Abstract
Introduction
Combination therapy with both basal insulin (BI) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) is an effective treatment in patients with uncontrolled type 2 diabetes mellitus (T2DM). The recent development and release of a fixed-ratio combination of slow-release insulin degludec and the GLP-1RA liraglutide (IDegLira) represents an improvement to this therapy. We have conducted a real-world evidence study in Italian patients with T2DM to evaluate whether the encouraging clinical trial results obtained with IDegLira, which became available in Italy in January 2018, can be confirmed in Italian clinical practice.
Methods
This was a multicenter, retrospective, observational study in patients with T2DM treated with IDegLira from January to December 2018. Prior to the initiation of IDegLira therapy, patients were treated with BI with or without one or more concomitant oral antidiabetic drugs (BOT group) or according to the basal bolus protocol (BI and rapid-acting insulin treatment; BB group).
Results
A total of 244 patients were included in the present study, of whom 186 were in the BOT group and 58 in the BB group. Following the switch to IDegLira therapy, glycemic control improved in both groups, with significant reductions in glycated hemoglobin after 6 and 12 months of treatment in the BOT group and after 6 months of treatment in the BB group. No gain in body weight and body mass index and reductions in fasting plasma glucose and number of concomitant diabetic medications (in BOT patients) were observed. All results obtained during the study were achieved at a moderate dose of IDegLira.
Conclusion
The findings from this study show that in a real-world setting, the switch to IDegLira treatment is a valid option for patients who are failing to achieve glycemic control targets and/or struggling with the side effects, such as weight gain and hypoglycemia, of other insulin therapies.
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00945-4) contains supplementary material, which is available to authorized users.
Keywords: Basal insulin, GLP-1 analogue, Glycemic control, Observational study, Type 2 diabetes mellitus
Key Summary Points
| Therapy with the fixed-ratio combination of basal insulin and glucagon-like peptide-1 receptor agonists (IDegLira) is an effective treatment in patients with uncontrolled type 2 diabetes mellitus (T2DM). |
| The aim of this real-world study was to evaluate IDegLira in an Italian population of T2DM patients. |
| Improvement of glycemic control, no gain in body weight and body mass index and reductions in fasting plasma glucose and number of concomitant diabetic medications were observed following the switch to IDegLira therapy. |
| The findings show that in a real-world setting, the switch to IDegLira treatment is a valid therapeutic option for patients with T2DM who are unable to achieve glycemic control targets and/or report side effects with other insulin therapies. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13034480.
Introduction
The combination therapy with a long-acting basal insulin (BI) and a glucagon-like protein-1 receptor agonist (GLP-1RA) in patients with type 2 diabetes mellitus (T2DM) is based on the solid understanding of their complementary mechanisms of actions [1], which potentiate each other by acting through different mechanisms in different tissues. This approach is also supported by full clinical data and is endorsed as a third-line treatment for the management of hyperglycemia in T2DM in the consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [2]. For patients already being treated with BI, the addition of a GLP-1RA to their therapeutic regimen is an option when intensification therapy is needed [3, 4]. This combination therapy shows the same efficacy as the basal bolus treatment regimen with respect to glycemic control, but with a lower risk of hypoglycemia and weight gain, and is obtained with a lower dose of insulin [5]. The timely addition of a GLP-1RA to monotherapy with BI also improves clinical and economic outcomes when it is taken into account that therapeutic inertia in treating patients with uncontrolled T2DM can result in worsening cardiovascular outcomes and that intensification of a basal oral regimen is often delayed in clinical practice [6, 7].
This co-administration therapy has recently been improved with the development and release of a fixed-ratio combination of slow-release insulin degludec and the GLP-1RA liraglutide (IDegLira) that avoids the burden of multiple daily injections [8]. IDegLira is administered once daily, independent of meals, up to a maximum daily dose of 50 dose steps (DS), with 1 DS containing 1 U of insulin degludec and 0.036 mg of liraglutide [9].
The clinical efficacy and safety of IDegLira were assessed in the phase III DUAL clinical trial program in T2DM patients with suboptimal glycemic control who were on oral antidiabetic drugs (OADs), GLP-1RA therapy and BI therapy [10]. The DUAL I–VII studies demonstrated that IDegLira is able to reduce blood glucose levels and, furthermore, to alleviate the major side effects of insulin therapy (hypoglycemia, body weight gain) and GLP-1RA therapy (nausea), combining the clinical advantages of both components [11–17].
