Abstract
Purpose
The present study was aimed at evaluating the role of Momordica charantia L. fruit and Genistein on beta cell, insulin resistance/sensitivity and lipid profile in type 2 diabetic rats.
Methods
Thirty-five (35) albino rats were divided into seven (7) groups of 5 rats each comprising of five (5) non-diabetic and thirty (30) diabetic rats. Groups 1 and 2 served as the normal control and diabetic control groups respectively and received distill water, groups 3 and 4 received Mormodica charantia L. at 250 mg/kg and 500 mg/kg respectively. Groups 5 and 6 received Genistein at 10 mg/kg and 20 mg/kg respectively while group 7 received Metformin at 500 mg/kg the experiment lasted for four weeks. All the rats were euthanized at the end of the fourth week.
Results
Lipid profile, glucose and insulin levels were determined from the analysis of serum parameters and the histology of the pancreas. A significant reduction (p < 0.05) in blood glucose levels was noticed in rats that received Momordica charantia L. (MC) and genistein when compared with diabetic control rats. A significant decrease (p < 0.05) in cholesterol, triglyceride, low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were also noted in rats that received MC and Genistein when compared with the diabetic control rats. MC and Genistein significantly increased (P < 0.05) serum insulin level compared to the diabetic control rats. MC and Genistein significantly decreased (p < 0.05) homeostatic model assessment-insulin resistance (HOMA-IR) level compared with the diabetic control group. Pancreas of rats that received MC and Genistein showed regenerating beta-cells.
Conclusion
Momordica charantia L. fruit and Genistein were able to enhance beta cell function and prevent lipid accumulation and insulin resistance in type 2 diabetic rats.
Keywords: Glucose, Hyperglycemia, Insulin, Isoflavones, Momordin, Pancreas
Introduction
Type 2 diabetes (T2D) is a chronic disorder characterized by insulin resistance and sensitivity due to the inability of insulin receptors to utilize insulin secreted from beta cell (β-cell) and/or the loss of pancreatic β-cell [1–3]. The complications that comes with T2D includes visual and cognitive impairment, neurological disorders, renal disease and coronary heart disease [4]. Diabetes is usually associated with abnormalities in the metabolism of lipid, protein and carbohydrate [5]. The sequence of events that leads to these metabolic disorders are highly debated but obesity is shown to be a predominant risk factor for insulin resistance due to the increase in free fatty acid levels in the liver, skeletal muscle and pancreas [6, 7]. Persistent lipotoxicity and insulin resistance could eventually lead to pancreatic β-cell dysfunction, which will subsequently cause type 2 diabetes [8]. Oxidative stress, inflammation and obesity are the common etiologies in T2D complication [9]. Inflammatory mediators and oxidative stress have been reported to modulate β-cell survival and function but the synthesis and secretion of insulin through the pancreatic β-cells is critical in maintaining normal blood glucose levels [10].
In 2017, it was estimated that 425 million people had diabetes [11]. In 2019, 463 million people were estimated to be living with diabetes with China topping the list, followed by India and USA [12]. The number of people living with diabetes is expected to increase to 578 million in 2030 and 700 million in 2045 [12]. Type 2 diabetes accounts for about 90% diabetes globally [12]. The increase in the prevalence of type 2 diabetes might be due to early detection and improved management and prevention of mortality rates associated with the disease [13]. Regular estimates and projections of future diabetes prevalence will promote researches that are aimed at management of diabetes and preventing death rates due to diabetic complications.
Momordica charantia L. commonly called bitter melon is rich in bioactive compounds such as charantin, momordin, cucurbitanes, polypetide-p, erythodiol and galacturonic acid which are known to possess hypoglycemic and antioxidant activities [14–16]. It is used for treatment and management of diabetes in India, South America and East Africa [17]. Genistein is an isoflavone (a class of phytoestrogen) found mostly in leguminous plants, isofavones are reported to increase serum insulin and prevent oxidative stress in diabetic rats [18]. Many prior studies have reported the role of Momordica charantia L. and genistein in the treatment and management of diabetes and its complications because of their antioxidant and anti-hyperglycemic properties [9, 16]. Momordica charantia L. juice was reported to increase the number of β-cells in diabetic rats [19]. The fruit of Momordica charantia L. was reported to increase insulin signaling in skeletal muscle and promote beta cell regeneration [20]. Genistein was reported to stimulate insulin secretion in the pancreatic islet of mice and insulin secreting cell lines [21]. It is also known to be effective in the prevention and treatment of metabolic disorders [22].
