Abstract
Background
This study proposed to compare the prevalence and risk factors for sarcopenia by EGWSOP-1 and EWGSOP-2 diagnostic criteria in Iran.
Methods
This cross-sectional study was conducted based on the data collected during the Bushehr Elderly Health (BEH) Program, stage II. Sarcopenia was defined as 3 definitions: EWGSOP-1(with Iranian cut off), EWGSOP-2(with Iranian cut off), EWGSOP-2(with European cut off) definition. We evaluated the age-standardized prevalence of sarcopenia in both genders. Regression analysis was used to show the associations in the adjusted models.
Results
Among 2426 participants, age-standardized prevalence of sarcopenia, and severe sarcopenia by EWGSOP-1 were 19.7%, and 12.9%, in men and 13.6%, and 16.7% in women, respectively. When we used EWGSOP-2 (with Iranian cut-off) criteria, these values were 10.5%, and 12.7% among men and 7.13% and 16.5% in women, respectively. The prevalence sarcopenia and severe sarcopenia by EWGSOP-2 (with European cut-off) were 12.7%, and 13.4% in men and 5.42%, and 13.7% in women, respectively. In both genders, getting older and high-fat mass were positively associated with sarcopenia, and BMI had a significant inverse association in both genders and all defintions.
Conclusions
Results showed that a prevalence of sarcopenia varied largely by using different criteria, in both sexes. EWGSOP2- defined sarcopenia prevalence was lower than that defined using EWGSOP-1 criteria due to different diagnostic factors to detect sarcopenia. Some adverse outcomes should be considered for evaluating sarcopenia to compare the accuracy of EWGSOP-1 and EWGSOP-2.
Keywords: Prevalence, Sarcopenia, EWGSOP-1, EWGSOP-2
Introduction
Sarcopenia is an age-related muscle disease in which muscle mass and muscle function are reduced, resulting in increased risk of disability, poor quality of life, and mortality [1, 2]. Compared to other health-related problems of the elderly, sarcopenia has a remarkable prevalence in the elderly population between 60 and 70 years, ranging from 5–13%, which increases up to 50% in over 80 population [1, 3]. The estimated annual muscular loss would be between 1–3% [4].
In spite of the fact that subject of sarcopenia is very interesting for clinicians and researchers, an operational definition covering different ethnic backgrounds is still under development. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) reported an operational definition of sarcopenia.
The similar approaches were carried out by the International Working Group on Sarcopenia (IWGS) [5] and Asian Working Group for Sarcopenia (AWGS) [6]. Based on these definitions, sarcopenia is characterized by low muscle mass in combination with poor muscle function.
Using different measurement tools, cutoff points and definitions cause different prevalence of sarcopenia in the world. On the other hand, these working groups of sarcopenia recommended the use of regional normative populations when available [7].
In 2018, the EWGSOP revised its consensus and proposed a new sarcopenia definition as the EWGSOP-2 [8]. In this updated definition, EWGSOP-2 focused on strength muscle as an important factor and recommended specific cut points for components of sarcopenia. Recently, some studies have published the prevalence of sarcopenia using the new criteria and factors related to sarcopenia [9–11]. However, there are no studies on the prevalence of sarcopenia and its related- factors using a new definition in Iranian older people.
So, the aim of this study was to compare the prevalence of sarcopenia and its associated factors by the EGWSOP-1 and EWGSOP-2 definitions in Iran. Additionally, we determined the agreement between definitions of sarcopenia.
Materials and methods
The study population
This cross-sectional study was conducted within the basis of the Bushehr Elderly Health (BEH) program, stage II. The methodology and protocol of the BEH program were previously described elsewhere [12]. In summary, the BEH study is a prospective population-based cohort study performed on a representative sample of older people in the urban population of Bushehr city, located in the south of Iran. The aim of this cohort is to determine the prevalence and risk factors of non-communicable diseases, including musculoskeletal disorders, and cognitive impairment and also assessment of their outcomes [13]. The study was approved by the Research Ethics Committee of both Bushehr University of Medical Sciences and Endocrinology & Metabolism Research Institute.
