Abstract
Type 2 diabetes mellitus (T2DM) is a heterogeneous condition that is related to both defective insulin secretion and peripheral insulin resistance. Beta cells are the major organ for secreting insulin hence, it is important to maintain an adequate beta-cell mass in response to various changes. Insulin resistance is a major cause of T2DM leads to elevated free fatty acid (FFA) levels which increases beta-cell mass and insulin secretion to compensate for insulin insensitivity. Chronic increase of plasma FFA levels results in disturbances in lipid metabolism, which contributes to decreased beta-cell function and lipotoxicity thus promoting T2DM. In the present review, we have discussed the process of beta-cell destruction, the role of genes in contributing to the fast increase in the progression of T2DM in detail. More than 130 variants in various T2DM susceptibility and candidate genes have been discovered to be associated with T2DM. Still, these variants elucidate only a small amount of total heritability of T2DM. Further, there is also an inventory of presently used therapeutic tools and a review of novel therapeutic approaches like incretin-based therapies or sodium-glucose transporter-2 inhibitors. Additionally, providing a concise but comprehensive update, this review will be essential to every clinician involved in the treatment of diabetes mellitus.
Keywords: Genetics, Type 2 diabetes mellitus, Insulin resistance, Obesity, Beta cell
Introduction
Type 2 diabetes mellitus (T2DM) persists to be one of the major health problems worldwide. It is a metabolic disorder caused by hyperglycemia, which occurs due to inadequate pancreatic insulin secretion or insulin resistance in peripheral tissues [1]. T2DM is the outcome of a diverse interaction between genetic, epigenetic and environmental factors [2]. Long term defects in β-cells can affect insulin levels and may result in severe glucotoxicity effects on pancreatic cells resulting in impaired insulin secretion [3]. Insulin resistance is a condition in which cells fail to react to insulin appropriately and inhibit the activity of lipoprotein lipase as a consequence of modifying lipids and apolipoprotein by altering the catabolism [4]. These changes may contribute to increasing lipolysis and free fatty acid (FFA) into the blood that results in the disease phenotype. Among the different known factors, the most general cause of developing insulin resistance is obesity and overweight [5, 6]. T2DM also shows a strong inheritable genetic association with a family history, where disease risk increases up to 40% if one of the close relatives had diabetes and doubles when the mother shows disease phenotype [7].
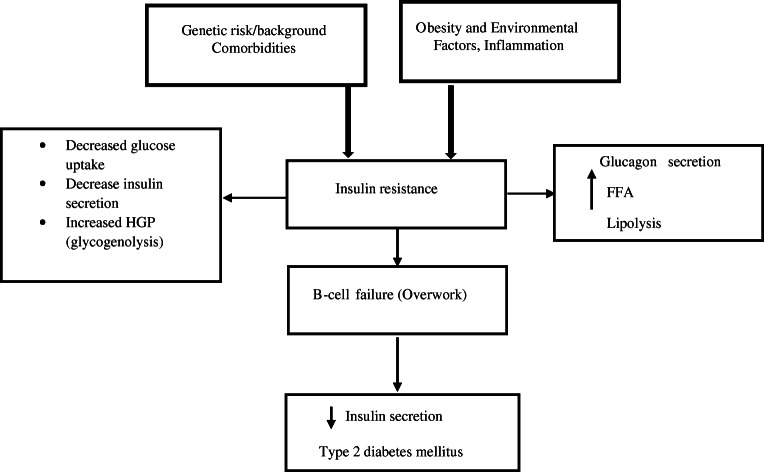
Recently, Genome-wide association (GWAS) study, suggests the involvement of more than 50 genetic variants with T2DM [8]. Several genes previously observed are significantly associated with T2DM including PON1, LCAT, APOE, FTO, and TCF7L2 [2, 9]. Polymorphisms in these genes have previously been shown to be significantly associated with T2DM by an increase in free fatty acid (FFA). Exposure of cells to increased FFA inhibits the phosphorylation of serine residues of the insulin receptor substrate (IRS). This phosphorylation diminishes the tyrosine phosphorylation of IRS-1 in reaction to insulin and loses the capability to bind with insulin receptors thus inhibiting insulin action and signaling. Mechanism and causes of T2DM are mostly unknown and various polymorphic studies in different ethnicities are being conducted to identify the genes responsible for the progression of diabetes [10]. However, the sequence of genes diverges in various regions, and the dissimilarity in nucleotide modifies a phenotypic trait that displays an increased risk of T2DM. Apart from coding genes, non-coding genes can also raise the risk of diabetes by modifying the regulation of genes which alters the protein product and functioning [11]. It has also been identified that the development of T2DM results from the involvement of genetic makeup and the environmental factors together with increased obesity (visceral fat) [12] (Fig.1). The population of different ethnicities is also at increased risk of diabetes such as African-Americans, Asian-Americans, and Hispanic-Americans [7]. However, the increase in a sedentary life, poor foodstuffs, stress, red meat, westernization culture, urbanization, and competition showed an increased frequency of T2DM among Indians [7]. This review will thus focus on a wide variety of significant aspects, from genetic background to topics related to the pathogenesis of diabetes mellitus such as dysfunctional beta cells and lipotoxicity. There is also an inventory of presently used therapeutic tools and a review of novel therapeutic approaches like incretin-based therapies or sodium-glucose transporter-2 inhibitors. Additionally, providing a concise but comprehensive update, this review will be essential to every clinician involved in the treatment of diabetes mellitus.