Previous studies highlight the role of Momordica charantia L. fruit juice and Genistein supplementation in promoting insulin signaling and beta cell regeneration in type 1 diabetes and diabetic complications [9, 18–20]. The present study examines the role of lyophilized Momordica charantia L. fruit and Genistein on type 2 diabetic rats, focusing on lipid profile, beta cell regeneration and homeostatic model assessment of insulin sensitivity, β-cells function and insulin resistance.
Materials and methods
Plant materials
Momordica charantia L. fruits were obtained from a local farm around Shika Dam, Sabon Gari Local Government (LGA) in Kaduna State, Nigeria and were authenticated in the Department of Biological Sciences, Ahmadu Bello University (ABU) Zaria (voucher number 01139). The fruits were washed, freeze dried and lyophilized with a high-speed hammer mill at a pressure of 30 pa. It was filtered through a 150 μm screen. The super fine powder was dissolved in distilled water before use.
Experimental animals
Thirty-five (35) male and female Wistar rats (Rattus norvegicus) approximately 7 weeks old (63 g–79 g body weight) were purchased from the Laboratory Animal Resource Center, Department of Pharmacology, ABU Zaria. They were kept in the laboratory animal facility, Department of Pharmacology, for two (2) weeks before the commencement of the study. Oral acute toxicity study of Momordica charantia L. fruit was carried out according to the method of Lorke [23], in order to determine the dosages to be used. The first phase consisted of nine rats divided into three groups of three rats each. The rats in each group received Momordica charantia L. fruit at (10, 100 & 1000) mg/kg respectively. The rats were monitored for 24 h for any sign of toxicity or mortality. İn the second phase, three rats were divided into three groups of one rat each. The rats received Momordica charantia L. fruit at (1600, 2900 & 5000) mg/kg. The rats were monitored for 24 h consistently and subsequently for the next 14 days for any sign of toxicity and/or mortality. The LD50 of Momordica charantia L. fruit was considered to be greater than 5000 mg/kg since no mortality was recorded.
Induction of type 2 diabetes
Type 2 diabetes was induced in 30 rats by mixing 40 g of animal-derived fats with 100 g of pelletized rat chow for the period of 10 weeks. At the end of the 10th week, diabetes was induced by injecting the rats with a freshly prepared streptozotocin injection (St. Louis, USA) in citrate buffer (pH 4.5) at 40 mg/kg. `Oral glucose tolerance test (OGTT) was performed on each rat, one week after diabetes induction. The rats were given 2.5 g/kg glucose and the blood glucose concentrations were measured at 30 min, 60 min, 90 min and 120 min after induction using a glucometer (AccuChek, Roche, Switzerland). Rats that displayed a sustained increase in blood glucose level up to ≥200 mg/dL were confirmed to be diabetic.
Experimental design
The thirty-five (35) rats were divided into seven (7) groups of five (5) rats in each group comprising of five (5) non-diabetic and thirty (30) diabetic rats. Groups 1 and 2 served as the normal control and diabetic control groups respectively and received 0.2 ml/kg distilled water, groups 3 and 4 received 250 mg/kg of Momordica charantia L. fruit (5% of LD50) and 500 mg/kg Momordica charantia L. fruit (10% of LD50) respectively. Groups 5 and 6 received (10 mg/kg and 20 mg/kg) Genistein respectively [24] while group 7 served as the standard drug group and received Metformin at 500 mg/kg. The experiment lasted for a period of four (4) weeks. At the end of the fourth week, each rat was given 2.5 g/kg glucose and the blood glucose concentrations were measured at 30 min, 60 min, 90 min and 120 min using a glucometer. The rats were euthanized with Ketamine hydrochloride injection (Pfizer, USA) and the blood was collected in a plain bottle. The blood samples were centrifuged at 3000 rpm for 5 Minutes. The serum was stored at −30 °C until analysis.