Data collection
Participants’ data were collected through the valid questionnaires including sociodemographic characteristics, and lifestyle data. A fixed stadiometer and a digital scale were used for the measurements of height and weight, respectively. Body mass index (BMI) was calculated by the formula weight (kg) / [height (m)]2. Waist circumference was measured above the iliac crest and the hip circumference was measured at the widest part of the hips. Blood pressure (BP) was measured twice by a standard mercury sphygmomanometer after 15 min of rest in the seated position and then the mean of the two measurements was considered as the participant’s systolic and diastolic blood pressures. The physical activity level was evaluated by a standard questionnaire-based on metabolic equivalent (MET) levels. [14]
Dual x-ray absorptiometry (DXA, Discovery WI, HologicInc, USA) was used to measure body composition. The skeletal muscle mass index (SMI) was defined as the sum of the muscle masses of the four limbs as appendicular skeletal muscle mass divided by squared height.
Maximum handgrip strength was measured in both hands by a digital grip strength dynamometer, 3 times, and the highest value was used [15]. Walking speed over 4.57 m was used for estimating physical performance [16].
Definition of terms
Sarcopenia
In this study, sarcopenia characterized by EWGSOP-1 and EWGSOP-2 criteria was compared.
Using EWGSOP- 1criteria, sarcopenia was defined as low muscle mass, additionally low muscle strength or low physical performance and when all three criteria of the definition were, the patient had severe sarcopenia [1].
On the other hand, EWGSOP and AWGS [6] recommended the use of reference data to decide cut- off values for sarcopenia components. Recently, a study showed that the reference data of the Iranian population for recognizing sarcopenia. the cut-off points for low SMI was 7.0 kg/m2 and 5.4 kg/m2 among men and women, respectively. The low muscle strength was < 26 kg for men and < 18 kg for women and the cut-off value for low physical performance was < 0.8 m/s for both sexes [17].
Based on EWGSOP-2 definition [8], sarcopenia was detected by the presence of low muscle mass with low muscle strength, and severe sarcopenia was considered when low muscle strength, low muscle mass, and low physical performance were all detected. According to new EWGSOP-2 definition, the European cut-off points for SMI were 7.0 kg/m2 for men and 5.5 kg/m2 among women, handgrip strength < 27 kg, and < 16 kg for men and women; respectively. The cut-off value for low physical performance was less of 0.8 m/s for both sexes [2].
Therefore, we compared three groups: (1) EWGSOP-1 (with Iranian cut-off points); (2) EWGSOP-2 (with Iranian cut-off points); (3) EWGSOP-2 (with European cut-off points) (Table 1].
Table 1.
Comparison of sarcopenia definitions
| Criteria | Components | Definitions | ||
|---|---|---|---|---|
| Low function | Low muscle mass | Sarcopenia | Severe Sarcopenia | |
|
EWGSOP-1 (with Iranian cut off) |
low muscle strength < 26 kg : men < 18 kg :women OR low physical performance was < 0.8 m/s for both sexes |
SMI < 7.0 kg/m2 :men SMI < 5.4 kg/m2 :women |
Low muscle mass + Low muscle strength OR Low physical performance |
Low muscle mass + Low muscle strength + Low physical performance |
|
EWGSOP-2 (with Iranian cut off) |
low muscle strength < 26 kg : men < 18 kg :women OR low physical performance was < 0.8 m/s for both sexes |
SMI < 7.0 kg/m2 :men SMI < 5.4 kg/m2 :women |
Low muscle strength + Low muscle mass |
Low muscle strength + Low muscle mass + Low physical performance |
|
EWGSOP-2 (with European cut off) |
low muscle strength < 27 kg : men < 16 kg :women OR low physical performance was < 0.8 m/s for both sexes |
SMI < 7.0 kg/m2 :men SMI < 5.5 kg/m2 :women |
Low muscle strength + Low muscle mass |
Low muscle strength + Low muscle mass + Low physical performance |
EGWSOP; European Working Group on Sarcopenia in Older People, SMI; Skeletal Muscle Index
Other associated clinical characteristics
Current smoker was defined as one who smokes at least one cigarette per day or uses a hookah or pipe once daily at the time of evaluation. The amount of physical activity was estimated based on metabolic equivalents (METs) score using a validated questionnaire for a single measurement of 24 h physical activity on an average weekday. High Fat Mass was outlined as total body percent fat > 30 for males and > 40 for females[18].