Fig. 1.
Proposed mechanism of genetic predisposition and obesity-induced insulin resistance and type 2 diabetes mellitus
Pathogenesis of T2DM
T2DM is a multifactorial disease involving the influence of multiple genes along with obesity, insulin resistance, and environmental factors [5]. In T2DM, insulin resistance contributes to increased glucose production in the liver and decreased glucose uptake in muscle and adipose tissues at a set insulin level. Additionally, dysfunctional β-cells also result in reduced insulin secretion which is not enough for maintaining normal glucose levels [13].
Genetic factors
The risk of growing T2DM also depends on lifestyle as well as genetic background. There is a higher probability of developing T2DM in individuals having diabetic parents and this ratio further increases if both the parents have T2DM [14]. Similarly, odds of having T2DM also increases up to 70% in monozygotic than in dizygotic twins [11]. Additionally, by purifying selection method alleles that are susceptible to the T2DM are reserved at low frequency and these inherited genes can be used for screening and early diagnosis in families with a history of T2DM [15, 16]. However, these variants are acting independently or in combination with common variants in different geographical areas [17].
Modern researches are meticulously working to discover a genetic variant that contributes to disease and increases the risk of T2DM. Genetic variations might add to the outcome of the possibility of T2DM, and modifications in these gene traits could also increase the chances of diabetes and obesity [18]. Also, past findings showed that Indians are at increased risk of diabetes due to increased obesity and waist circumference in comparison to other racial populations [19]. Even though Indians had decreased body mass index (BMI), but, increased waist circumference and body fat upsurge their risk of developing T2DM in the future [10, 20]. However, an increase in physical activity and a healthy diet, maintaining normal BMI may perhaps decrease the risk of diabetes in individuals with a family history of diabetes [21]. In addition, epigenetics also modify and alter genes by altering gene expression patterns through DNA methylation and histone modification [22]. Mixing altogether, genome sequencing, and epigenetic examination might increase the understanding of genetic risk models and T2DM pathogenesis. Since the process to identify whole-genome methylation is in progress and discovery of only a limited portion of the methylation region that could be valuable in the early diagnosis of diabetes [22]. Advancement in scientific and research approach might contribute to examine genome-wide methylation more precisely since identification of whole-genome methylation is in progress and only a limited portion of the methylation region associated with T2DM had been discovered.
Increased lipolysis
The insulin peptide hormone is a potent inhibitor of lipolysis and inhibits the release of FFA from adipocytes by inhibiting the enzyme hormone-sensitive lipase. It is also observed that a long term increase in FFA could raise the prevalence of insulin resistance and impair insulin secretion [23]. Furthermore, increase circulation of FFA could markedly raise the storage of triglycerides (TG) in muscles and induces insulin resistance in these tissues. Fatty acyl CoA of FFA has been shown to damage insulin action due to lipotoxicity which results in the destruction of beta-cell [1]. Additionally, FFA oxidation prevents glucose oxidation in muscles by inhibiting glycolytic enzymes and excessive FFA oxidation leads to the accumulation of acetyl CoA, inhibitor of pyruvate dehydrogenase, and increases the NADH/NAD ratio hindering Krebs’s cycle [1]. As a result accumulation of glucose-6–phosphate inhibits Phosphofructokinase (PFK) leading to the accumulation of glucose-6–phosphate that in turn inhibits hexokinase II. Further, the blockage of glucose phosphorylation causes an upsurge in glucose level and obstructs the transportation of glucose inside the cells.