Biochemical parameters
Serum insulin level and lipid profile were analyzed with enzyme linked immunosorbent assay (ELISA) kit according to manufacturer’s instruction (W&Z Biotech, China and ACON Labs, USA). Serum insulin homeostatic model assessment (HOMA-IR, HOMA-β and HOMA-IS) were estimated using the following formulae: HOMA-IR =
Where HOMA-IR = homeostatic model assessment insulin resistance, HOMA-β = homeostatic model assessment beta-cell function and HOMA-IS = homeostatic model assessment insulin sensitivity [25]. The pancreas of each rat was harvested, fixed in 10% neutral buffered formalin and processed for light microscopic study. Five slides from each rat were observed under the microscope and five pancreatic islets were observed per slide.
Statistical analysis
Values were expressed as mean ± standard error of the mean (SEM). Statistical package for social sciences (SPSS) Version 21 was used for the analysis. One-way analysis of variance (ANOVA) followed by Tukey multiple comparison post hoc test was used to determine the difference between groups and P < 0.05 was considered statistically significant.
Results
Oral glucose tolerance test (pre-treatment)
Figure 1 present the result of OGTT before commencement of the experiment, blood glucose levels in all the experimental groups were significantly higher (P < 0.05) than the baseline of 200 mg/dL at 30 min (470 mg/dL) the glucose level started to decrease at 60 min and 90 min with a range of (320–355) mg/dL at 120 min. This indicated that hyperglycemia was successfully induced in all experimental groups. The mean blood glucose level of the normal control rats was 110 mg/dL at 120 min. There was no significant change (P > 0.05) in the glucose tolerance between the experimental groups (Fig. 1).
Fig. 1.
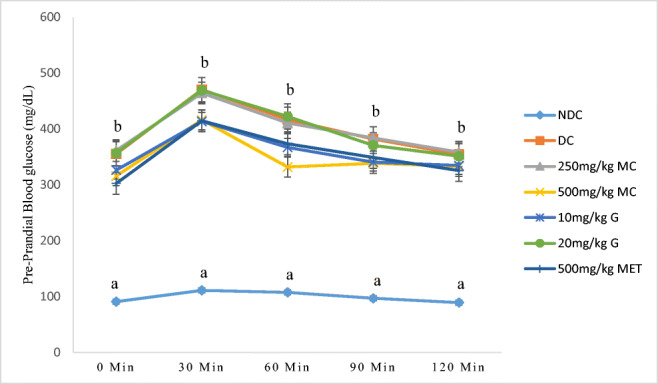
Oral glucose tolerance test of rats treated with high fat diet and 40 mg/kg sreptozotocin. Values along the same line with different superscripts are significantly different at (P < 0.05). n = 5, error bars indicate standard error of the mean, NC = normal control, DC = diabetic control, MC = Momordica charantia L., G = Genistein, MET = Metformin
Oral glucose tolerance test (post-treatment)
The result from fig. 2 showed significant elevation (p < 0.05) in blood glucose levels of rats in diabetic control rats at (30–120) minutes when compared with the normal control. A significant reduction (p < 0.05) in blood glucose levels was noticed in rats that received Mormodica charantia L., Genistein and metformin over a period of 120 min. The rats that received 500 mg/kg Mormodica charantia L. and 20 mg/kg Genistein had a significantly lower blood glucose level (p < 0.05) when compared with rats that received 250 mg/kg Mormodica charantia L. and 10 mg/kg Genistein. Mormodica charantia L. administered at 500 mg/kg and 20 mg/kg Genistein were as effective as Metformin in the control of blood glucose level in diabetic rats (Fig. 2).
Fig. 2.