Based on these encouraging results, the use of IDeglira was approved in Europe in September 2014, in combination with OADs for those patients who do not reach an adequate glycemic control with OADs alone or combined with a GLP-1RA or BI. IDeglira was approved for use in the USA in November 2016, while in Italy it has been on the market since January 2018 [9, 18].
In contrast to data from clinical trials, real-world evidence on IDegLira is limited. Real-world retrospective studies are particularly attractive, as they provide the means to rapidly gather data from large heterogeneous patient populations that are representative of those seen in routine clinical practice. The aim of this real-world cohort study was, therefore, to evaluate whether the clinical trial results obtained with IDegLira can be confirmed in clinical practice, in an Italian T2DM patient population, with the final aim to achieve the recommended glycated hemoglobin (HbA1c) target without increasing the risk for hypoglycemia compared to titration of BI alone, and without worsening the extra-glycemic parameters related to cardiovascular risk.
Methods
Study Design
This is a multicenter retrospective observational cohort study conducted at seven Italian diabetes centers. Patients with T2DM attending clinics at these diabetes centers and treated with IDegLira in the period of January–December 2018 were enrolled. The duration of the observation phase was 12 months.
The study was performed in accordance with the current legislation on Observational Studies and the Helsinki Declaration of 1964 and its later amendments.The study was approved by and the data was been processed at the U.O. of Diabetology of the IRCCS of the Sacred Heart Hospital—Don Calabria di Negrar, in compliance with privacy regulations. Patients have been asked for their consent to the processing of personal data. A formal approval by the Ethics Committee was considered for patients who did not sign the informed consent form because they did not visit the center after the approval of this retrospective study.
Patients
Patients with T2DM considered for enrollment in the present study had started IDegLira therapy following the switch from prior treatments of either BI with or without concomitant ≥ 1 OADs (BOT group) or according to the basal bolus protocol (BI and rapid-acting insulin treatment; BB group). The patients included in the study were not in metabolic compensation according to the criteria of the guidelines provided by Italian and international diabetic societies. The initial dose of IDegLira, as well as the recommended titration scheme, were evaluated by the treating clinician.
Exclusion criteria were: age < 18 years; BI-naïve patients; concomitant or suspected malignant diseases (within 3 months of the baseline visit); recent acute diseases (within 3 months of the baseline visit); severe renal failure (glomerular filtration rate < 15 mL/min); severe liver failure; congestive heart failure (New York Heart Association functional class IV); high degree of fragility; and chronic pancreatitis.
Study Measures
Variation in HbA1c, based on HbA1c values provided by blood analyses performed as part of the routine diabetic control visits, was considered to be the primary endpoint. The proportion of patients with HbA1c < 7% was also assessed.
Variations in the following parameters were evaluated as secondary endpoints: (1) body weight and body mass index (BMI); (2) mean fasting plasma glucose (FPG), collected as the mean FPG level for the 28 days of treatment immediately preceding the day of measurement (baseline and 6- and 12-month follow-up visits); (3) systolic blood pressure; (4) lipid profile (total and high-density lipoprotein [HDL] cholesterol and triglycerides).
Variations in IDegLira dose during the study, compared to previous BI dosage, were also evaluated.
All measures were assessed at the baseline visit (corresponding to the start of IDegLira treatment) and after 6 and 12 months of treatment (6- and 12-month follow-up visits, respectively). To obtain data on change in the parameters (delta values), only patients with available data at both relevant time points and baseline values were included in the analysis. This restriction resulted in a different population dimension (n) for each parameter at each time point, which is a characteristic of a real-world study.
Statistical Analysis
Statistical evaluation of all continuous data was performed using a paired t test or Wilcoxon Signed Ranks test. Discrete paired data were evaluated with the McNemar test. All analyses were performed using SPSS v26 software (IBM Corp., Armonk, NY, USA).
Results
Study Population and Clinical Characteristics
Among the 275 patients with T2DM considered for enrollment in the screening phase, 31 were excluded because they were naïve to insulin therapy. Thus, a total of 244 patients were included in the study, 186 in the BOT group and 58 in the BB group. Included patients had comparable baseline characteristics regarding age, disease duration, HbA1c levels, weight, BMI, FPG, systolic blood pressure and lipid profile. Symptomatic hypoglycemia episodes were significantly higher in the BB group.
Detail son the baseline demographics and clinical characteristics of the patients enrolled in the study are presented in Table 1. Previous diabetic therapies followed by patients are summarized in Table 2.
Table 1.