Adiposity (obesity)
Increased FFA is known to cause insulin resistance in tissues by modifying perilipin expression that is found in adipocytes on the exterior of triacylglycerol, inhibiting lipases from hydrolyzing triacylglycerol to enable the release of FFAs [24]. Likewise, in obese individuals, adipose tissues produce more proinflammatory markers and increased expression of pro-inflammatory proteins like TNF- α, monocyte chemotactic protein1, interleukin 6 (IL-6), macrophages and reduce leptin levels. Moreover, adiponectin as well as IL-10 secreted by adipose tissues also increases along with retinol-binding protein 4 and secreted frizzled-related protein 5 (Sfrp5) [1]. In adipose tissue, Leukotrienes (LTs) are potent pro-inflammatory mediators that inhibit 5-lipoxygenase, an enzyme involved in the biosynthesis of LTs that protects obese mice from insulin resistance by reducing AT macrophages and T cell infiltration. In the presence of excess nutrients, the adiponectin receptor is down-regulated by the activation of the JNK pathway by TNF-α, IL-6, and oxidative stress [7].
Further, after dietary intake adipose tissues store FFA as TG in adipocytes and discharges FFA to fuel other organs through lipolysis and lipogenesis during starvation [25]. However, FFA binds to toll-like receptor 4 (TLR4) triggers proinflammatory action, causes the build-up of adipose tissue macrophages but excess FFA released from adipocytes stored in the liver, together with glucose could be a consequence of fatty liver disease [26]. FFAs can also provoke IR by hindering glucose transport activity, which may be a result of decreased insulin receptor substrate-1 (IRS-1)-mediated phosphatidylinositol 3-kinase (PI3-k) activity. Increased FFA up regulates intracellular fatty acyl-CoA and diacylglycerol (DAG) concentrations, which lead to activation of protein kinase C (PKC)-theta. Further, PKC-theta stimulates IRS-1-mediated PI3-k activity, which decreases insulin-stimulated glucose transport activity [27]. FFAs from dietary fat is also the main signaling molecules that binds to free fatty acid receptors (FFARs), which are G protein-coupled receptors (GPCRs) and these long-chain fatty acids (FAs) triggers GPR40/FFAR1 and GPR120/FFAR4, whereas short-chain FAs stimulate GPR43/FFAR2 and used as possible beneficial targets to decrease FFA-mediated IR [23].
Insulin resistance
Insulin resistance is a pathological condition of the body in which cells are unable to sense insulin hormone and the body cells lose sensitivity to insulin [28]. Insulin resistance causes decrease glycogen synthesis and protein catabolism in muscles as well as inhibits lipoprotein lipase activity in adipocytes thus increasing FFA and inflammatory markers cytokines like Interleukin-6 (IL-6), tumor necrosis factor (TNF). Increased circulation of cytokines causes an alteration in insulin sensitivity and interruption of glucose metabolism [29]. Further, TNF is the main cause that activates the secretion of FFAs from the adipose tissue into the circulation and mediates the suppression of many genes responsible for glucose and FFA storage. On the other hand, TNF has also been shown to down-regulate the genes encoding adiponectin, GLUT4, IRS-1, etc. as well as recovers insulin sensitivity by various mechanisms, causing a decrease in plasma FFA and glucose levels. In the liver, adiponectin stimulates FFA oxidation, decreases lipid synthesis, reduces uptake of FFA and inhibits gluconeogenesis. In skeletal muscle, adiponectin increases glucose and FFA oxidation as well as restrain the secretion of TNF [30], affecting insulin sensitivity in either a helpful (adiponectin, leptin, interleukin-10) or as a destructive way (TNF, resistin, interleukin-6, retinol-binding protein 4, monocyte chemoattractant protein-1, plasminogen activator inhibitor-1) [20]. Recently, it was noticed that adiponectin may improve the survival of pancreatic islets by acting as an antiapoptotic and inhibits the cytokine effect on FA induced β- cell apoptosis to prevent lipotoxicity provoked dysfunction. It also reduces the formation of toxic lipids and neutralizes the lipotoxicity induced beta-cell destruction [7].