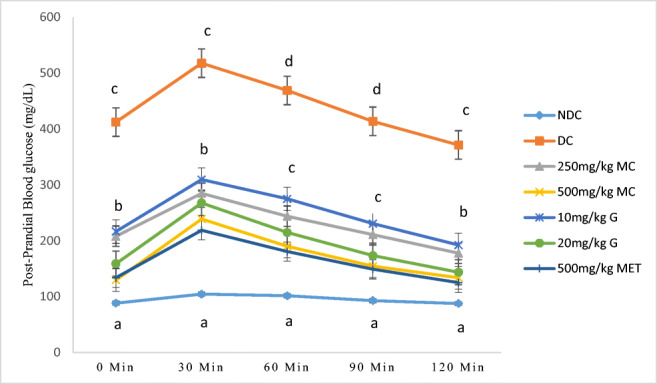
Oral glucose tolerance test of non-diabetic and diabetic rats treated with Momordica charantia L., genistein and metformin. Values along the same line with different superscripts are significantly different at (P < 0.05). n = 5, error bars indicate standard error of the mean, NC = normal control, DC = diabetic control, MC = Mormodica charantia L., G = Genistein, MET = Metformin
Biochemical parameters
Table 1 shows that there was a significant increase (P < 0.05) in cholesterol (170.65 ± 0.78), triglyceride (136.60 ± 8.78), LDL (88.97 ± 2.15) and VLDL (27.32 ± 1.75) levels in diabetic control rats when compared to normal control (131.40 ± 6.19, 36.85 ± 2.63, 41.45 ± 4.78 and 7.37 ± 0.52 respectively) with a significant decrease (P < 0.05) in HDL level in diabetic control rats (44.73 ± 4.01) compared with the normal control (81.30 ± 2.54). A significant decrease (p < 0.05) in cholesterol, triglyceride, LDL and VLDL levels were also noted in rats that received Momordica charantia L., Genistein and Metformin when compared with the diabetic control rats. For the HDL level, a significant increase (p < 0.05) was observed in rats that received Momordica charantia L., (70.63 ± 6.67 and 72.92 ± 1.12) Genistein (61.35 ± 2.55 and 79.77 ± 2.33) and Metformin (66.34 ± 4.85) compared with diabetic control rats. There was no significant change (p > 0.05) in the levels of HDL between the rats that received metformin and diabetic control rats (Table 1).
Table 1.
Biochemical analysis of serum lipid profile of diabetic rats treated with Momordica charantia L., Genistein and Metformin
| Groups | Cholesterol mg/dl | Triglyceride (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|---|---|---|
| Normal control | 131.40 ± 6.19a | 36.85 ± 2.63a | 81.30 ± 2.54d | 41.45 ± 4.78a | 7.37 ± 0.52a |
| Diabetic control | 170.65 ± 0.78e | 136.60 ± 8.78c | 44.73 ± 4.01a | 88.97 ± 2.15c | 27.32 ± 1.75d |
| 250 mg/kg Momordica charantia L. | 155.17 ± 3.52cd | 67.70 ± 15.89a | 70.63 ± 6.67cd | 64.25 ± 6.19b | 16.04 ± 2.13bc |
| 500 mg/kg Momordica charantia L. | 141.23 ± 3.67ab | 52.12 ± 9.73a | 72.92 ± 1.12cd | 48.65 ± 6.61ab | 10.47 ± 1.91ab |
| 10 mg/kg Genistein | 146.35 ± 2.16bc | 65.83 ± 10.71a | 61.35 ± 2.55bc | 57.60 ± 6.60ab | 14.91 ± 1.67b |
| 20 mg/kg Genistein | 134.37 ± 3.00a | 51.05 ± 6.05a | 79.77 ± 2.33d | 42.82 ± 4.60a | 11.46 ± 0.41ab |
| 500 mg/kg Metformin | 159.12 ± 0.51d | 102.17 ± 13.56b | 66.34 ± 4.85ab | 61.55 ± 2.71b | 20.43 ± 2.71c |
All values were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey multiple comparison post hoc test. Values along the same column with different with superscripts a,b,c,d,e are significantly different (p < 0.05), n = 5.