Baseline demographics and clinical characteristics of patients enrolled in the study
| Baseline demographics and clinical characteristics | BOT groupa | BB groupa |
|---|---|---|
| Full analysis set (N) | 186 | 58 |
| Male, n (%) | 98 (52.7) | 34 (58.6) |
| Age (years) |
64 ± 9 n = 185 |
63 ± 11 n = 58 |
| Disease duration (years) |
15 ± 8 n = 182 |
16 ± 9 n = 58 |
| HbA1c (%) |
8.5 ± 1.2 n = 184 |
8.2 ± 1.2 n = 58 |
| Weight (kg) |
93.4 ± 19.2 n = 172 |
101.1 ± 21.3 n = 58 |
| BMI (kg/m2) |
33.0 ± 6.2 n = 172 |
34.9 ± 6.9 n = 58 |
| FPG (mg/dL) |
162.4 ± 46.8 n = 154 |
154.2 ± 49.0 n = 58 |
| Systolic blood pressure (mmHg) |
136.3 ± 19.5 n = 165 |
137.6 ± 16.9 n = 58 |
| Lipid profile (mg/dL) | ||
| Total cholesterol |
168.7 ± 41.3 n = 150 |
171.4 ± 42.8 n = 52 |
| HDL cholesterol |
45.8 ± 12.5 n = 145 |
47.2 ± 11.1 n = 49 |
| Triglycerides |
152.2 ± 81.6 n = 147 |
160.0 ± 95.6 n = 52 |
| Hypoglycemia episodes | ||
| Symptomatic |
0.3 ± 1.1 n = 99 |
1.3 ± 2.0 n = 32 |
| Severe |
0.3 ± 1.1 n = 99 |
0 n = 32 |
Values in table are presented as the mean ± standard deviation (SD), unless indicated otherwise
BMI Body mass index, FPG fasting plasma glucose, HbA1c glycated glucose, HDL high-density lipoprotein
aThe patient groups comprised those patients with type 2 diabetes mellitus (T2DM) whose previous therapy prior to the switch to IDeglira (fixed-ratio combination of basal insulin [BI] and glucagon-like peptide-1 receptor agonist [GLP1-RA]) was either BI with or without concomitant ≥ 1 oral antidiabetic drugs (OADs) (BOT group) or those treated according to the basal bolus protocol (BI and rapid-acting insulin treatment; BB group)
Table 2.
Previous therapy for diabetes
| Previous therapy for diabetes | n (%) |
|---|---|
| Basal insulin | 244 (100) |
| Glargine | 175 (71.7) |
| Detemir | 14 (5.7) |
| Degludec | 29 (11.9) |
| Glargine U300 | 22 (9.0) |
| NPH insulin | 4 (1.6) |
| Basal bolus | 58 (23.8) |
| OADs | |
| Metformin | 165 (67.6) |
| Sulfonylurea | 72 (29.5) |
| DPP-4 inhibitors | 39 (16.0) |
| Pioglitazone | 11 (4.5) |
| Acarbose | 4 (1.6) |
| SGLT-2i | 25 (10.2) |
| GLP1-RA | 9 (3.7) |
DPP-4 Dipeptidyl peptidase-4 inhibitors, NPH Neutral Protamine Hagedorn, SGLT-2i sodium/glucose cotransporter-2 inhibitor
Before the switch to IDegLira, most of the patients in the BOT group were treated with two additional OADs (52.7%); 33.3% of patients were treated with one additional OAD, 9.7% with three OADs and only a small percentage were treated with four OADs (0.5%) or BI alone (3.8%).
Effectiveness Outcomes
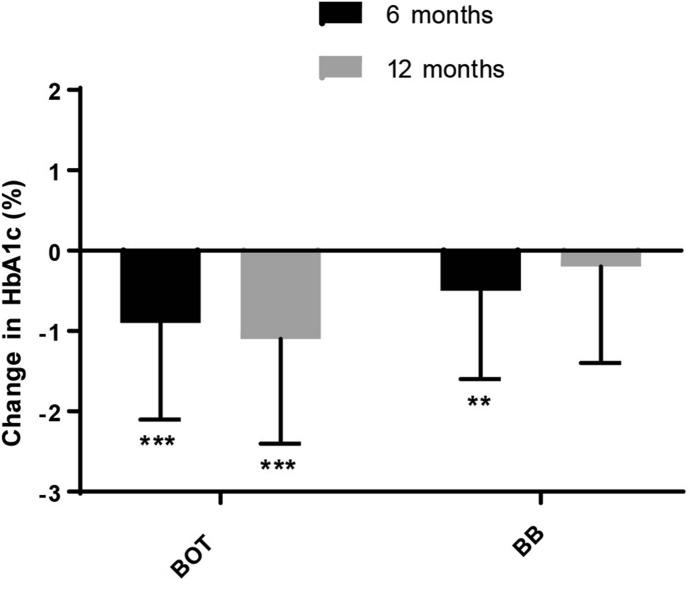
In the BOT patient group, a significant reduction in HbA1c level was observed at each follow-up visit versus baseline (6 months mean ± standard deviation [SD] reduction − 0.9 ± 1.2%; 12 months mean ± SD reduction − 1.1 ± 1.3%; p < 0.001) (Fig. 1; Table 3). The proportion of patients in the BOT group with HbA1c < 7% was significantly higher after 6 and 12 months of IDegLira treatment (+ 21.6 and + 27.5%, respectively) than at baseline (p < 0.001) (Table 3).