Loss of β-cell mass and β-cell destruction
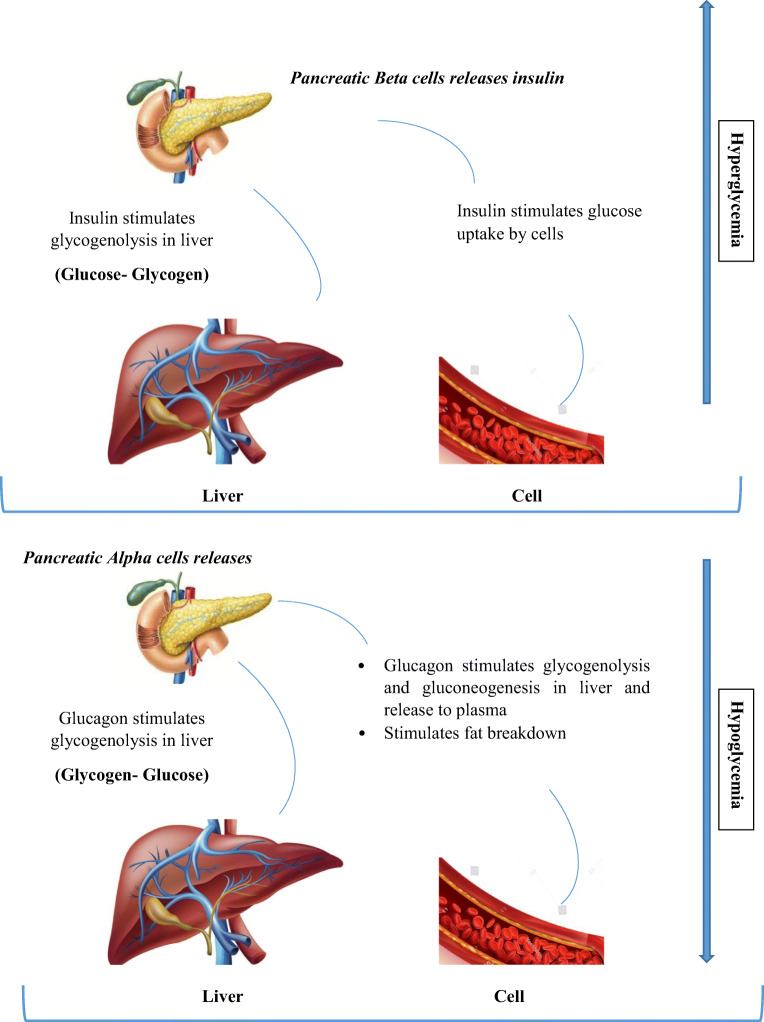
Insufficient functional beta cell mass is the main factor for type 1 diabetes and a major contributor to T2DM, highlighting the significance of understanding beta cell dynamics in the occurrence of diabetes. Increased FFA and saturated fats can harm beta-cell through cytokine-induced inflammation with progressive weakening of beta-cell function leading to beta-cell collapse and death [27]. Proinflammatory cytokines cause beta cell death due to mitochondrial stress leading to beta-cell destruction [1]. Furthermore, saturated fatty acid encourages islet inflammation and proliferates cytokines expression in beta cells thus making beta-cell dysfunction. Beta-cell hypertrophy (decreased beta-cell size) and hyperplasia (increased beta cell number) arise during beta-cell compensation to increase beta-cell mass in response to hyperglycemia [27] (Fig.2). Interestingly, when cell death happens, beta-cell compensation occurs to repair cell physiology. Previous studies showed that saturated fats increase intramuscular palmitic acid accumulation that may lead to insulin resistance and extended exposure to palmitate (PA), that hinders the secretory ability of beta cells and increases beta-cell apoptosis [1]. In T2DM, reduced beta-cell mass occurs through apoptosis, necrosis, autophagy and increase apoptosis together with reduced replication [7]. The cytokine, IL1 induces beta-cell necrosis signifies that macrophage-derived cytokines contribute to the early pathogenesis of diabetes by inducing beta-cell death through a mechanism that promotes necrosis and islet inflammation. Additionally, in obese individuals, adipose tissue is a primary source of inflammation with the penetration of macrophages that numbs the glucose recipient organs to the action of insulin thus raising the likelihood of T2DM [31]. Saturated fats in the blood resulting from diet or due to lipolysis of fat depots lead to fatty acid and glucose rival for uptake and metabolism in tissues. Long term increased blood sugar, high FFA causes glucotoxicity and damage beta cells by elevating oxidative stress. Further, the uncontrolled growth of beta cells enhances beta cell numbers and improves the functional capacity through increased blood glucose that might result in uncontrolled beta cell growth due to constant secretion of insulin [6]. Additionally, islet inflammation (insulitis) occurring due to excess FFA might leading to metabolic collapse in beta cells resulting in synthesis of cytokines, and recruitment of immune cells to the site of assembly which initiates beta cell dysfunction and impair insulin secretion [22].
Fig. 2.