Serum insulin level
The serum insulin level of diabetic control (23.01 ± 0.15) rats decreased significantly (p < 0.05) as compared with normal control (58.15 ± 2.57) rats (Table 2). Oral administration of Momordica charantia L. (36.13 ± 3.14 and 45.43 ± 1.77) and Genistein (41.88 ± 1.79 and 42.24 ± 2.44) significantly increased (P < 0.05) the serum insulin level compared to the diabetic control rats (23.01 ± 0.15). Also the serum insulin level of rats that received Metformin (39.56 ± 1.87) were significantly higher (p < 0.05) than those of normal control rats (58.15 ± 2.57) but had no significant difference (p > 0.05) when compared with rats that received Momordica charantia L. (36.13 ± 3.14 and 45.43 ± 1.77) and Genistein (41.88 ± 1.79 and 42.24 ± 2.44) (Table 2).
Table 2.
Serum insulin level and Homeostasis Model Assessment of diabetic rats treated with Momordica charantia L., Genistein and Metformin
| Groups | Insulin (ng/dl) | HOMA-IR | HOMA-β | HOMA-IS |
|---|---|---|---|---|
| Normal control | 58.15 ± 2.57d | 12.43 ± 0.77a | 14.04 ± 0.67e | 2.00 ± 0.12d |
| Diabetic control | 23.01 ± 0.15a | 24.29 ± 1.77c | 1.10 ± 0.07a | 1.03 ± 0.01a |
| 250 mg/kg Momordica charantia L. | 36.13 ± 3.14b | 18.47 ± 1.43b | 3.69 ± 0.64b | 1.36 ± 0.11ab |
| 500 mg/kg Momordica charantia L. | 45.43 ± 1.77c | 13.97 ± 0.89ab | 7.57 ± 0.46d | 1.79 ± 0.12bc |
| 10 mg/kg Genistein | 41.88 ± 1.79b | 16.74 ± 0.83ab | 5.31 ± 0.32bc | 1.49 ± 0.07bc |
| 20 mg/kg Genistein | 42.24 ± 2.44bc | 13.70 ± 0.63ab | 6.62 ± 0.48cd | 1.81 ± 0.08bc |
| 500 mg/kg Metformin | 39.56 ± 1.87bc | 14.91 ± 0.63ab | 5.31 ± 0.29b | 1.66 ± 0.07bcd |
All values were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey multiple comparison post hoc test. Values along the same column with different with superscripts a,b,c,d,e are significantly different (p < 0.05), n = 5.
Homeostasis model assessment (HOMA)
The result of HOMA-IR showed a significant increase (p < 0.05) in diabetic control rats (24.29 ± 1.77) when compared with the normal control rats (12.43 ± 0.77). Rats that received Momordica charantia L. (18.47 ± 1.43 and 13.97 ± 0.89), Genistein (16.74 ± 0.83 and 13.70 ± 0.63) and Metformin (14.91 ± 0.63) had a significantly decreased (p < 0.05) in HOMA-IR level compared with the diabetic control (24.29 ± 1.77) group (Table 2). The HOMA-IS and HOMA-β results showed a significantly lower level (p < 0.05) in diabetic control rats (1.10 ± 0.07 and 1.03 ± 0.01) compared with the normal control (14.04 ± 0.67 and 2.00 ± 0.12) and rats that received 250 mg/kg Momordica charantia L. (3.69 ± 0.64 and 1.36 ± 0.11), 500 mg/kg Momordica charantia L. (7.57 ± 0.46 and1.79 ± 0.12), 10 mg/kg Genistein (5.31 ± 0.32 and 1.49 ± 0.07) and 20 mg/kg Genistein (6.62 ± 0.48 and 1.81 ± 0.08). The rats that received 250 mg/kg Momordica charantia L. (3.69 ± 0.64 and 1.36 ± 0.11), 500 mg/kg Momordica charantia L. (7.57 ± 0.46 and1.79 ± 0.12), 10 mg/kg Genistein (5.31 ± 0.32 and 1.49 ± 0.07), 20 mg/kg Genistein (6.62 ± 0.48 and 1.81 ± 0.08) and Metformin (5.31 ± 0.29 and 1.66 ± 0.07) showed a significantly high (p < 0.05) HOMA-IS and HOMA-β levels compared with diabetic control rats (14.04 ± 0.67 and 2.00 ± 0.12) (Table 2).