Statistical analysis
The data was presented as mean ± standard deviation (SD) for continuous and the percentage for categorical variables. Comparisons between continuous variables were done by t-test and comparisons of categorical data were performed using Pearson’s Chi-square test.
Cohen’s kappa coefficient was used to evaluate the degree of agreement between different sarcopenia definitions [19]. Age-standardized prevalence rates were measured using the population distribution of Iranian census data of 2016.
Stepwise logistic regression was used to select the independent risk factors of sarcopenia by different definitions. All the multivariate analyses included variables with p < 0.2; the final significance level for multivariate analyses was at P less than 0.05. All tests were two-sided, and a P-value < 0.05 was defined as statistically significant. The Stata 12 software (StataCorp, Texas, USA) was used for all statistical analyses.
Results
A total of 2426 elderly participants aged 69.34 ± 6.40 years (51.9% women) were considered for analyses. Table 2 shows the baseline characteristics of both genders. Overall, men had more appendicular muscle mass, less total fat mass, and lower BMI, waist, and hip circumferences than women. In handgrip muscle strength and walking speed, men walked faster and had more muscle strength than women (all P-value < 0.001). Also, 20.8% of men and 14.1% of women smoke daily.
Table 2.
General characteristics of the study participants
| Men (n = 1166) |
Women (n = 1260) |
Total (n = 2426) |
P- value | |
|---|---|---|---|---|
| Age (Years) | 69.54 ± 6.44 | 69.16 ± 6.35 | 69.34 ± 6.40 | 0.14 |
| Weight (Kg) | 72.30 ± 12.40 | 66.61 ± 13.13 | 69.35 ± 13.09 | < 0.001 |
| Height (cm) | 165.87 ± 6.31 | 152.24 ± 6.12 | 158.80 ± 9.22 | < 0.001 |
| Body mass index (Kg/m2) | 26.24 ± 4.02 | 28.70 ± 5.34 | 27.52 ± 4.90 | < 0.001 |
| Appendicular muscle mass (Kg) | 18.60 ± 2.80 | 13.37 ± 2.17 | 15.89 ± 3.61 | < 0.001 |
| SMI (Kg/m2) | 5.75 ± 0.84 | 6.76 ± 0.85 | 6.23 ± 0.98 | < 0.001 |
| Walking speed (m/s) | 0.95 ± 0.30 | 0.74 ± 0.27 | 0.84 ± 0.31 | < 0.001 |
| Hand grip strength (Kg) | 30.52 ± 8.38 | 17.34 ± 5.22 | 23.68 ± 9.56 | < 0.001 |
| Fat mass (Kg) | 31.24 ± 5.3 | 43.5 ± 5.3 | 37.59 ± 8.12 | < 0.001 |
| Waist circumference(cm) | 97.08 ± 11.23 | 100.23 ± 12.52 | 98.72 ± 12.02 | < 0.001 |
| Hip circumference(cm) | 99.33 ± 7.68 | 105.57 ± 11.21 | 102.57 ± 10.16 | < 0.001 |
| Mitral status (%) | ||||
| Married | 1113(95.5) | 751(59.6) | 1864(76.8) | < 0.001 |
| Single/widow/Divorced | 53(4.5) | 509(40.4) | 562(23.2 | |
| Education (%) | ||||
| None | 213(18.3) | 587(46.7) | 800(33.0) | < 0.001 |
| Under Diploma | 554(47.5 | 549(43.6) | 1103(45.5) | |
| Diploma and over | 399(34.2) | 122(9.7) | 521(21.5) | |
| Smoking (%) | 242(20.8) | 176(14.1) | 418(17.4) | < 0.001 |
| Physical activity (%) | 271(23.2) | 284(22.6) | 555(22.9) | 0.47 |
Data are presented as mean ± standard deviation or number(percent), SMI; Skeletal Muscle Index
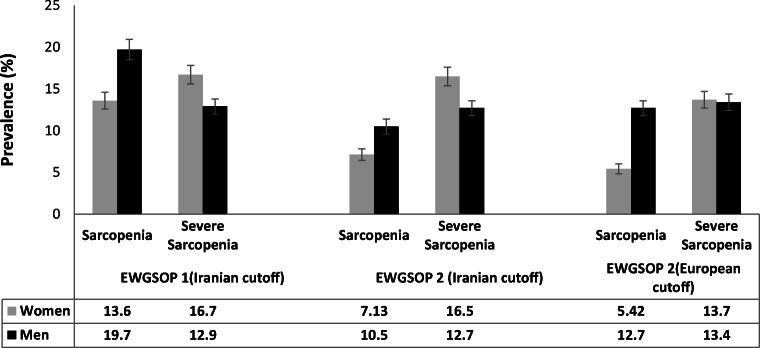