Pancreatic cell response to hyperglycemia and hypoglycaemia
Early detection of T2DM
Nowadays, exome sequencing, whole-genome sequencing, Tag SNP sites show the way to determine the increased risk of T2DM and also detecting the locus associated with genes responsible for the progression of diabetes. Polymorphic studies are developed and designed to compare the genes of T2DM subjects with matched healthy controls [30]. The gene sequences of T2DM and controls differ in various regions and this dissimilarity in nucleotide sequences modify a phenotypic trait that displays an increased risk of T2DM. Population-based findings and advances in the statistical approach will enhance the examination of genes associated with T2DM. Earlier than GWAS phase, association studies of genetic variants with T2DM, was dependent on SNPs identification [32]. Nowadays signs of progress in genotyping have extensively enhanced the chance of identifying the genetic architecture of T2DM. The latest advancement in this field is Metabochip and Illumina Exome chip that can identify 100,000 SNPs variants associated with T2DM and which could be helpful in the identification of early diagnostic biomarkers for T2DM. [33]. Large population-based meta-analysis studies with similar genetic backgrounds can minimize genotyping errors. Investigation on the Japanese population showed that after taking carbohydrates they had a lower ability of insulin secretion with minor pancreatic cell function than the western population [10]. These findings proved that most Japanese individuals have diabetes sensitive genes including thrifty genes which make them more predisposed to diabetes [2]. Also, noncoding region genes and several SNPs in the open reading frame (ORF) alter the protein stability, conformation, and function that might also increase the susceptibility of T2DM [34].
Detection of susceptible genes
Genetic testing of susceptible genes is helpful in early diagnosis and the management of T2DM. Further, genes present in coding and non-coding regions do not separately manipulate diabetes risk but they act in combination with multiple genes and jointly with environmental factors. Earlier findings suggested that Calpain10 impacts insulin secretion [35] and numerous ethnic populations showed involvement of Calpain10 with T2DM [36]. Moreover, some candidate gene is also used in pharmacogenomics to study how genes affect a person’s response to drugs that might aid in developing useful medications and doses that will be personalized to a person’s genetic constituents [35]. Screening of exonic region genes and its association with diabetes will provide a mechanism to prevent T2DM and delay its progression. Further sequencing of important susceptible genes in the earlier stages of life could be helpful in the diagnosis and prevention of T2DM. Association of common number variation (CNV), insertion, and deletions in diabetes are still inadequately identified with no strong association of CNV with T2DM reported [11]. In this context, Next-generation sequencing has been proved to be much better than polymorphism and CNV, which could decipher novel pathways to identify T2DM possibility. Hence, CNV is not considered as an important genetic risk marker in identifying diabetes and is mainly assumed to be only 5% in the human genome [11].
Screening of associated genetic variants
Earlier findings prove that rare variants are a causative factor in the development of diabetes and we still need to find out the effect and frequency in different populations because the frequency of alleles varies from population to population [37]. More possibly, Next-generation sequencing (NGS) can provide an insight into the exposure of genetic variation as whole-genome sequencing (WGS) proved to be a hallmark for early diagnosis of T2DM [38]. Previous studies established that early sequencing of candidate genes can be helpful in early detection of the susceptibility of diabetes [39]. Exome sequencing and Array-based genotyping provide noteworthy results in the early detection of T2DM. Also, whole-exome sequencing, in the Latin population observed a significant association of T2DM risk with uncommon variants [37].
Common variants are the genes that are found in coding regions as well as in regulatory sequences of the gene. These genes are susceptible to complex disease and are valuable in manipulating disease phenotype [14]. Some common variants are significantly associated with T2DM and are important in identifying its risk. There are various common variations observed in humans like copy number variations, variable number of tandem repeats, microsatellite, minisatellite [40].
Additionally, SNPs in the intronic region also affect gene expression and could also result in disease phenotype by alternative splicing, separately or in combination with multiple genes that expresses and generates numerous mRNA’s through alternative splicing. Splicing can also alter gene expression by placing in or removing regulatory elements that affect mRNA strength and translation [41]. Large numbers of splicing are an outcome of manipulation in the encoded proteins and alternative splicing produces a fragment of mRNA that brings in termination codon. Each intron in genes needs splicing, the majority of the gene coding for protein are alternatively spliced [42]. Earlier studies proved that the intronic region gene and silent synonymous showed no effect on protein modification however, recent meta-analysis findings on diabetes confirmed a potential association of these intronic regions with the increased risk of diabetes [30].
