGM groups i.e. 47.50 ± 9.05 and 42.50 ± 3.50 mg/dl. Like LDL, levels of uric acid were also found significantly very high in the HG group (7.10 ± 0.98 mg/dl) which reduced significantly to 5.15 ± 0.90 mg/dl after treatment with TCE. However, no such significant decrease in uric acid level (6.10 ± 0.77 mg/dl) was observed in the metformin-treated group (HGM). Metformin-treatment in the HG group reduced the levels of urea (24.15 ± 2.22 mg/dl), creatinine (0.88 ± 0.39 mg/dl), albumin (3.20 ± 0.43 g/dl), and glucose (89.56 ± 6.22 mg/dl) as compared to non- treated HG group. TCE treatment also reduced glucose levels (102.05 ± 7.83 mg/dl) but enhanced the Hb% (14.25 ± 0.63), number of RBCs and platelets in both HGT as well in HT groups. Overall clinical data illustrated that metformin was noticed to better in glucose reduction, but have negative effects on Hb% (11.02 ± 1.03) in the HGM group. However, TCE was found more effective as compared to metformin in controlling HG by downregulating the level of TG (380.45 ± 17.44 mg/dl), TCE (160.55 ± 3.36 mg/dl), and LDL (133.25 ± 3.18 mg/dl) in the HGT group.
Table 1.
The blood biochemistry, lipid profiles and hematocrit of all the groups employed in the study are expressed as mean ± SD(p < 0.05)
| S. no. | Parameters | Healthy (H) | Hypertriglyceridemia (HG) | ||||
|---|---|---|---|---|---|---|---|
| (Day 0) | TCE-treated plasma (Day 14) | HG (Day 0) | TCE-treated HG (Day 14) | Metformin-treated HG (Day 0) | Metformin-treated HG (Day 14) | ||
| 1. | Age (years) | 39.2 ± 2.4 | 39.2 ± 2.4 | 39.4 ± 1.6 | 39.3 ± 1.4 | 39.2 ± 2.0 | 39.2 ± 2.0 |
| 2. | BMI | 22.1 ± −2.2 | 22.3 ± −1.6 | 25.05 ± 1.65 | 24.56 ± 2.87 | 25.25 ± 2.6 | 24.8 ± 2.2 |
| 3. | TG (mg/dl) | 98.36 ± 3.01 | 85.20 ± 4.30 | 550.00 ± 18.58 | 380.45 ± 17.44 | 552.00 ± 17.28 | 433.00 ± 13.56 |
| 4. | TC (mg/dl) | 155.13 ± 4.43 | 140.20 ± 4.52 | 167.26 ± 6.06 | 160.55 ± 3.36 | 170.01 ± 5.05 | 169.70 ± 5.32 |
| 5. | HDL (mg/dl) | 45.10 ± 2.52 | 48.66 ± 5.82 | 35.50 ± 6.50 | 47.50 ± 9.05 | 33.23 ± 8.09 | 42.50 ± 3.50 |
| 6. | LDL (mg/dl) | 108.50 ± 6.11 | 99.73 ± 3.16 | 157.91 ± 11.10 | 133.25 ± 3.18 | 155.39 ± 23.09 | 129.12 ± 13.59 |
| 7. | VLDL (mg/dl) | 18.26 ± 3.65 | 14.65 ± 2.89 | 69.56 ± 5.66 | 31.85 ± 5.88 | 42.0 ± 2.43 | 25.081 ± 6.89 |
| 8. | Urea (mg/dl) | 17.56 ± 8.90 | 16.10 ± 4.00 | 30.26 ± 1.76 | 27.60 ± 5.93 | 26.20 ± 5.76 | 24.15 ± 2.22 |
| 9. | Creatinine (mg/dl) | 0.89 ± 0.66 | 0.83 ± 0.40 | 1.35 ± 0.43 | 0.95 ± 0.07 | 0.92 ± 3.33 | 0.88 ± 0.39 |
| 10. | Total Protein (g/dl) | 5.6 ± 0.36 | 6.3 ± 0.36 | 5.35 ± 0.47 | 5.0 ± 0.56 | 5.23 ± 0.71 | 5.05 ± 0.55 |
| 11. | Albumin (g/dl) | 4.06 ± 0.40 | 3.30 ± 2.04 | 5.20 ± 0.17 | 3.90 ± 1.27 | 3.98 ± 2.02 | 3.20 ± 0.43 |
| 12. | ALT (U/L) | 21.46 ± 2.22 | 21.06 ± 6.50 | 36.05 ± 6.37 | 24.95 ± 4.01 | 39.10 ± 6.34 | 38.12 ± 5.43 |
| 13. | AST (IU/L) | 31.10 ± 3.62 | 18.96 ± 7.50 | 23.45 ± 4.21 | 20.55 ± 6.33 | 22.67 ± 6.01 | 22.13 ± 6.23 |
| 14. | Uric acid (mg/dl) | 4.93 ± 0.91 | 4.16 ± 0.65 | 7.10 ± 0.98 | 5.15 ± 0.90 | 5.51 ± 0.41 | 6.10 ± 0.77 |
| 15. | Glucose (mg/dl) | 97.06 ± 7.06 | 85.16 ± 6.55 | 105.95 ± 8.55 | 102.05 ± 7.83 | 95.23 ± 6.43 | 89.56 ± 6.22 |
| 16. | Hemoglobin % (g/dl) | 13.63 ± 1.60 | 14.90 ± 1.72 | 12.65 ± 1.34 | 14.25 ± 0.63 | 10.34 ± 1.05 | 11.02 ± 1.03 |
| 17. | RBCs (× 106) | 4.79 ± 0.37 | 5.23 ± 0.12 | 4.66 ± 0.67 | 5.06 ± 0.07 | 4.02 ± 0.80 | 4.73 ± 0.78 |
| 18. | WBCs (× 103) | 7.35 ± 1.01 | 6.48 ± 1.22 | 7.95 ± 0.35 | 7.35 ± 1.76 | 8.98 ± 0.40 | 8.13 ± 0.54 |
| 19. | Platelets (× 103) | 296.40 ± 12.36 | 313.60 ± 8.27 | 276.50 ± 9.45 | 342.50 ± 4.74 | 275.87 ± 56 | 280.50 ± 8.66 |
BMI Body Mass Index, TG Triglycerides, TC Total Cholesterol, HDL High Density Lipoprotein, LDL Low Density Lipoprotein, VLDL Very Low Density Lipoprotein, AST Aspartate transaminase, ALT Alanine transaminase, RBCs Red Blood Cells, WBCs White Blood Cells
Oxidative burden measurement
IsoPs levels in the urine samples from all groups were analyzed using a selective ion monitoring method with predetermined LCMS parameters including fixed collision energy (eV) in both positive and negative modes of ion polarities. The average LOD and LOQ for all the IsoPs were observed 0.175 μg/ml and 0.8774 μg/ml, respectively, with >0.90 correlation coefficient (R2) under both ionic modes (Table 2). In the present study it was observed that the levels of most of the IsoPs were increased (about 1.5–3.0 fold, p < 0.001) in the HG patients compared to control and it reached normal or below normal upon treatment with TCE in the HGT group (Table 3).