Figure-1 compares the age-standardized prevalence of sarcopenia using EWGSOP-1 (with Iranian cut-off points), EWGSOP-2 (with Iranian cut-off points), and EWGSOP-2 (with European cut-off points). The prevalence of sarcopenia and severe sarcopenia by EWGSOP-1 was 19.7%, and 12.9%, among men and 13.6%, 16.7% in women, respectively. When we used EWGSOP-2 (with Iranian cut-off points) criteria, the prevalence of sarcopenia and severe sarcopenia was 10.5% and 12.7% among men and 7.13%, and 16.5% in women, respectively. The prevalence of sarcopenia, and severe sarcopenia by EWGSOP-2 (with European cut-off points) were 12.7%, and 13.4%, in men and 5.42%, and 13.7% in women, respectively. By comparing the three different criteria, the Cohen’s kappa coefficient between EWGSOP-1 (Iranian cut off points) and EWGSOP-2 (with Iranian cut-off points) was 0.33 in women and 0.34 in men. Also, the Cohen’s kappa coefficient between EWGSOP-2 (Iranian cut off points) and EWGSOP-2 (with European cut-off points) was 0.73 and 0.92 among women and men, respectively.
Fig. 1.
Age-standardized prevalence rates of sarcopenia according to different definitions in both genders
Table 3 also reports the prevalence of each stage of sarcopenia among four different age groups in different diagnostic criteria. The prevalence of sarcopenia was between 3.20% and 16.10% among women in three criteria While the prevalence of sarcopenia in men was higher than women in all ages by three criteria. As it highlights, among the oldest population ( > = 75 years), the prevalence of severe sarcopenia is higher than the other age groups in both genders using all three criteria.
Table 3.
Prevalence of Sarcopenia according to definition of EWGSOP-1 and EWGSOP-2 by Iranian and European cut- off values in Different Age Groups and gender
| EWGSOP V1 (Iranian cutoff) | EWGSOP V2 (Iranian cutoff) | EWGSOP V2 (European cutoff) | ||||
|---|---|---|---|---|---|---|
| Sarcopenia | Severe Sarcopenia | Sarcopenia | Severe Sarcopenia | Sarcopenia | Severe Sarcopenia | |
| Men (Years) | ||||||
| 60–64 |
11.80 (8.25–16.15) |
2.50 (1.01–5.08) |
6.30 (3.80–9.83) |
2.50 (1.00-5.01) |
7.70 (4.92–11.49) |
2.50 (1.00-5.01) |
| 65–69 |
20.10 (16.40-24.32) |
5.00 (3.14–7.59) |
10.10 (7.42–13.39) |
4.90 (3.08–7.45) |
12.30 (9.30-15.77) |
5.90 (3.85–8.58) |
| 70–74 |
20.00 (14.63–26.31) |
12.30 (8.04–17.76) |
10.00 (6.22–15.02) |
12.00 (7.84–17.33) |
13.00 (8.67–18.47) |
12.50 (8.26–17.90) |
| ≥ 75 |
27.50 (22.06–33.46) |
38.20 (32.21–44.57) |
16.30 (11.94–21.42) |
38.10 (32.07–44.40) |
19.00 (14.39–24.45) |
39.30 (33.22–45.61) |
| Women (Years) | ||||||
| 60–64 |
11.50 (8.15–15.64) |
7.90 (5.12–11.52) |
4.90 (2.74–7.88) |
7.80 (5.04–11.34) |
3.20 (1.55–5.85) |
3.87 (2.02–6.66) |
| 65–69 |
16.10 (12.99–19.56) |
10.00 (9.13–14.97) |
8.30 (6.08–11.04) |
9.90 (7.43–12.77) |
6.40 (4.43–8.85) |
6.60 (4.60–9.07) |
| 70–74 |
14.80 (9.88–20.89) |
25.00 (18.79–32.07) |
8.00 (4.42–12.99) |
25.00 (18.79–32.07) |
6.30 (3.16–10.91) |
20.50 (14.76–27.18) |
| ≥ 75 |
10.00 (6.44–14.62) |
37.00 (30.70-43.55) |
6.90 (4.01-11.00) |
36.80 (30.57–43.37) |
5.60 (3.03–9.43) |