Fig. 1.
Change (delta reduction, %) in glycated hemoglobin (HbA1c) levels versus baseline (dark horizontal line) at each follow-up visit in the BOT and BB patient populations. Bars and whiskers represent the mean and standard deviation (SD), respectively. Asterisks indicate statistical significance versus baseline levels at **p < 0.01 and ***p < 0.001. Number of measurements: BOT group: 6 months, n = 156; 12 months, n = 51; BB group: 6 months, n = 45; 12 months n = 17. See text (section Patients) for description of BOT and BB patient groups
Table 3.
Effectiveness outcomes
| Outcome | Follow-up visit at 6 months | Follow-up visit at 12 months | ||||
|---|---|---|---|---|---|---|
| n | Baseline value | Follow-up value | n | Baseline value | Follow-up value | |
| BOT group | ||||||
| HbA1c (%) | 156 | 8.4 ± 1.2 | 7.5 ± 1.1*** | 51 | 8.4 ± 1.0 | 7.3 ± 0.9*** |
| HbA1c < 7%, n (%) | 157 | 15 (9.6) | 49 (31.2)*** | 51 | 2 (3.9) | 16 (31.4)*** |
| Weight (kg) | 138 | 93.0 ± 18.8 | 92.0 ± 18.9* | 50 | 98.0 ± 21.9 | 96.6 ± 20.4 |
| BMI (kg/m2) | 138 | 33.0 ± 6.3 | 32.7 ± 6.2* | 50 | 34.4 ± 7.2 | 33.9 ± 6.5 |
| FPG (mg/dL | 103 | 160.8 ± 48.2 | 122.8 ± 41.1*** | 37 | 139.6 ± 36.5 | 118.4 ± 34.5*** |
| Lipid profile (mg/dL) | ||||||
| Total cholesterol | 104 | 167.9 ± 43.3 | 154.1 ± 39.5*** | 33 | 161.2 ± 41.9 | 145.7 ± 33.7** |
| Triglycerides | 102 | 144.1 ± 82.5 | 138.3 ± 89.8 | 33 | 151.5 ± 72.4 | 118.5 ± 51.2** |
| BB | ||||||
| HbA1c (%) | 45 | 8.2 ± 1.3 | 7.7 ± 1.2** | 17 | 7.6 ± 0.8 | 7.3 ± 1.1 |
| HbA1c < 7%, n (%) | 45 | 11 (24.4) | 15 (33.3) | 17 | 6 (35.3) | 7 (41.2) |
| Weight (kg) | 46 | 103.0 ± 22.9 | 100.1 ± 22.5*** | 17 | 106.3 ± 22.8 | 100.1 ± 20.8*** |
| BMI (kg/m2) | 46 | 35.9 ± 7.2 | 35.0 ± 7.1** | 17 | 36.2 ± 7.0 | 34.1 ± 6.1** |
| FPG (mg/dL) | 38 | 153.3 ± 50.8 | 143.1 ± 45.3 | 15 | 136.9 ± 29.9 | 127.5 ± 32.9 |
| Lipid profile (mg/dL) | ||||||
| Total cholesterol | 27 | 169.9 ± 43.9 | 163.7 ± 39.9 | 14 | 168.6 ± 48.8 | 145.0 ± 25.0 |
| Triglycerides | 26 | 141.0 ± 75.7 | 153.3 ± 85.3 | 14 | 142.3 ± 72.7 | 154.3 ± 65.0 |
Values are presented as the mean ± SD, unless indicated otherwise
*p < 0.05, **p < 0.01, ***p < 0.001 versus baseline value
In the BB patient group, a significant reduction in HbA1c level was observed after 6 months of treatment versus baseline (mean ± SD reduction − 0.5 ± 1.1%; p = 0.005), while at 12 months, although the number of observed patients was small, a slight reduction was observed (mean ± SD reduction − 0.2 ± 1.2%) (Fig. 1; Table 3). The proportion of BB patients with HbA1c < 7% at 6 and 12 months of treatment was comparable to the relative baseline values (Table 3).