Table 3.
Table with quantity of each of IsoPs analyzed under present study in both ion polarity modes and expressed in μg/ml with mean ± SD (p < 0.01)
| S. no. | Name of IsoPs analyzed | Urine lipid positive | Urine lipid negative | ||||
|---|---|---|---|---|---|---|---|
| H | HG | HGT | H | HG | HGT | ||
| 1 | 8-iPGA1 | – | – | – | 6.126±0.707 | 19.924±0.707 | 1.742±0.464 |
| 2 | PGF2α | – | – | – | 0.092±.038 | 0.129±0.045 | 0.097±0.026 |
| 3 | 11-epi -PGF2α | 5.549±.869 | 8.821±1.626 | 6.357±1.414 | – | – | – |
| 4 | PGF1α | 4.262±2.875 | 5.563±1.414 | 4.084±1.414 | – | – | – |
| 5 | PGF2α-d4 | – | – | – | – | – | – |
| 6 | 8-iso − 16-cyt-PGE2 | – | – | – | 0.219±0.07 | 0.719±0.063 | 0.036±0.014 |
8-iPGA1 8-iso Prostaglandin A1, PGF2α Prostaglandin F2α, 11-epi -PGF2α 11-epi-Prostaglandin F2α, PGF1α Prostaglandin F1α, PGF2α-d4 Prostaglandin F2α-d4 (Dinoprost-d4), 8-iso −16-cyt-PGE2 8-iso-16-cyclohexyl-tetranor- Prostaglandin E2
Multivariate analysis of urinary metabolome
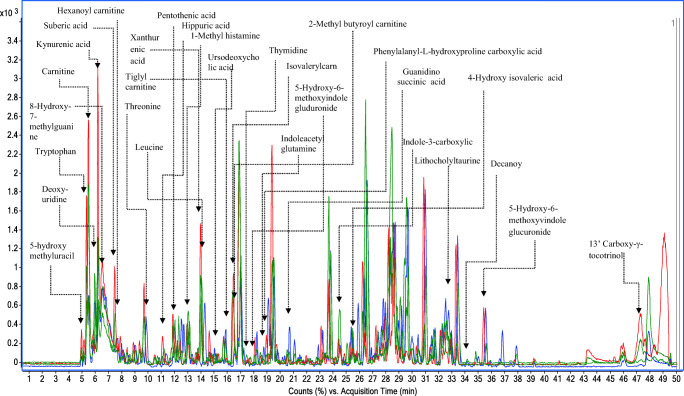
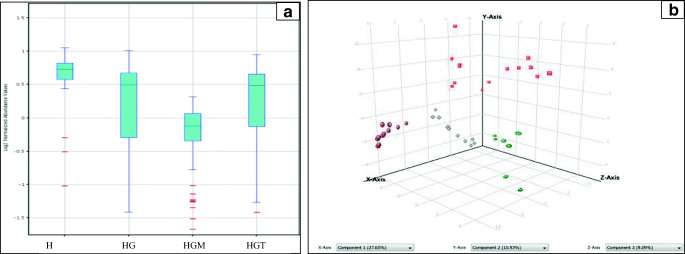
Analysis of total ion chromatograms showed an average of 273 peaks containing around 1567 compounds/spectrum. The analysis method was optimized and the results were reproducible with minimum variations across the samples. The extracted base peak chromatograms of 4 study groups are shown in (Fig. 2). Variability in the higher peaks was normalized successfully using internal standards and Z-transforms. The overall percent variance was represented as a box-whisker plot showing altered metabolites across all the 4 groups, i.e. H, HG, HGM, and HGT. The box-and-whisker (Fig. 3a) and PCA score (Fig. 3b) plots didn’t show drift during the HPLC-QTOF-MS data analysis. Supervised PCA of H, HG, HGT, and HGM groups showed 27.65%, 10.93%, variability along the X and Y-axis. Reproducible and stable molecular features were subsequently used for statistical analysis. The well-established PLS-DA and support vector machines (SVMs) models demonstrated satisfactory modeling for LC-MS (R2 = 0.97, and Q2 = 0.86). The results of the sample classification presented in terms of discrimination and recognition abilities were found to be 93.34% and 97.33% accurate, representing the percentage of the samples correctly classified during model training and cross-validation. Data were further subjected to Bonferroni FWER multiple testing correction to decrease the false discovery rate. The R2 and Q2 values of the original SVMs and PLS-DA models were higher than the randomly classified permutation distribution; this shows that both the original models were valid.