Pharmacological treatment
Numerous drugs are prescribed to T2DM subjects depending on disease severity. Biguanides are a class of drugs that are primarily prescribed for the management of T2DM [43]. Incretin-based treatment motivates secretion and further restrains glucagon production from the liver. It is the most widely used therapy in the European population [44]. Also, Sodium-glucose co-transporter type 2 (SGLT2) used as an inhibitor in the treatment of T2DM since it helps in reabsorption of renal filter glucose and raises urinary glucose by retaining glucose levels. Apart from this, Epoxyoukalide, a marine product, was found to be protective against cytokine-induced apoptosis, preserves beta-cell function, and persuade beta cell proliferation mediated by extracellular signal-regulated kinases (ERK1/2) activation and targets, cyclin D2, and cyclin R [27]. C-Kit is also significant in the progression and functioning of islets, especially in support of beta-cell proliferation, maturation, and survival. c-Kit role in overexpression of beta cells confers improve glucose metabolism by enhancing insulin secretion and increase beta-cell mass along with proliferation, probably through the activation of the phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway [45].
Dipeptidyl peptidase-4 (DPP-4) inhibitors are useful in blocking and degrading the DPP-4 enzymes. Sitagliptin, regulates T2DM insulin secretory capacity while, Vildagliptin help in the preservation of α- and β-cell functions by inhibiting lipolysis and triglyceride storage in the muscles, pancreas, and liver [46]. These drugs mainly act on the glucagon secretion and inhibit the progression of T2DM. These drugs are also efficient as compared to other anti-diabetic drugs which cause gastrointestinal disturbances [47, 48]. Mimitin, a small 20 kDa mitochondrial protein that targets c-myc, implicated in cell proliferation also acts as a modulator of GSIS and prevents its inhibition by proinflammatory cytokines [49].
Exenatide, GLP-1 receptor agonists are useful in lowering HbA1c by 0.9–1.1% and useful in maintaining blood glucose levels by lowering blood glucose. GLP-1 receptor functions by inhibiting glucagon discharge and prompting insulin release [50]. These are mainly prescribed in combination with Sulfonylureas and Biguanides when the insulin hormone becomes insensitive and the blood glucose levels remain uncontrolled for a prolonged period [46]. Further, insulin maintains lipid metabolism and directs the hepatic glucose production. Metabolic abnormalities and hyperglycemia causes defect in insulin receptor and activity. Additionally, insulin therapy is effective in managing insulin sensitivity and functioning by decreasing blood glucose levels, thus reducing glucose toxicity and suppressing the production of ketosis [1]. However, in Caucasians and African Americans oral antidiabetic drugs failed to act on cells in some conditions thus maintaining the glucose levels by shifting the patient to insulin therapy [51]. Oral antidiabetic agents and insulin are currently used for the treatment of T2DM and have brought about promising outcomes but few problems like efficacy and adverse effect of these drugs still exist which calls for further research to identify novel therapeutic strategies. We still need to examine the impact of novel therapies on diabetes subjects without any side effects.
Adjuvant therapies for T2DM management
Exercise
Regular aerobic exercise, physical activity is beneficial in inhibition of onset of T2DM [52]. Additionally, regular exercise such as walking or dancing is more beneficial for insulin sensitivity and reducing abdominal fat, reduced free fatty acid in muscle, and shown good results in restoring insulin sensitivity [53].
Meditation
Research evidence proved the role of yoga-based lifestyle amendments in managing T2DM. It has also been observed that psychoneuro endocrine and immune mechanisms have complete effects on T2DM control. Parasympathetic activation and the related anti-stress mechanisms recover overall metabolic and psychological profiles, upsurge insulin sensitivity, and improve glucose tolerance [54].
Diet
The T2DM food plan should follow a variable ratio of fats, proteins, and carbohydrates, where the carbohydrates consumed, must be of low glycemic load and the fat along with proteins consumed should primarily come from plant sources. Both the amount and quality of dietary fat may alter glucose tolerance and insulin sensitivity [7]. Interestingly, reduced fat intake (particularly saturated fat) may lower the risk of T2DM by improving insulin resistance as well as promoting weight loss. Previous evidence verified that changing dietary fats with unsaturated (polyunsaturated and/or monounsaturated) fatty acids had notably improved insulin sensitivity by decreasing low density lipoprotein (LDL) [52].
Conclusion
T2DM needs to be prevented on a priority basis especially in India and China to resist constant growth. Previous trials and follow up findings show that lifestyle and dietary modifications play a vital role in the regression of diabetes and its complications. However, a large proportion of diabetes susceptibility gene is still incomplete and unidentified with minor extents is recognized. To overcome this ambiguity computational biology approach should also be utilized to gain a clear understanding of how polymorphism and variation in genes lead to diabetes. Besides, the inclusion of risk populations in the developing world may expand understanding of gene-environment interaction and is more likely to lead to the discovery of rare variants and unique variants that associate with the disease.