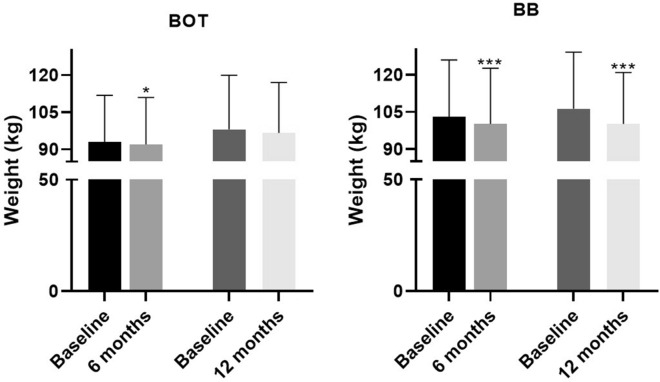
A significant reduction in body weight was reported for both patient groups after 6 months of treatment versus baseline values (BOT group mean ± SD reduction − 1.1 ± 4.9 kg, p = 0.011; BB group mean ± SD reduction − 2.8 ± 5.4 kg, p = 0.001). At the end of the study, a significant reduction in body weight was observed in the BB group, although the number of patients was small (mean ± SD reduction − 6.2 ± 6.6 kg; p = 0.001) (Fig. 2; Table 3).
Fig. 2.
Trend for change in body weight according to follow-up in the BOT and BB groups. Asterisks indicate statistical significance versus baseline levels at *p < 0.05 and ***p < 0.001. Bars and whiskers represent the mean and SD, respectively
In the BOT group, a significant reduction in BMI was observed after 6 months of treatment (mean ± SD reduction − 0.4 ± 2.0 kg/m2; p = 0.042). A significant reduction in BMI was also observed at each follow-up visit for the BB patient group, despite the small number of patients observed at 12 months, compared to the baseline values (6 months mean ± SD reduction − 0.9 ± 1.9 kg/m2; 12 months mean ± SD reduction − 2.1 ± 2.4 kg/m2; p = 0.002) (Table 3).
A further analysis of HbA1C and weight variations was performed according to patient baseline BMI (cut-off BMI value 30). No statistically significant differences in delta HbA1c and delta weight after 6 and 12 months between patients with baseline BMI > 30 and those with baseline BMI ≤ 30 were observed.
In the BOT group, a significant reduction in FPG was observed at each follow-up visit, compared to baseline values (6 months mean ± SD reduction − 38.0 ± 54.0 mg/dL; 12 months mean ± SD reduction − 21.2 ± 34.8 mg/dL; p ≤ 0.001). FPG levels also decreased in the BB group, but the changes were not significantly different from the baseline values (Table 3).
A significant reduction in total cholesterol was observed in the BOT group at each follow-up visit, compared to baseline values (6 months mean ± SD reduction − 13.9 ± 35.8 mg/dl; p < 0.001; 12 months mean ± SD reduction − 15.5 ± 31.7 mg/dl; p = 0.009) (Table 5). In the BB group, only a slight decrease of total cholesterol values was observed at each follow-up visit (Table 3).
Table 5.
Proportion of patients in the BOT group receiving a specific combination of oral antidiabetic drugs + basal insulin therapy or IDegLira
| Therapeutic regimen | Previous therapy (n = 186) | Baseline visit (n = 186) | 6-month follow-up (n = 157) | 12-month follow-up (n = 51) |
|---|---|---|---|---|
| Basal insulin only | 3.8% | 3.2% | 4.5% | – |
| Basal insulin + 1 OAD | 33.3% | 57.0% | 66.2% | 72.5% |
| Basal insulin + 2 OAD | 52.7% | 37.1% | 26.8% | 27.5% |
| Basal insulin + 3 OAD | 9.7% | 2.7% | 2.5% | – |
| Basal insulin + 4 OAD | 0.5% | – | – | – |
Triglyceride values were slightly decreased in BOT group after 6 months of treatment (mean ± SD reduction − 5.8 ± 65.9 mg/dl). This reduction became significant at the end of the study (mean ± SD reduction − 33.1 ± 65.6 mg/dl; p = 0.006) (Table 3).
During the study, no significant variation in systolic blood pressure and HDL cholesterol values were reported in both populations (Electronic Supplementary Material Table 1).