37.70 (31.40-44.25) |
EGWSOP; European Working Group on Sarcopenia in Older People
Table 4 shows the results of the logistic regression model to define the associate variables with sarcopenia. In all sarcopenia definitions, getting older increases the odds of sarcopenia in both genders. In both sexes, sarcopenia was more likely in individuals with high-fat mass in all definitions (OR between 2.17 and 3.06 in men, 3.91 and 5.42 in women). As expected BMI is inversely associated with sarcopenia, as having sarcropenia is less likely with increasing BMI in all definitions among both genders. Moreover, having physical activity decreases the odds of sarcopenia in all definitions.
Table 4.
The odds ratio of independent associate factors for sarcopenia defined by EWGSOP-1 and EWGSOP-2 using Iranian and European cut- off values in Different genders
| EWGSOP-V1 (Iranian cutoff) |
EWGSOP-V2 (Iranian cutoff) |
EWGSOP-V2 (European cutoff) |
||||
|---|---|---|---|---|---|---|
| OR(95% CI) | P- Value | OR(95% CI) | P- Value | OR(95% CI) | P- Value | |
| Men | ||||||
| Age | 1.14(1.11–1.17) | < 0.001 | 1.14(1.11–1.17) | < 0.001 | 1.13(1.10–1.16) | < 0.001 |
| Body mass index (Kg/m2) | 0.73(0.67–0.80) | < 0.001 | 0.75(0.71–0.86) | < 0.001 | 0.77(0.71-0.84) | < 0.001 |
| Waist circumference(cm) | 1.03(0.99–1.06) | 0.065 | 1.02(0.99–1.05) | 0.156 | 1.02(0.99–1.05) | 0.093 |
| Hip circumference(cm) | 0.95(0.92–0.98) | 0.004 | 0.95(0.91–0.98) | 0.016 | 0.94(0.90–0.97) | 0.001 |
| High Fat mass (Kg) | 2.17(1.47–3.20) | < 0.001 | 2.47(1.64–3.69) | < 0.001 | 3.06(2.01–4.64) | < 0.001 |
| Smoking (%) | Not included | Not included | Not included | |||
| Physical activity (%) | 0.41(0.28–0.61) | < 0.001 | 0.48(0.31–0.75) | 0.001 | 0.52(0.35–0.79) | 0.002 |
| Education(Yr) | 0.98(0.95–1.01) | 0.199 | 0.95(0.92–0.98) | 0.006 | 0.96(0.93–0.99) | 0.010 |
| Triglyceride (mg/dl) | 1.00(0.99–1.04) | 0.056 | 1.00(0.99–1.004) | 0.126 | Not included | |
| Women | ||||||
| Age | 1.03(1.00-1.06) | 0.057 | 1.05(1.02–1.08) | < 0.001 | 1.10(1.07–1.13) | < 0.001 |
| Body mass index (Kg/m2) | 0.55(0.51–0.60) | < 0.001 | 0.58(0.54–0.63) | < 0.001 | 0.64(0.60–0.69) | < 0.001 |
| Waist circumference(cm) | Not included | Not included | Not included | |||
| Hip circumference(cm) | Not included | Not included | Not included | |||
| High Fat mass (Kg) | 5.42(3.38–8.71) | < 0.001 | 3.91(2.44–6.27) | < 0.001 | 4.21 (2.58–6.86) | < 0.001 |
| Smoking (%) | 1.48(0.91–2.41) | 0.116 | Not included | Not included | ||
| Physical activity (%) | Not included | 0.61(0.39–0.95) | 0.015 | 0.70(0.44–1.12) | 0.137 | |
| Education (Yr) | 0.95(0.91–0.99) | 0.036 | 0.95(0.90–0.99 | 0.026 | 0.96(0.91–1.01) | 0.100 |
| Triglyceride(mg/dl) | Not included | Not included | Not included | |||
EGWSOP; European Working Group on Sarcopenia in Older People
Discussion
This study was performed to evaluate the prevalence and associated factors of sarcopenia defined the EWGSOP-2 and 1 criteria with different cut-offs in Iranian community-dwelling adults. The prevalence sarcopenia based on EWGSOP-1(with Iranian cut-off points) was 13.6% in women and 19.7% in men respectively. Similar results were found in other our study in Iran that showed a prevalence of about 21% in men and 15% among women, respectively [20].