Compliance with ethical standards
Conflict of interest
No conflict of interest exists.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front Endocrinol. 2018;9(07):1–10. doi: 10.3389/fendo.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad RB, Groop L. Genetics of type 2 diabetes—pitfalls and possibilities. Genes. 2015;6(1):87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong D. Advanced protocols in oxidative stress III. Advanced Protocols in Oxidative Stress III. 2014;1208:1–477. doi: 10.1007/978-1-4939-1441-8. [DOI] [PubMed] [Google Scholar]
- 4.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes : Indian scenario. 2007;(03):217–30. [PubMed]
- 5.Zatalia SR, Sanusi H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta medica Indonesiana. 2013;45(2):141–147. [PubMed] [Google Scholar]
- 6.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2014;7:587–591. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie DG. AMP-activated protein kinase — an energy sensor that regulates all aspects of cell function. 2011;1895–908. [DOI] [PMC free article] [PubMed]
- 9.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9):1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 10.Abate N, Chandalia M, Satija P, Adams-huet B, Grundy SM, Sandeep S, et al. Susceptibility to type 2 diabetes. Diabetes. 2005;54(04):1207–1213. doi: 10.2337/diabetes.54.4.1207. [DOI] [PubMed] [Google Scholar]
- 11.Lyssenko V, Laakso M. Genetic Screening for the Risk of Type 2 Diabetes: Worthless or valuable? Diabetes Care. 2013;36(Supplement_2):S120–6. [DOI] [PMC free article] [PubMed]
- 12.Jayashree S, Arindam M, Vijay K V. Genetic epidemiology of coronary artery disease : an Asian Indian perspective 2015;94(3):539–49. [DOI] [PubMed]
- 13.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharmaceutical Journal. 2016;24(5):547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mccarthy M, Menzel S. The genetics of type 2 diabetes. 2010;195–9. [DOI] [PMC free article] [PubMed]
- 15.Berumen J, Orozco L, Betancourt-cravioto M, Gallardo H, Zulueta M, Mendizabal L, et al. Influence of obesity , parental history of diabetes and genes in type 2 diabetes : A case-control study. 2019;(1):1–15. [DOI] [PMC free article] [PubMed]
- 16.Meigs JB, Cupples LA, Wilson PWF. The Framingham Offspring Study. (23):2201–7. [DOI] [PubMed]
- 17.Moran Y, Labrador L, Camargo ME, Fernández D, Chiurillo MA. type 2 diabetes in Venezuelans. 2016; [DOI] [PMC free article] [PubMed]
- 18.Wilding JPH, Bastien A., Norwood P, list JF, T’Joen C, Fiedorek FT. a study of Dapagliflozin in patients with type 2 diabetes receiving high doses of. Emerging Treatments and Technologies 2009;32(9):1656–62. [DOI] [PMC free article] [PubMed]
- 19.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australasian Medical Journal. 2014;7(1):45–48. doi: 10.4066/AMJ.2014.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in south Asians. Lancet. 1991;337(8):382–386. doi: 10.1016/0140-6736(91)91164-P. [DOI] [PubMed] [Google Scholar]
- 21.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(11):5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 22.Introduction I. Monogenic Diabetes : What It Teaches Us on the Common Forms of Type 1 and Type 2 Diabetes. 2016;37(June):190–222. [DOI] [PMC free article] [PubMed]
- 23.Hevener AL, Olefsky JM, Reichart D, Nguyen MTA, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Investig. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wamique M, Ali W. Rizvi Fatima Nishat. Role of free fatty acid and elevated VLDL in type II diabetes mellitus - a review. EJPMR. 2015;2(7):115–119. [Google Scholar]
- 25.Iraj G. Clinical review 124: diabetic dyslipidemia : causes and consequences. J Clin Endocrinol Metab. 2001;86(3):965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 26.Kitasato L, Tojo T, Hatakeyama Y, Kameda R, Hashikata T, Yamaoka-Tojo M, et al. Postprandial hyperglycemia and endothelial function in type 2 diabetes: focus on mitiglinide. Cardiovasc Diabetol. 2012;11:79. doi: 10.1186/1475-2840-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4(03):1–12. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ripsin CM, Kang H, Urban RJ. Management of blood glucose in type 2 diabetes mellitus. Am Fam Physician. 2009;79(1):29–36. [PubMed] [Google Scholar]
- 29.Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of high-density lipoprotein: alterations in cardiovascular disease. EMBO Molecular Medicine. 2012;4(4):251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena M, Agrawal CG, Srivastava N, Banerjee M. gene polymorphisms in type 2 diabetes. 2014;6(07):60–8. [PMC free article] [PubMed]
- 31.Han SJ, Boyko EJ. The evidence for an obesity paradox in type 2 diabetes mellitus. Diabetes Metab J. 2018;42(3):179–187. doi: 10.4093/dmj.2018.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nat Rev Genet. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 33.Avenue G. Heterogeneity in Healthy Women 2002;667–73.