Variation in IDegLira Dose and Concomitant OADs During the Study
Previous to the switch to IDegLira treatment, the mean dose of BI was 21.1 ± 9.8 IU in the BOT group and 55.9 ± 26.3 IU of total daily insulin (BI + rapid-acting insulin) in the BB group (Table 4). The mean start-up dose of IDegLira in the BOT and BB groups was 18.7 ± 6.8 SU (Step Unit) and 20.2 ± 7.6 SU, respectively (Fig. 3; Table 4).
Table 4.
Insulin dose before and after the switch to IDegLira
| Patient groups and measurement time points | n | Insulin (IU) before switch | IDegLira dose (SU) | Change (delta) after switch |
|---|---|---|---|---|
| BOT | ||||
| Baseline visit | 184 | 21.1 ± 9.8 | 18.7 ± 6.8 | − 2.4 ± 8.6*** |
| 6-month follow-up | 158 | 21.4 ± 10.1 | 25.1 ± 9.5 | 3.7 ± 10.7*** |
| 12-month follow-up | 51 | 24.4 ± 11.6 | 27.2 ± 10.8 | 2.8 ± 13.4 |
| BB | ||||
| Baseline visit | 57 | 55.9 ± 26.3 | 20.2 ± 7.6 | − 35.7 ± 24.4*** |
| 6-month follow-up | 46 | 55.9 ± 25.6 | 28.9 ± 12.6 | − 27.0 ± 21.7*** |
| 12-month follow-up | 17 | 57.5 ± 28.6 | 25.9 ± 10.3 | − 31.6 ± 25.1*** |
Values in table are presented as the mean ± SD
SU Step Unit
***p < 0.001 versus before switch
Fig. 3.
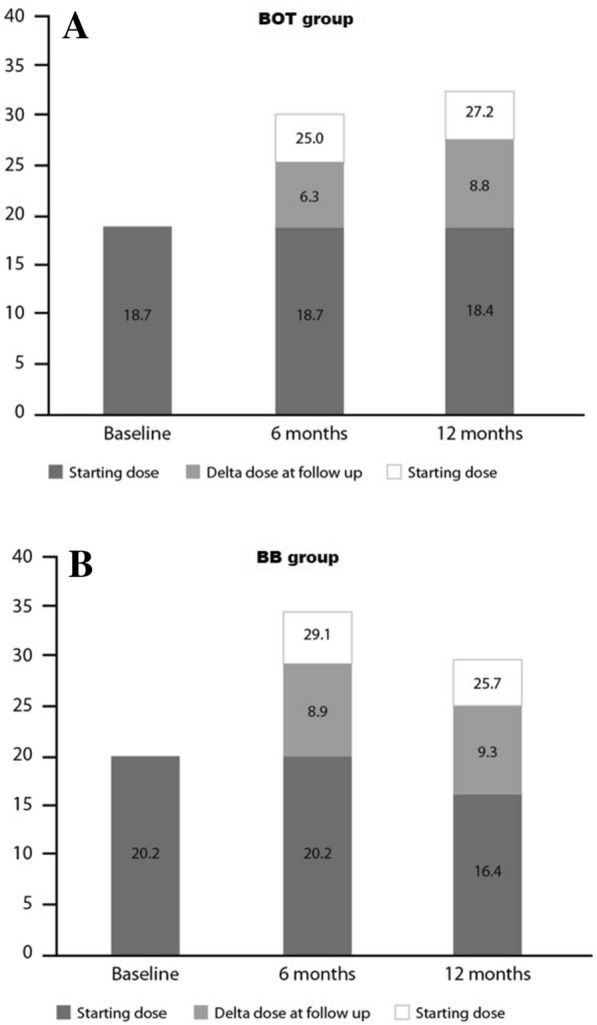
IDegLira mean dose steps (expressed as SU) at baseline and at the 6- and 12-month follow-ups in the BOT (a) and BB (b) groups. Final mean dosages (expressed as SU) at visit are reported above columns, taking into account the relative baseline values of patients
The mean dose of IDegLira increased to 25.1 ± 9.5 SU in the BOT group after 6 months of treatment and to 27.2 ± 10.8 SU after 12 months of treatment(Fig. 3a; Table 4). In the BB group, the mean dose of IDegLira was 28.9 ± 12.6 SU (p < 0.001) after 6 months and 25.9 ± 10.3 SU (p < 0.001) after 12 months of treatment; these values represent a significant reduction compared to previous BI therapy (Fig. 3b; Table 4).