Fig 2.
Extracted +ESI base peak chromatograms (BPC) are showing percent abundance of peaks in the urine samples of healthy (H, dark black), hypertriglyceridemic (HG, red), TCE-treated hypertriglyceridemic (HGT, green) and metformin-treated hypertriglyceridemic (HGM, blue) groups
Fig. 3.
a The box-and-whisker plot showing distribution of metabolites in healthy (H), hypertriglyceridemic (HG), and metformin treated (HGM), and TCE treated (HGT) groups of hypertriglyceridemic patients. b PCA plot of metabolic variables in samples where brown dots indicates healthy (H) population, red squares indicates hypertriglyceridemic (HG) population, grey squares indicates metformin treated hypertriglyceridemic (HGM) population, and green circles indicates TCE treated hypertriglyceridemic (HGT) population
Differentially expressed metabolites in different groups
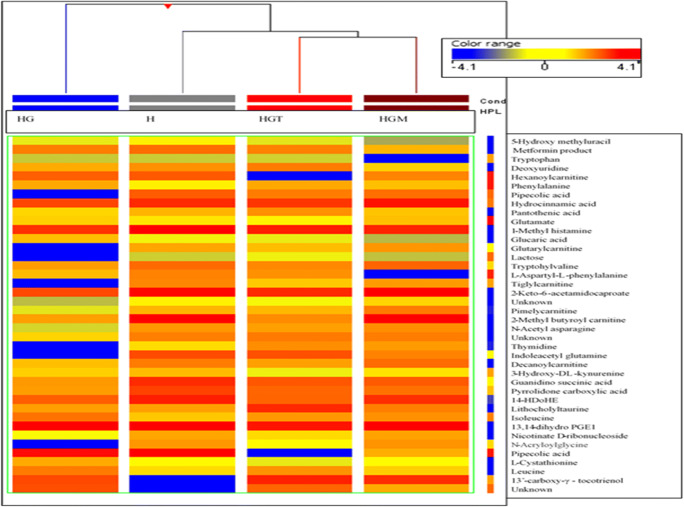
Further correction of the data obtained from PLS-DA with Bonferroni FWER multiple testing corrections to omit false values and less significant metabolites showed 105 differentially expressed metabolites. Data were further filtered and metabolites with any missing values (n = 72) were filtered out. Finally, 40 differentially expressed metabolites present in all groups were obtained and identified using Metlin (accurate ms/ms) library (metabolite.cdb) having 19,608 human metabolites and standards (Table 4). A Pearson co-relation heat map of these selected metabolites was also generated to understand the level of relatedness between these metabolites across 4 study groups (Fig. 4). The correlation map also showed that the HG group was the most discrete while the treated groups, i.e. HGM and HGT were near to the healthy group indicate the regulatory effect of TCE and metformin on HG. Moreover, TCE treatment was found more effective than metformin, and as a result, the HGT group appeared closer to the healthy group as compared to the HGM group. Pantothenic acid is an important variable of hyper-triglyceridemia and it was found upregulated in HG (3.88 fold) group while reverted significantly in HGM (1.17 fold) and HGT (2.42 fold) groups. Hexanoylcarnitine was observed to be increased significantly by 3.96 fold in the HG group, which decreased to −1.43 and − 1.16 fold in HGT and HGM groups respectively. Other carnitine derivatives like tiglylcarnitine and glutarylcarnitine were found to be increased by more than four folds in HG patients (Table 4). All these above-mentioned carnitine derivatives were found to be significantly down-regulated in HGT and HGM groups. D-Lactose, a primary disaccharide in the human diet, was found to be depleted in HG (−1.52 fold), HGM (−1.07 fold), and HGT (−1.70 fold) groups. Urinary glucaric acid was found to be increased in HG by 2.21 fold as well as in the HGT group by 1.49 fold, whereas, HGM group showed a decrease in its level by 1.79 fold. Proteins are referred as building blocks for numerous compounds and influence structural and signaling activities. Urinary levels of leucine and isoleucine were increased in HG patients by 1.52 and 3.88 folds and decreased in both treated groups (Table 4). Phenylalanine was found to be increased in the HG group by 2.12 folds and depleted in HGM (1.04 fold) and HGT (−1.61 fold) groups. N-Acetyl asparagine, L-aspartyl-L- phenylalanine, and indole acetyl glutamine showed 2.07, 1.77, and − 3.55 fold changes in HG groups, respectively. 13’-Carboxy-gamma-tocotrienol, an antioxidant, found slightly upregulated in HG (1.73 fold), whereas, more reduced levels in HGT (−3.85 fold) than HGM (−2.68 fold) were observed. Thymidine nucleotide levels were found to be increased in HG (3.93) and decreased in HGM (−2.11 fold) and HGT (−1.68 fold) groups. Increased levels of mitochondrial dysfunction and DNA damage marker, i.e. 5-hydroxy methyluracil (nucleotide lesion product), were noticed in HG (1.50 fold), while HGM and HGT groups showed 1.79 and 1.17 folds regulation respectively. Inflammatory marker’s like 1-methyl histamine level was increased in HG (4.02 fold) but found depleted in HGM (1.68 fold) and HGT (2.10 fold) as compared to the healthy group. Level of L- cystathionine, a biomarker of increased oxidative stress, impaired methylation, and the manifestation of vitamin-B6 deficiency, was found to be elevated in HG (3.34 fold) and decreased in HGM (−2.16 fold) and HGT (−2.87 fold) groups, maybe because of cystathionuria in HG. Deoxyuridine, a naturally occurring nucleoside, was found to be reduced in HG (−1.03 fold) and HGT (−1.25 fold) but upregulated in HGM (1.79 fold) groups. Nicotinate D-ribonucleoside was found to be decreased in HG (−3.82 fold) and the HGT (−3.59 fold) group along with a slight increase in HGM (−2.13 fold) level. Guanidinosuccinic acid, synthesized by oxidation of argininosuccinic acid by free radicals, was found to be elevated in HG (2.12 fold), in HGT (1.61 fold) group but not in HGM (2.05 fold) (Table 4).