- 34.Kayıkcıoglu M. Polymorphisms of lipid metabolism enzyme-coding genes in patients with diabetic dyslipidemia. The Anatolian Journal of Cardiology. 2017;6:313–321. doi: 10.14744/AnatolJCardiol.2016.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khodaeian M, Enayati S, Tabatabaei-malazy O, Amoli MM. Association between genetic variants and diabetes mellitus in Iranian populations : a systematic review of observational studies. 2015. [DOI] [PMC free article] [PubMed]
- 36.Dasgupta S, Sirisha PVS, Neelaveni K, Anuradha K, Reddy BM. Association of CAPN10 SNPs and Haplotypes with Polycystic Ovary Syndrome among South Indian Women 2012;7(2):1–8. [DOI] [PMC free article] [PubMed]
- 37.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell KJ. What is complex about complex disorders? Genome Biol. 2012;13(1):1–11. doi: 10.1186/gb-2012-13-1-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N. NIH Public Access. 2013;41(1):82–88. [Google Scholar]
- 40.Singh S. The genetics of type 2 diabetes mellitus : a review. JSR. 2011;55:35–48. [Google Scholar]
- 41.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. 2014;11. [DOI] [PMC free article] [PubMed]
- 42.Ganza ML, Wintfeld N, Li Q, Alas V, Langer J, Hammer M. The association of body mass index with the risk of type 2 diabetes: a case-control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6(1):1–8. doi: 10.1186/1758-5996-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka H, Yoshida S, Oshima H, Minoura H, Negoro K, Yamazaki T, et al. Chronic Treatment with Novel GPR40 Agonists Improve Whole- Body Glucose Metabolism Based on the Glucose-Dependent Insulin Secretion. 2013;40(09):443–452. doi: 10.1124/jpet.113.206466. [DOI] [PubMed] [Google Scholar]
- 44.Solun B, Marcoviciu D, Dicker D. Dipeptidyl peptidase-4 inhibitors and their effects on the cardiovascular system. Curr Cardiol Rep 2013;15(8). [DOI] [PubMed]
- 45.Feng ZC, Li J, Turco BA, Riopel M, Yee SP, Wang R. Critical role of c-kit in beta cell function: increased insulin secretion and protection against diabetes in a mouse model. Diabetologia. 2012;55(8):2214–2225. doi: 10.1007/s00125-012-2566-5. [DOI] [PubMed] [Google Scholar]
- 46.Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Practice and Research: Clinical Endocrinology and Metabolism [Internet] 2009;23(4):479–486. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Hanefeld M, Forst T. Dapagliflozin, an SGLT2 inhibitor, for diabetes. Lancet. 2010;375(9):2196–2198. doi: 10.1016/S0140-6736(10)60749-0. [DOI] [PubMed] [Google Scholar]
- 48.Trevisan R. The role of Vildagliptin in the therapy of type 2 diabetic patients with renal dysfunction. Diabetes Therapy. 2017;8(6):1215–1226. doi: 10.1007/s13300-017-0302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wegrzyn P, Yarwood SJ, Fiegler N, Bzowska M, Koj A, Mizgalska D, Malicki S, Pajak M, Kasza A, Kachamakova-Trojanowska N, Bereta J, Jura J, Jura J. Mimitin - a novel cytokine-regulated mitochondrial protein. BMC Cell Biol. 2009;10:23. doi: 10.1186/1471-2121-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park YJ, Ao Z, Kieffer TJ, Chen H, Safikhan N, Thompson DM, Meloche M, Warnock GL, Marzban L. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: implications in type 2 diabetes and islet transplantation. Diabetologia. 2013;56(3):508–519. doi: 10.1007/s00125-012-2802-z. [DOI] [PubMed] [Google Scholar]
- 51.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91(2):151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Zhang W, Saraf SL, Nouraie M, Han J, Gowhari M, et al. HHS Public Access. 2015;134(8):895–904. doi: 10.1007/s00439-015-1572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medicine S. Exercise and Type 2 Diabetes. 2010;33(12).
- 54.Raveendran AV, Deshpandae A, Joshi SR. Therapeutic role of yoga in type 2 diabetes. Endocrinol Metab. 2018;33(3):307–317. doi: 10.3803/EnM.2018.33.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]