The number of OADs taken by patients in the BOT group concomitant to IDegLira treatment is shown in Table 5. At the end of the study, 27.5% of patients in the BOT group were being treated with two concomitant OADs, which is a reduction of 25.2% compared to the previous therapy regimen. Most patients in the BOT group (72.5%) were treated with one additional OAD. None of the patients were treated with three or four OADs (Table 5).
Safety Data
In the BOT group, two patients suspended the IDegLira treatment for persistent nausea and vomiting and four patients stopped the treatment for poor glycemic control. In the BB group, one patient reported an exacerbation of chronic obstructive pulmonary disease and, therefore, suspended the IDegLira treatment.
Discussion
The DUAL I–VII clinical trial program provides encouraging results regarding the efficacy and safety of IDegLira therapy in patients with T2DM and suboptimal glycemic control. In particular, in the DUAL II and V studies, the efficacy of IDegLira was assessed in patients who switched from BI degludec or glargine treatment, respectively, and the results demonstrat a greater reduction in HbA1c, hypoglycemia and weight in the IDegLira-treated group [11, 13]. The effectiveness of IDegLira was also compared to the therapeutic intensification with the rapid-acting insulin analogue (basal-bolus protocol) in the DUAL VII study: at the end of the study, HbA1c levels in patients switched to IDegLira was comparable to that in the basal-bolus group. A greater reduction in hypoglycemia and body weight in favor of IDegLira was also observed at the end of the 6-month follow-up. These results were obtained with a significantly lower quantity of insulin: 40 IU/day for the IDegLira group, compared to 84 IU/day for the basal-bolus group [14].
However, real-world observational studies are needed to confirm the results obtained in the clinical trials. In this context, the present work, which is the second real-world study involving IDegLira, was carried out in an Italian context, with the aim to compare the effectiveness of fixed and flexible BI/GLP-1RA combinations using routinely accumulated clinical data. The results show that in specialist routine care, treatment with fixed or flexible BI/GLP-1RA combinations provide comparable improvement in glycemic control, with lower treatment costs with the fixed combination [19].
Specifically, our results show that the IDegLira therapy, following its substitution for previous insulin regimens, improved glycemic control, with significant reductions in HbA1c after 6 and 12 months of treatment with IDegLira in the BOT group and after 6 months in the BB group. They also show that the IDegLira treatment was associated with no weight and BMI gain, reductions in FPG and a reduction in the number of concomitant diabetic medications compared to previous regimens in patients from the BOT group.
Of note, the obtained results were achieved at a moderate dose of IDegLira (mean ± SD IDegLira dose at the end of the study 27.1 ± 10.8 SU for the BOT group and 25.9 ± 10.3 SU for the BB group), taking into account that the reference IDegLira maximum dose is 50 SU daily, which also represents a significant reduction in the amount of BI taken by BB patients (− 31.6 ± 25.1 IU at the end of the study).
The data obtained in this study are in line with the results of the EXTRA study, a real-word evidence study conducted in a number of European diabetes centers [20]. In the EXTRA study, the multidose injection (MDI) subgroup demonstrated a significant reduction in HbA1c of − 0.7% and a significant reduction in body weight of 2.4 kg [20]. Another small real-word evidence study examined the safety and efficacy of switching from the MDI regimen to once-daily IDegLira in relatively well-controlled (HbA1c baseline 7.5%) T2DM subjects, thereby reducing the total daily insulin dose [21]. The conclusions drawn by the authors were that in everyday clinical practice, switching from low-dose MDI to IDegLira in patients with well-controlled T2DM is safe, may result in weight loss and similar or better glycemic control and substantially reduces the insulin requirement [21].
The present study has a number of limitations, typical for observational studies. These include the lack of data related to glycemic self-control and the high dropout rate (> 50%) in both observational groups over time, which resulted in fewer patients at each follow-up visit. In particular, for the 12-month evaluations of the BB group, the reduced number of patients does not allow any conclusions to be drawn, but the data are useful in that they suggest a trend during the time the reported parameters were measured.
Conclusions
In conclusion, the findings from this study show that in a real-world setting the switch to IDegLira treatment is pertinent to physicians who are considering the most proper therapy for patients who are failing to achieve glycemic control targets and/or struggling with side effects, such as weight gain and hypoglycemia. Of note, both patient populations considered in this real-world study (BOT group and BB group) can benefit the treatment.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank patients for participating in the current study and for allowing access to their clinical data records for the purpose of this research.