Table 4.
Urinary metabolites without missing valuesextracted from PLS-DA data and according to their significance and variability
| S. no. | Compound mass | RT | Tentative identification | p (Corr) | FC HG | FC HGM | FC HGT |
|---|---|---|---|---|---|---|---|
| 1. | 315.23328 | 33.99 | Decanoylcarnitine | 0.011 | −4.04 | −2.16 | 3.03 |
| 2. | 256.14423 | 33.32 | Nicotinate D-ribonucleoside | 0.011 | −3.82 | −2.13 | −1.59 |
| 3. | 303.11615 | 18.62 | Indoleacetyl glutamine | 0.011 | −3.55 | −3.69 | −3.59 |
| 4. | 491.99985 | 33.22 | Unknown | 0.020 | −3.03 | −2.97 | −2.28 |
| 5. | 129.04219 | 19.16 | N-Acryloylglycine | 0.011 | −2.72 | 1.83 | 2.05 |
| 6. | 129.04288 | 17.45 | Unknown | 0.011 | −2.72 | 1.13 | 1.05 |
| 7. | 357.2402 | 32.85 | 14-HDoHE | 0.011 | −2.22 | −3.98 | −4.26 |
| 8. | 342.08438 | 14.64 | D-Lactose | 0.011 | −1.52 | −1.07 | −1.70 |
| 9. | 303.16367 | 14.82 | Unknown | 0.011 | −1.29 | 1.96 | 2.27 |
| 10. | 228.10681 | 5.45 | Deoxyuridine | 0.011 | −1.03 | 1.79 | −1.25 |
| 11. | 150.05238 | 8.90 | Hydrocinnamic acid | 0.020 | 1.02 | 1.29 | −2.15 |
| 12. | 241.98705 | 4.91 | Metformin product | 0.011 | 1.03 | −1.79 | 1.25 |
| 13. | 187.0607 | 15.72 | 2-Keto-6-acetamidocaproate | 0.011 | 1.09 | −3.54 | −3.08 |
| 14. | 243.14482 | 15.70 | Tiglylcarnitine | 0.011 | 1.24 | −3.29 | −3.15 |
| 15. | 142.03287 | 4.85 | 5-Hydroxy methyluracil | 0.020 | 1.50 | 1.79 | 1.17 |
| 16. | 147.10214 | 12.71 | Glutamate | 0.020 | 1.50 | 1.79 | −1.17 |
| 17. | 131.09428 | 17.45 | Leucine | 0.011 | 1.52 | −1.07 | 1.70 |
| 18. | 224.10178 | 19.65 | 3-Hydroxy-DL-kynurenine | 0.011 | 1.69 | −2.61 | −1.25 |
| 19 | 440.30023 | 47.23 | 13’-Carboxy-γ-tocotrienol | 0.011 | 1.73 | −2.68 | −3.85 |
| 20. | 280.08563 | 15.15 | L-Aspartyl-L-phenylalanine | 0.011 | 1.77 | 3.98 | −1.00 |
| 21. | 483.26508 | 32.82 | Lithocholyltaurine | 0.011 | 1.79 | 1.39 | 1.54 |
| 22. | 357.2444 | 33.30 | 13,14-dihydro PGE1 | 0.02 | 1.84 | −3.39 | −3.92 |
| 23. | 303.28528 | 45.27 | Pimelylcarnitine | 0.011 | 1.91 | −2.27 | −1.97 |
| 24. | 174.06883 | 17.45 | FN-Acetyl asparagine | 0.011 | 2.07 | 3.22 | 2.89 |
| 25. | 165.04526 | 7.98 | Phenylalanine | 0.011 | 2.12 | 1.04 | −1.61 |
| 26. | 175.0612 | 20.54 | Guanidinosuccinic acid | 0.011 | 2.12 | 2.05 | 1.61 |
| 27. | 204.1424 | 5.08 | Tryptophan | 0.011 | 2.21 | 2.74 | 2.09 |
| 28. | 210.04611 | 14.01 | Glucaric acid | 0.011 | 2.21 | 1.79 | 1.49 |
| 29. | 245.1568 | 16.73 | 2-Methyl butyroyl carnitine | 0.011 | 2.59 | −1.88 | −1.81 |
| 30. | 129.05501 | 20.54 | Pyrrolidone carboxylic acid | 0.011 | 2.98 | 2.06 | 1.93 |
| 31. | 129.04146 | 16.55 | Pipecolic acid | 0.015 | 3.03 | 1.98 | 1.03 |
| 32. | 222.05516 | 41.05 | L-Cystathionine | 0.011 | 3.34 | −2.16 | −2.87 |
| 33. | 275.17236 | 13.92 | Glutarylcarnitine | 0.020 | 3.59 | −3.30 | −1.18 |
| 34. | 219.11171 | 10.72 | Pantothenic acid | 0.011 | 3.88 | 1.17 | 2.42 |
| 35. | 131.09246 | 16.55 | Isoleucine | 0.03 | 3.88 | −1.29 | −1.68 |
| 36. | 242.12325 | 17.48 | Thymidine | 0.011 | 3.93 | −2.11 | −1.68 |
| 37. | 259.13837 | 7.74 | Hexanoyl carnitine | 0.011 | 3.96 | −1.16 | −1.43 |
| 38. | 125.08251 | 12.95 | 1-Methyl histamine | 0.030 | 4.02 | 1.68 | 2.10 |
| 39. | 261.15375 | 8.09 | Gamma glutamylornithine | 0.011 | 4.13 | −1.54 | −1.30 |
| 40. | 315.15875 | 16.48 | Tryptophylvaline | 0.011 | 4.84 | −3.16 | −3.03 |
Metabolites were identified using various metabolomics databases in ESI + mode (p < 0.05). 14-HDoHE 14-hydroxydocosahexaenoate, PGE1 Prostaglandin E1
Fig. 4.