The prevalence sarcopenia based on EWGSOP-2(with Iranian cut-off points) was 7.13% in women and 10.5% in men and based on EWGSOP-2(with European cut-off points) was 5.42% and 12.7% among women and men, respectively. Until the time of writing this article, none study found the prevalence of sarcopenia with the EWGSOP-2 criteria in Iran.
According to the difference in the prevalence of sarcopenia between the two consensus, EWGSOP-1 criteria utilized low muscle mass as the main diagnostic factor and EWGSOP-2 emphasized low muscle strength as a major component of sarcopenia. Also, some studies have shown that muscle strength is a better predictor of adverse results such as poor quality of life, disability, and mortality than muscle mass [15, 21, 22].
In this study, we found that EWGSOP2- defined sarcopenia prevalence was lower than that defined using EWGSOP-1 criteria due to different diagnostic factors to detect sarcopenia. Based on EWGSOP-2 criteria, low muscle performance was used only to classify the severe sarcopenia, while people with low muscle mass with low muscle performance without low strength would be classified as sarcopenia using the EWGSOP-1 criteria. Besides, the variation in prevalence between EWGSOP-1 and 2 was due to the difference between cut off values for muscle mass and strength in both gender. On the other hand, EWGSOP and AWGS recommended the use of reference values of SMI and muscle strength of the same population to get valid cut-off points to improve the diagnosis of sarcopenia[1, 6]. In order to fill the gap of cut- off points of components sarcopenia in the Iranian population, a study was conducted to determine cut-off points of low muscle mass which lead to reference values in Iranian people. Calculated cutoff values of low SMI were 7.0 Kg/m2 and 5.4 Kg/m2 among Iranian men and women, respectively which were alike to AWGS recommendation [6]. Therefore, in the current study, we compare the prevalence of sarcopenia by the EWGSOP-1 and 2 with Iranian and European cut off points. Although all cutoffs of muscle mass are the same in EWGSOP-1 and EWGSOP-2 (both with Iranian cut off), it seems that Priority muscle strength to muscle mass leads to a lower prevalence of sarcopenia by EWGSOP-2 definition in both genders. Similar results were found in another study that showed a prevalence of 4% based on EWGSOP-2 definition vs. 14% by EWGSOP-1 criteria in a Brazilian population [23]. Also, Yang et al. found that the prevalence of EWGSOP-2-defined sarcopenia was lower than that defined by the EWGSOP-1 in both genders [9].
Our study showed the low agreement between the EWGSOP-1 and EWGSOP-2 criteria with Iranian cut off points. In addition, the EWGSOP-2 with European cut off points had a higher agreement with EWGSOP-1 with Iranian cut off points because update cut points for the EWGSOP-2 criteria were closer to the Iranian cut off points.