Histological study
Photomicrograph of the pancreas of normal control rats showed normal beta-cells (Fig. 3a), photomicrograph of pancreas of diabetic control rats showed severe degranulation of the β-cells with eosinophilic material (Fig. 3b). The pancreas of rats that received Momordica charantia L. showed centrally situated normal β-cells and regeneration of β-cells (Figs. 3c, d). The pancreas of rats that received Genistein showed two adjacent islets with regular contours both showing slight regeneration of β-cells (Figs. 3e, f), while the pancreas of rats that received metformin showed normal beta cells (Fig. 3g).
Fig. 3.
Composite photomicrographs of pancreas of non-diabetic and diabetic rats showing normal beta-cells in a, degranulation of beta-cells in b and regenerating beta-cells in c, d, e, f & g. H&E ×250. a = pancreas of normal control rats, b = pancreas of diabetic control rats, c = pancreas of rats treated with 250 mg/kg Momordica charantia L., d = pancreas of rats treated with 500 mg/kg Momordica charantia L., e = pancreas of rats treated with 10 mg/kg Genistein, f = 20 mg/kg Genistein, g = pancreas of rats treated with Metformin
Discussion
The outcome of the present result indicated that type 2 diabetes was successfully induced which is in agreement with the results of earlier studies [26] where type 2 diabetes was induced in canine and rat models with high-fat diet and 35-40 mg/kg streptozotocin injection. Streptozotocin is known to deplete pancreatic β-cells by transferring methyl group to DNA leading to DNA fragmentation [27]. Insulin resistance is linked to glucose intolerance which occurs as a result of reduced/impaired insulin action at normal glucose levels, these are all seen in type 2 diabetes [28]. Destruction of IRS-1 and GLUT-4 signaling pathways might also lead to insulin resistance [29]. The blood glucose level of the normal control rats was within the normal blood glucose ranged from 70 to 110 mg/dL [30].
The hypoglycemic activity of Momordica charantia L. that was observed in the present study is in line with previous studies [31–33] that reported anti-hyperglycemic effect of Momordica charantia L. in different diabetic animal model. The beneficial effects of the Momordica charantia L. fruit could be attributed to improved insulin sensitivity and beta-cell function [34, 35]. The antihyperglycemic effect of genistein that was reported in the present study may be attributed to its ability to improve glucose tolerance and circulating serum insulin level as well as the ability to activate cAMP/PKA signaling cascades to exert its insulinotropic effect [36, 37]. These are in agreement with studies which reported that soy protein consumption was effective in reducing blood glucose level due to its Genistein content [38, 39].
Accumulation of free fatty acids in the liver can lead to different abnormalities that contribute to dyslipidemia as observed in diabetic patients [40]. The observed increase in the serum level of cholesterol, triglyceride, LDL and VLDL of diabetic control rats as observed in the present study is in agreement with previous studies that reported increase in serum cholesterol, triglyceride, LDL in type 2 diabetic [41, 42]. Low serum level of HDL was observed in the diabetic control rats in the current study. Prior research studies also suggest that HDL dysfunctions in the diabetic state leading to decrease in serum HDL level [43, 44]. The esterification of free fatty acids leads to triglycerides accumulation within hepatocytes, promoting the uptake of fatty acid by liver cells leading to steatosis [45]. The present study reported that Momordica charantia L. fruit and Genistein were effective in preventing steatosis by decreasing serum levels of cholesterol, triglyceride, LDL and VLDL and preventing lipid accumulation within the liver tissue. While some studies also confirmed that Momordica charantia L. and genistein can revert the usual increase in lipid profile that is common in the diabetic state [46, 47], other studies reported that Genistein supplement did not improve plasma lipid profile of mice [48].