Pearson heat map alignment denoting fold changes of 40 listed metabolites across healthy (H), hypertriglyceridemic (HG), metformin-treated HG (HGM) and TCE-treated HG (HGT) groups. The columns correspond to different individual groups and rows correspond to the altered metabolites. Correlation map is showing HG group as most discrete group and metformin /TCE treated groups were near to healthy group. Moreover, TCE treatment is more effective than metformin and as a result HGT group is closer to the H group than HGM group
Pyrrolidone carboxylic acid was found to be elevated in the HG, HGM, and HGT groups by 2.98, 2.06, and 1.93 folds respectively. No significant alteration in the lithocholyltaurine levels was observed in the treated groups and found to be depleted in all the study groups. 2-Keto-6-acetamidocaproate, an intermediate in lysine degradation, was also found to be elevated considerably in HG (1.09 fold) as compared to HGM (−3.54 fold) and HGT (−3.08 fold) groups. Levels of gamma glutamylornithine were increased in HG by 4.13 folds and decreased in HGM and HGT groups by −1.54 and − 1.30 fold. 3-Hydroxy-DL-kynurenine, a tryptophan metabolite, and free radical generator showed increased levels in HG (1.69 fold) and decreased levels in HGT (−1.25 fold) and HGM (−2.61 fold) groups as compared to healthy individuals (Table 4).
Pathway analysis
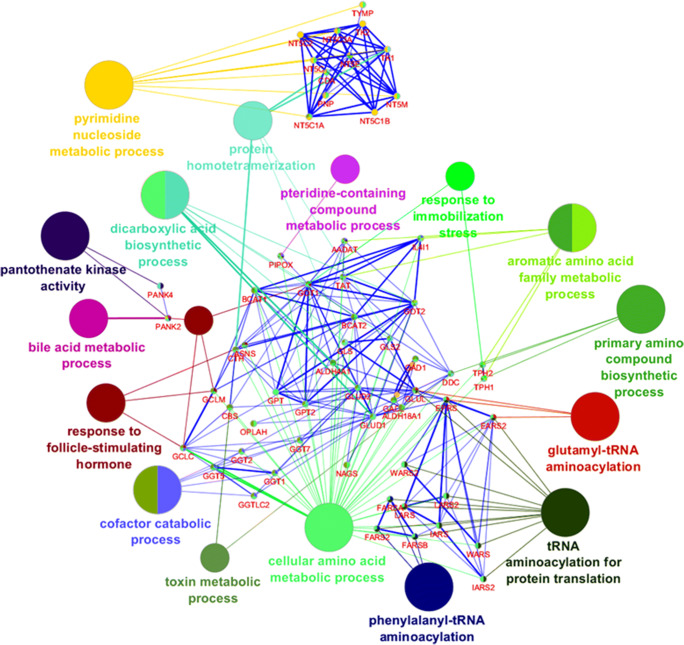
The total number of pathways affected in the HG group and further altered upon drug-treatments was identified by analyzing the level of altered metabolites using Cytoscape. Analysis from Cytoscape showed that in the HG patient’s bile acid, vitamin B5 - CoA biosynthesis, biopterin, butanoate, vitamin B3, vitamin B9, histidine, lysine, methionine, cysteine, pyrimidine, tryptophan, tyrosine metabolisms, urea cycle, and metabolism of arginine, proline, glutamate, aspartate, and asparagine are the most affected metabolomics pathways. Along with these metabolic pathways valine, leucine, and isoleucine degradation were also found to be elevated significantly. Altered metabolic pathways also altered biological processes and further analysis with Cytoscapeplugin ClueGo showed significantly altered pathways in HG and restored by TCE (Fig. 5).
Fig. 5.
Major biological processes found to be affected in HG pateints and modulated after treatment with metformin and TCE
Discussion
T. cordifolia is being used as an immunomodulatory drug and to treat various disease conditions including metabolic disorders in the traditional medicinal systems across the world [3]. In the present study, clinical trials were conducted employing standard protocols to evaluate the efficacy and mechanism of TCE in HG patients (Fig. 1). Being a metabolic disorder, HG leads to several interrelated disorders like cardiovascular diseases and diabetes mellitus acute pancreatitis, etc. [15, 16]. This progressive disease starts from disturbed basal parameters and inflammation. The present study revealed that the TCE intervention reduced the TG, LDL, and VLDL levels significantly in the HG patients and normalized the levels near to control group (Table 1). TCE intervention also significantly improved the plasma HDL content, decreased abnormal lipid biomes, and subsequently altered the associated parameters such as creatinine, AST, ALT, uric acid, etc., in TCE treated group.