Funding
Publication charges were covered by Polistudium through a Novo Nordisk S.p.A. unconditional grant. Novo Nordisk S.p.A. did not influence and were not involved in data collection, interpretation and analysis. No funding or sponsorship was received for this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The authors of this article contributed by providing data and/or reviewing data and article and have approved the final version of the manuscript before the submission.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Sara Di Nunzio, PhD, Valentina Mirisola, PhD, Simonetta Papa, PhD and Aashni Shah (Polistudium SRL, Milan, Italy). Support for this assistance was funded by Novo Nordisk.
Disclosures
Luciano Zenari, Cesare Miranda and Vera Frisonreceived consultancy fees from Novo Nordisk. Andrea Da Porto, Lorena De Moliner, Francesca Lugli, Valeria Guazzoni, Gloria Groppelli, Laura Molteni, Massimo Bracaccia, Natalino Simioni, Barbara Bonsembiante and Annunziata Lapolla have nothing to disclose.
Compliance with Ethics Guidelines
The study was performed in accordance with the current legislation on Observational Studies and the Helsinki Declaration of 1964 and its later amendments.The study was approved by and the data was been processed at the U.O. of Diabetology of the IRCCS of the Sacred Heart Hospital–Don Calabria di Negrar, in compliance with privacy regulations. Patients were asked for their consent to the processing of their personal data. A formal approval by the Institutional Ethics Committee was considered for patients who did not sign the informed consent form because they did not visit the center after the approval had been granted by the Ethics Committee for this retrospective study.
Data Availability
The authors have full control of all primary data and agree to allow the journal to review the data, if requested.
References
- 1.Berlie H, Hurren KM, Pinelli NR. Glucagon-like peptide-1 receptor agonists as add-on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2012;5:165–174. doi: 10.2147/DMSO.S27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;2018(41):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485–502. doi: 10.1111/dom.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cariou B. Pleiotropic effects of insulin and GLP-1 receptor agonists: potential benefits of the association. Diabetes Metab. 2015;41(6 Suppl 1):6S28–6S35. doi: 10.1016/S1262-3636(16)30006-4. [DOI] [PubMed] [Google Scholar]
- 5.Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3082. doi: 10.1002/dmrr.3082. [DOI] [PubMed] [Google Scholar]
- 6.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong L, Pan C, Wang H, Bertolini M, Lew E, Meneghini LF. Impact of delaying treatment intensification with a glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes uncontrolled on basal insulin: a longitudinal study of a US administrative claims database. Diabetes Obes Metab. 2018;20:831–839. doi: 10.1111/dom.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes E. IDegLira: Redefining insulin optimisation using a single injection in patients with type 2 diabetes. Prim Care Diabetes. 2016;10:202–209. doi: 10.1016/j.pcd.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Novo Nordisk. Xultophy® Summary of Product Characteristics. 2017. www.medicines.org.uk/emc/medicine/29493. Accessed 30 July 2020.
- 10.Vedtofte L, Knop FK, Vilsbøll T. Efficacy and safety of fixed-ratio combination of insulin degludec and liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Opin Drug Saf. 2017;16:387–396. doi: 10.1080/14740338.2017.1288715. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) Diabetes Care. 2014;37:2926–2933. doi: 10.2337/dc14-0785. [DOI] [PubMed] [Google Scholar]
- 12.Gough SCL, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 13.Lingvay I, Manghi FP, García-Hernández P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898–907. doi: 10.1001/jama.2016.1252. [DOI] [PubMed] [Google Scholar]
- 14.Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41:1009–1016. doi: 10.2337/dc17-1114. [DOI] [PubMed] [Google Scholar]
- 15.Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab. 2017;19:858–865. doi: 10.1111/dom.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101–114. doi: 10.1007/s13300-016-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with Type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34:189–196. doi: 10.1111/dme.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novo Nordisk. Xultophy® Prescribing Information (PI). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208583s000lbl.pdf. Accessed 31 July 2020.
- 19.Morieri ML, Rigato M, Frison V, et al. Fixed versus flexible combination of GLP-1 receptor agonists with basal insulin in type 2 diabetes: a retrospective multicentre comparative effectiveness study. Diabetes Obes Metab. 2019;21:2542–2552. doi: 10.1111/dom.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price H, Blüher M, Prager R, et al. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: Results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20:954–962. doi: 10.1111/dom.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taybani Z, Bótyik B, Katkó M, Gyimesi A, Várkonyi T. Simplifying complex insulin regimens while preserving good glycemic control in type 2 diabetes. Diabetes Ther. 2019;10(5):1869–1878. doi: 10.1007/s13300-019-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors have full control of all primary data and agree to allow the journal to review the data, if requested.