Some publications have shown low to moderate agreement between EWGSOP-1 and revised EWGSOP-2 definition [24, 25]. A study in a Chinese older people found the kappa value 0.32 and 0.37 among men and women similar to our results [9].
Our findings are in agreement with other studies indicating that the older age, high fat mass, physical activity, lower BMI were independently associated with sarcopenia in both genders regardless of the definitions [20, 26]. It seems that there is no difference in sarcopenia associated- factors between EWGSOP-1 and revised EWGSOP-2 definition in our population. However, further prospective studies need to confirm that the EWGSOP-2 operational definition is more suitable than other criteria to guide clinical practice and scientific research. In all models, age was an important factor with an increase of about 5 to 14 percent in the prevalence of sarcopenia in both genders. Evidences indicated a decline of muscle mass, strength, and physical function begin in the third decade of life and more rapidly in the fifth decade of life [4, 27]. Lifestyle factors such as physical inactivity, smoking, and poor diet have been shown to be the risk factors for muscle mass and muscle function [28]. In our results, fat mass was a stronger factor which stayed in both sexes independent of BMI. Scientific evidence indicates that BMI does not address adipose tissue or distinguish between lean and fat mass, and different methods are required to indicate body adiposity [29]. In this regard, the percentage of fat mass is a real measure of adiposity tissue since it has been demonstrated to be related to metabolic dysregulation, regardless of body weight [30]. Sarcopenia characterized by fat infiltration into muscle, an expansion in fibrosis, changes in muscle metabolism, oxidative stress, and degeneration of the neuromuscular junction. This finally leads to progressive loss of muscle quality and function [31]. These results clarify why a more elevated level of BMI, with more muscle mass and less fat mass, decreases the risk of sarcopenia.
In this study, some limitations should be recognized. The cross-sectional design limited the possibilities of determining the most optimal cut off values and suitable definition through outcome-based approaches. Also, further research with a longitudinal design is required to recognize any causal relationship. This study with a great sample size from a population-based study provided data on musculoskeletal disorders such as sarcopenia in Iran.
Conclusions
This study is the first study to compare the prevalence of sarcopenia using the EWGSOP-1 and 2 criteria in Iran. Different diagnostic approaches and cut off points of components of sarcopenia have made a substantial impact on the prevalence of sarcopenia. Some adverse outcomes should be taken into the account for estimating the Iranian probable sarcopenia in order to compare the accuracy of EWGSOP-1 and EWGSOP-2.
Acknowledgements
We would like to thank all the personnel of the Bushehr Elderly Health program and all the individuals who took part in the study. Also, the authors thank the “World Congress on Osteoporosis, osteoarthritis, and Musculoskeletal Diseases (WCO-IOF-ESCEO 2018) that the preliminary of this study as a poster abstract has been published in proceeding.
Authors’ contributions
All authors read and approved the final paper.
Compliance with ethical standards
Conflict of interest
Gita Shafiee, Ramin Heshmat, Afshin Ostovar, Fatemeh Khatami, Noushin Fahimfar, Seyed Masoud Arzaghi, Safoora Gharibzadeh, Sara Hanaei, Iraj Nabipour, Bagher Larijani declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramin Heshmat, Email: rheshmat@tums.ac.ir.
Afshin Ostovar, Email: afshin.ostovar@gmail.com.