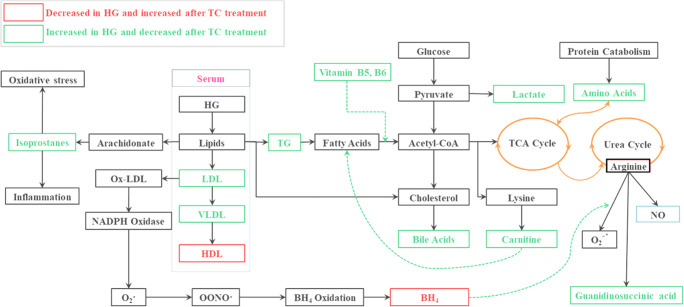
Arginine plays an important role in the urea cycle and in the removal of oxidative stress. Oxidative stress converts arginine into guanidinosuccinic acid due to the uncoupling of eNOS by free oxygen radicals [22]. Hence, increased levels of guanidinosuccinic acid and cystathione in HG patients confirmed the increased oxidative stress (Table 4). Arginine depletion and homocysteine accumulation have been reported to lead liver cirrhosis [23]. Elevated eNOS in HG individuals signified the abnormal regulation of biopterin metabolism, which adversely affected the urea cycle. Super-oxides released by eNOS contribute to atherogenesis [24, 25]. This is due to the depletion of tetrahydrobiopterin (BH4) [26] that point was confirmed by the decreased levels of BH4 and elevated levels of guanidinosuccinic acid in HG patients (Table 4, Figs. 4, 5). While altered urea cycle causes the release of more butanoate or dicarboxylic acids due to decrease fatty acid oxidation [27]. Observed folate deficiency in HG patients can also be correlated with increased urinary cystathionine ascribed to dysregulation of sulfur-containing amino acid metabolism [28]. This fact was confirmed through an elevated level of cystathionine in HG patients in the present study also (Table 4). Besides, oxidative stress produces the cyclooxygenase derived prostaglandin F2-like compounds commonly called isoprostanes [29–31]. Urinary metabolome showed that isoprostanes i.e. PGF2α, 11-epi-PGF2α, PGF1α, PGA1 and 8-iso −16-cyt-PGE2 were significantly up-regulated in HG individuals. While TCE intervention significantly suppressed the isoprostanes levels (Table 3).
Outcomes from biochemistry and hematology were further confirmed by urine metabolome analysis. Box-whisker and PCA plot signifies the variability among the groups and showed the shift of TCE treated HG group near to healthy group (Fig. 3a, b). Further, the clear shift of TCE treated HG group toward healthy control was also observed on the Pearson heat map constructed from 40 significantly altered metabolites. The correlation map depicted the HG group as the most discrete group and metformin or TCE treated groups were near to the healthy group. Moreover, TCE treatment was more effective than metformin, and as a result HGT group was closer enough towards the healthy suspects than HGM treated individuals (Fig. 4). Increased excretion of branched-chain amino acids (BCAA), tryptophan, phenylalanine, lysine, and glutamine metabolism were noticed in HG patients (Table 4). It is a good fact that increased levels of BCAA are linked not only to insulin resistance in skeletal muscle but also to the pathophysiological conditions of the heart [32, 33]. By contrast, disturbed branched-chain amino acids (BCAA) metabolism leads to ketosis in diabetes mellitus [34]. Therefore, increased levels of urinary BCAAs have been reported in hyper-triglyceridemia independent of fasting blood glucose levels. Hence, BCAAs play an important role in hyper-triglyceridemia. Another important observation of the present investigation strongly supports the association between amino acids and HG but could not detect a relationship between glucose and HG, as glucose level remained normal. Therefore, the study supports the earlier findings [35, 36] that HG does not appear to be directly related to insulin sensitivity in this study.
Likewise, the correlation of BCAA and aromatic amino acid metabolism with obesity (an intermediate stage of HG) is well established, but their role in HG is needed to be explored further [37]. TCE intervention reduced the excretion of BCAA to a great extent. Low levels of BCAA have been reported to decrease fatty acid synthesis and increase β–oxidation [38, 39]. TCE intervention also upregulated the intermediate markers of PPARα and PPARγ activation i.e. decanoylcarnitine, glutarylcarnitine, tiglylcarnitine and acylcarnitines (Table 4). Carnitines help in transportation of the activated form of fatty acid into the mitochondrion for its further metabolism via β-oxidation to generate energy [40]. Activation of PPARγ, AMPK, and increased translocation of GLUT-1 by one of the Tinospora species has been reported previously by Noipha et al. [41].
Similarly, increased excretory levels of pantothenic acid and pyridoxal 5′-phosphate (B6) in the HG patients indicated blocked fatty acid activations i.e. conversion of fatty acids into fatty acyl-CoA [42]. These findings were supported through an increased urinary level of N- acryloyl glycine in HG patients, a biomarker of disorders associated with mitochondrial fatty acid beta-oxidation. Reduced levels of pantothenate and pyridoxine in serum also slow down the conversion of pyruvate to acetyl-CoA, resulting in the accumulation of pyruvate and poor formation of citric acid and TCA cycle intermediates. Hence, higher levels of serum triglycerides and urinary carnitines, pantothenic acid, vitamin B6, and acryloyl glycine in HG patients support the possibility of a mitochondrial disorder and liver dysfunction. Therefore, TC administration restored the normal levels of these metabolites and reduced their excretion further. This is under a previous study where TCE was observed to increase the retaining power of the liver [5].
Metabolic pathways analysis with ClueGo plugins of Cytoscape 3.6.0 showed 14 metabolic pathways were affected significantly (p < 0.05) in HG patients and restored to normal by TCE (Fig. 5). These pathways can also be categorized as lipids, amino acids, vitamins, nucleosides related to metabolic pathways. Bile acids synthesis was found to be affected by HG patients. Increased excretion of lithocholyltaurine was observed in HG patients and it was reduced by metformin and more effectively by TCE. Existed literature showed that inhibition of PPARα in HG patients increased bile acid synthesis and their conjugation [42, 43]. Although the present study has some drawbacks due to the low number of participants which might be the source of some inadequate statistical conflicts. T. cordifolia intervention needs to be addressed more comprehensively which will be helpful in the promotion of existed reports towards the usage of herbal supplementation.