References
- 1.Cruz-Jentoft A, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis. Report of the European Workign Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. Journal of diabetes metabolic disorders. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–33. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Shafiee G, Heshmat R, Ostovar A, Nabipour I, Larijani B. Sarcopenia disease in Iran: an overview. J Diabetes Metab Disord. 2019;18(2):665–74. doi: 10.1007/s40200-019-00452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Yao X, Shen J, Sun G, Sun Q, Tian X, et al. Comparison of revised EWGSOP criteria and four other diagnostic criteria of sarcopenia in Chinese community-dwelling elderly residents. Exp Gerontol. 2020;130:110798. doi: 10.1016/j.exger.2019.110798. [DOI] [PubMed] [Google Scholar]
- 10.Saeki C, Takano K, Oikawa T, Aoki Y, Kanai T, Takakura K, et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord. 2019;20(1):615. doi: 10.1186/s12891-019-2983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kassmann H, et al. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing. 2019;48(5):719–24. doi: 10.1093/ageing/afz035. [DOI] [PubMed] [Google Scholar]
- 12.Ostovar A, Nabipour I, Larijani B, Heshmat R, Darabi H, Vahdat K, et al. Bushehr Elderly Health (BEH) Programme, phase I (cardiovascular system). BMJ Open. 2015;5(12):e009597. [DOI] [PMC free article] [PubMed]
- 13.Shafiee G, Ostovar A, Heshmat R, Darabi H, Sharifi F, Raeisi A, et al. Bushehr Elderly Health (BEH) programme: study protocol and design of musculoskeletal system and cognitive function (stage II). BMJ Open. 2017;7(8):e013606. [DOI] [PMC free article] [PubMed]
- 14.Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196–202. [DOI] [PubMed]
- 15.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 16.Rydwik E, Bergland A, Forsen L, Frandin K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: a systematic review. Physiother Theory Pract. 2012;28(3):238–56. doi: 10.3109/09593985.2011.601804. [DOI] [PubMed] [Google Scholar]
- 17.Shafiee G, Ostovar A, Heshmat R, Keshtkar AA, Sharifi F, Shadman Z, et al. Appendicular Skeletal Muscle Mass Reference Values and the Peak Muscle Mass to Identify Sarcopenia among Iranian Healthy Population. Int J Prev Med. 2018;9. [DOI] [PMC free article] [PubMed]
- 18.Hill KD, Farrier K, Russell M, Burton E. Dysmobility syndrome: current perspectives. Clin Interv Aging. 2017;12:145–52. doi: 10.2147/CIA.S102961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–82. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemi R, Shafiee G, Motlagh AD, Pasalar P, Esmailzadeh A, Siassi F, et al. Sarcopenia and its associated factors in Iranian older individuals: results of SARIR study. Arch Gerontol Geriatr. 2016;66:18–22. [DOI] [PubMed]
- 21.Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. 2016;16(1):5–20. [DOI] [PubMed]
- 22.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35(4):409–15. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 23.de Freitas MM, de Oliveira VLP, Grassi T, Valduga K, Miller MEP, Schuchmann RA, et al. Difference in sarcopenia prevalence and associated factors according to 2010 and 2018 European consensus (EWGSOP) in elderly patients with type 2 diabetes mellitus. Exp Gerontol. 2020;132:110835. doi: 10.1016/j.exger.2020.110835. [DOI] [PubMed] [Google Scholar]
- 24.Locquet M, Beaudart C, Petermans J, Reginster JY, Bruyere O. EWGSOP2 Versus EWGSOP1: impact on the prevalence of sarcopenia and its major health consequences. J Am Med Dir Assoc. 2019;20(3):384–5. doi: 10.1016/j.jamda.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Phu S, Vogrin S, Zanker J, Bani Hassan E, Al Saedi A, Duque G. Agreement between initial and revised european working group on sarcopenia in older people definitions. J Am Med Dir Assoc. 2019;20(3):382–3 e1. [DOI] [PubMed]
- 26.Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ., 3rd Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol Ser A Biol Sci Med Sci. 2005;60(2):213–6. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 27.Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcif Tissue Int. 2017;101(3):229–47. [DOI] [PMC free article] [PubMed]
- 28.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol Ser A Biol Sci Med Sci. 2010;65(5):495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the heritage family study. Int J Obes Relat Metab Disord. 2002;26(6):789–96. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 30.Lichtash CT, Cui J, Guo X, Chen YD, Hsueh WA, Rotter JI, et al. Body adiposity index versus body mass index and other anthropometric traits as correlates of cardiometabolic risk factors. PloS One. 2013;8(6):e65954. [DOI] [PMC free article] [PubMed]
- 31.Dhillon RJ, Hasni S. Pathogenesis and Management of Sarcopenia. Clin Geriatr Med. 2017;33(1):17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]