Overall, data present showed that TCE supplementation maintained the primary parameters in HG patients such as lipid profiles, liver function enzymes, urea cycle, and creatinine levels. TCE retained primary parameters through modulation of various key metabolic pathways and signaling. Primarily, the metabolomics profiles indicated the activation of PPARα and PPARγ to control mitochondria and fatty acid metabolism that was evident by increased levels of carnitines and decreased levels of BCAA and bile acids. Notably, TCE also alleviated the oxidative burden by suppressing the isoprostanes, cystathionine, and guanidinosuccinic acid formation and elevating the levels of BH4 and vitamin B5 and B6 (Fig. 6). These findings were endorsed by PLS-DA of metabolomics data. PLS-DA identified forty endogenic excreted compounds as markers of hypertriglyceridemia and most of them are different from hyperlipidemia. Also, the study supports the previous finding that branched-chain amino acid elevation in HG is independent of fasting blood glucose levels. The study provided strong evidence that TCE intervention was an effective way to treat hypertriglyceridemia and worked through the modulation of various metabolic pathways, oxidative stress, and inflammation.
Fig. 6.
Interconnectivity of metabolic pathways restored by TC intervention in HG pateints
Electronic supplementary material
(DOCX 101 kb)
Acknowledgements
Authors wish to acknowledge the CCRAS, Ministry of AYUSH, Govt of India for supporting the work.
Abbreviations
- HG
Hyper-triglyceridemia
- PPAR
Peroxisome proliferator-activated receptors
- PLS-DA
Partial least-squares-discriminant analysis
- HL
Hyperlipidemia
- H
Healthy
- RRLC
Rapid Resolution Liquid Chromatography
- ESI-Q-TOF-MS
Electrospray Ionization Quadrupole Time of Flight Mass Spectrometry
- TCE
Tinospora cordifolia Extract
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- RT
Retention time
Author’s contributions
AS and RD design the study and conducted the experiment. AY, RD and TK Mandal interpret the data and design the manuscript.
Compliance with ethical standards
Ethics approval
Human Ethics Committee of the PDDYP Ayurveda College, Pune, India approved the study wide letter no. RRI/2011/HEC/2023 dated 18-02-2011.
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Upadhyay A, Kumar K, Kumar A, Mishra H. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) -validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010:112. [DOI] [PMC free article] [PubMed]
- 2.Pathak P, Vyas M, Vyas H, Naria M. Rasayana effect of Guduchi Churna on the life span of Drosophila melanogaster. AYU (An Int Q J Res Ayurveda) 2016;37:67. doi: 10.4103/ayu.AYU_11_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque MA, Jantan I, Abbas Bukhari SN. Tinospora species: an overview of their modulating effects on the immune system. J Ethnopharmacol. 2017:67–85. [DOI] [PubMed]
- 4.Sannegowda KM, Venkatesha SH, Moudgil KD. Tinospora cordifolia inhibits autoimmune arthritis by regulating key immune mediators of inflammation and bone damage. Int J Immunopathol Pharmacol. 2015;28(4):521–531. doi: 10.1177/0394632015608248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma B, Dabur R. Protective effects of Tinospora cordifolia on hepatic and gastrointestinal toxicity induced by chronic and moderate alcoholism. Alcohol Alcohol. 2015;51:1–10. doi: 10.1093/alcalc/agv130. [DOI] [PubMed] [Google Scholar]
- 6.Parveen A, Wang YH, Fantoukh O, Alhusban M, Raman V, Ali Z, Khan IA. Development of a chemical fingerprint as a tool to distinguish closely related Tinospora species and quantitation of marker compounds. J Pharm Biomed Anal. 2020;178:112894. doi: 10.1016/j.jpba.2019.112894. [DOI] [PubMed] [Google Scholar]
- 7.Bajpai V, Kumar S, Singh A, Bano N, Pathak M, Kumar N, Misra-Bhattacharya S, Kumar B. Metabolic fingerprinting of dioecious Tinospora cordifolia (Thunb) Miers stem using DART TOF MS and differential pharmacological efficacy of its male and female plants. Ind Crop Prod. 2017;101:46–53. doi: 10.1016/j.indcrop.2017.02.037. [DOI] [Google Scholar]
- 8.Bhalerao BM, Vishwakarma KS, Maheshwari VL. Tinospora cordifolia (Willd.) Miers ex Hook.f. & Thoms.-plant tissue culture and comparative chemo-profiling study as a function of different supporting trees. Indian J Nat Prod Resour. 2013;4:380–386. [Google Scholar]
- 9.Tiwari M, Dwivedi UN, Kakkar P. Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1, pro-inflammatory cytokines and redox status in murine model of asthma. J Ethnopharmacol. 2014;153(2):326–337. doi: 10.1016/j.jep.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Reddy SS, Ramatholisamma P, Karuna R, Saralakumari D. Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male Wistar rats. Food Chem Toxicol. 2009;47(9):2224–2229. doi: 10.1016/j.fct.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Sharma AK, Gupta M, Singh AP, Kaur G. Tinospora cordifolia attenuates high fat diet-induced obesity and associated hepatic and renal dysfunctions in rats. PharmaNutrition. 2020;26:100189. doi: 10.1016/j.phanu.2020.100189. [DOI] [Google Scholar]
- 12.Baskaran R, Priya LB, Kumar VS, Padma VV. Tinospora cordifolia extract prevents cadmium-induced oxidative stress and hepatotoxicity in experimental rats. J Ayurveda Integr Med. 2018;9(4):252–257. doi: 10.1016/j.jaim.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosaraju J, Chinni S, Roy PD, Kannan E, Antony AS, Kumar MS. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced parkinsonism. Indian J Pharmacol. 2014;46(2):176–180. doi: 10.4103/0253-7613.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 1999;13(4):275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Kuchay MS, Farooqui KJ, Bano T, Khandelwal M, Gill H, Mithal A. Heparin and insulin in the management of hypertriglyceridemia-associated pancreatitis: Case series and literature review. Arch Endocrinol Metab. 2017:198–201. [DOI] [PMC free article] [PubMed]
- 16.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, REDUCE-IT Investigators Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 17.Chu AHY, Moy FM. Joint association of sitting time and physical activity with metabolic risk factors among middle-aged Malays in a developing country: a cross-sectional study. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed]
- 18.Hasani-Ranjbar S, Jouyandeh Z, Abdollahi M. A systematic review of anti-obesity medicinal plants – an update. J Diabetes Metab Disord. 2013. [DOI] [PMC free article] [PubMed]
- 19.Shirolkar A, Gahlaut A, Hooda V, Dabur R. Phytochemical composition changes in untreated stem juice of Tinospora cordifolia (W) Mier during refrigerated storage. J Pharm Res. 2013;7:1–6. [Google Scholar]
- 20.Chernushevich IV, Loboda AV, Thomson BA. An introduction to quadrupole-time-of-flight mass spectrometry. J Mass Spectrom. 2001;36:849–865. doi: 10.1002/jms.207. [DOI] [PubMed] [Google Scholar]
- 21.Shirolkar A, Sharma B, Lata S, Dabur R. Guduchi Sawras (Tinospora cordifolia): an Ayurvedic drug treatment modulates the impaired lipid metabolism in alcoholics through dopaminergic neurotransmission and anti-oxidant defense system. Biomed Pharmacother. 2016;83:1265–1277. doi: 10.1016/j.biopha.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 22.Aoyagi K. Inhibition of arginine synthesis by urea: a mechanism for arginine deficiency in renal failure which leads to increased hydroxyl radical generation. InGuanidino Compounds in Biology and Medicine. 2003:11–5. [PubMed]
- 23.Pacana T, Cazanave S, Verdianelli A, Patel V, Min HK, Mirshahi F, Quinlivan E, Sanyal AJ. Dysregulated hepatic methionine metabolism drives homocysteine elevation in diet-induced nonalcoholic fatty liver disease. PLoS One. 2015;10:e0136822. doi: 10.1371/journal.pone.0136822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31(2):113–126. doi: 10.1002/dmrr.2558. [DOI] [PubMed] [Google Scholar]
- 25.Radosinska J, Bacova B, Bernatova I, Navarova J, Zhukovska A, Shysh A, Okruhlicova L, Tribulova N. Myocardial NOS activity and connexin-43 expression in untreated and omega-3 fatty acids-treated spontaneously hypertensive and hereditary hypertriglyceridemic rats. Mol Cell Biochem. 2011;347(1–2):163–173. doi: 10.1007/s11010-010-0625-0. [DOI] [PubMed] [Google Scholar]
- 26.Tejero J, Stuehr D. Tetrahydrobiopterin in nitric oxide synthase. IUBMB Life. 2013;65(4):358–365. doi: 10.1002/iub.1136. [DOI] [PubMed] [Google Scholar]
- 27.Mortensen PB. Formation and degradation of dicarboxylic acids in relation to alterations in fatty acid oxidation in rats. Biochim Biophys Acta (BBA)/Lipids Lipid Metab. 1992;1124:71–79. doi: 10.1016/0005-2760(92)90128-I. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, He H, Wang H, Wang S, Li X, Liu Y, Jiang H, Jiang H, Yan Y, Wang Y, Liu X. Activation of transsulfuration pathway by salvianolic acid a treatment: a homocysteine-lowering approach with beneficial effects on redox homeostasis in high-fat diet-induced hyperlipidemic rats. Nutr Metab. 2013;10(1):1–1. doi: 10.1186/1743-7075-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J [Internet] 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 30.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005:10–23. [DOI] [PubMed]
- 31.van’t Erve TJ, Kadiiska MB, London SJ, Mason RP. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biol. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bifari F, Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol. 2017:1366–77. [DOI] [PMC free article] [PubMed]
- 33.Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG, Rabinowitz JD, Arany Z. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019;29:417–429. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arany Z, Neinast M. Branched chain amino acids in metabolic disease. Curr Diab Rep. 2018;18(10):76. doi: 10.1007/s11892-018-1048-7. [DOI] [PubMed] [Google Scholar]
- 35.Kametani T, Koshida H, Nagaoka T, Miyakoshi H. Hypertriglyceridemia is an independent risk factor for development of impaired fasting glucose and diabetes mellitus: a 9-year longitudinal study in Japanese. Inter Med. 2002;41:516–521. doi: 10.2169/internalmedicine.41.516. [DOI] [PubMed] [Google Scholar]
- 36.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The hypertriglyceridemia is associated with isolated impaired glucose tolerance in subjects without insulin resistance. Endocr Res. 2015;40:70–73. doi: 10.3109/07435800.2014.934963. [DOI] [PubMed] [Google Scholar]
- 37.Mook-Kanamori DO, Römisch-Margl W, Kastenmüller G, Prehn C, Petersen AK, Illig T, Gieger C, Wang-Sattler R, Meisinger C, Peters A, Adamski J, Suhre K. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J Endocrinol Investig. 2014;37:369–374. doi: 10.1007/s40618-013-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 39.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012:606–14. [DOI] [PMC free article] [PubMed]
- 40.Abdoli N, Azarmi Y, Eghbal MA. Mitigation of statins-induced cytotoxicity and mitochondrial dysfunction by L-carnitine in freshly-isolated rat hepatocytes. Res Pharm Sci. 2015;10:143–151. [PMC free article] [PubMed] [Google Scholar]
- 41.Noipha K, Ratanachaiyavong S, Purintrapiban J, Herunsalee A, Ninla-Aesong P. Effect of Tinospora crispa on glucose uptake in skeletal muscle: role of glucose transporter 1 expression and extracellular signal-regulated kinase1/2 activation. Asian Biomed. 2011;5:361–369. [Google Scholar]
- 42.El-Hattab AW, Scaglia F. Disorders of carnitine biosynthesis and transport. Mol Genet Metab. 2015:107–12. [DOI] [PubMed]
- 43.Kersten S, Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. 2017:75–84. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 101 kb)