The decrease in serum insulin level and hyperglycemia seen in the diabetic control rats of the present study may be as a result of beta-cell depletion and/or excessive hepatic glucose output due to insulin insensitivity. Studies have shown that insulin resistance and depletion of the beta-cells are the hallmark of type 2 diabetes [2, 49]. The present study showed that Momordica charantia L. and Genistein were able to significantly increase the syntheses of insulin, this may be because of the capacity of these substances to protect pancreatic beta cell and enhance the regeneration of damaged beta cells leading to an increase in insulin secretion and sensitivity. Earlier studies have revealed that Genistein protects β-cell, induces proliferation and increases insulin secretion [24, 50]. This further confirms the anti-hyperglycemic potential of Genistein by its effect on blood insulin level by elevating it as observed in the present study.
The significant increase in the levels of HOMA-β and HOMA-IS that was observed in diabetic rats treated with Momordica charantia L. and genistein in the present study is an indication that Momordica charantia L. and Genistein can protect beta islet cells and increase insulin sensitivity. The homeostasis model assessment (HOMA-β) is used to assume the feedback loop between the beta-cell and the liver [25]. Previous studies have reported lower HOMA-β values in individuals who developed diabetes, suggesting that higher HOMA-β values is a sign of protection against diabetes [51]. A significant decrease in HOMA-IR was noticed in diabetic rats while treatment with Momordica charantia L. and Genistein attenuated T2D by preventing insulin resistance. HOMA-IR can be used for the early evaluation of insulin resistance [52]. High levels of HOMA-IR was reported as a predictive sign of developing type 2 diabetes [53]. The reduction observed in the HOMA-IR is in agreement with the work carried out by [54] that observed that Genistein reduced HOMA-IR. This could be achieved by increasing the uptake of glucose through the promotion of GLUT 4 to the cell membrane [55]. The significant increase in the HOMA-IR might be due to ectopic lipid accumulation in the liver and muscle tissue which induces insulin resistance by increasing the level of diacyglycerol (DAG) in the tissue [56].
The degranulation of the β-cells and presence eosinophilic material that was observed in the pancreas of diabetic control rats confirms the specificity of streptozotocin in beta cell toxicity that eventually leads to diabetes mellitus. This present study showed that Momordica charantia L. and Genistein were able to induce the regeneration of damaged beta cells. This is in agreement with a previous study by [57] who reported that Momordica charantia L. has a glucose lowering effect on mice by either preventing beta cell damage or enhancing the regeneration of depleted beta cells. Beta cell proliferation was also observed in cell culture model and in pancreatic islet cell of Genistein-treated mice model following the administration of Genistein [48].
In conclusion, administration of Momordica charantia L. fruit at 250 mg/kg and 500 mg/kg and (10 mg/kg and 20 mg/kg) Genistein were able to enhance beta cell function and prevent lipid accumulation and insulin resistance in type 2 diabetic rats. It is recommended that Momordica charantia L. fruit and Genistein should be subjected to clinical trials at different doses for possible translation to Human trials for the prevention and management of type 2 diabetes.
Authors’ contribution
Conception and design: All authors; Administrative support: JOH, JAT, UEU, AII & NID; Provision of study materials: WM & NID; Collection and assembly of data: WM & NID; Data analysis and interpretation: All authors; Initial draft of manuscript: WM; Critical review of the manuscript: JOH, JAT, UEU, AII & NID; Final approval of manuscript: All authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication
Ethics approval
The research was approved by ABU Directorate of Academic Planning and Monitoring (Approval No: ABUCAUC/2019/22) and was conducted according to the ARRIVE Guidelines
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care. 2014;37:81–90. [Google Scholar]
- 2.Boland BB, Brown JC, Boland ML, Cann J, Sulikowski M, Hansen G, Grønlund RV, et al. Pancreatic b-cell rest replenishes insulin secretory capacity and attenuates diabetes in an extreme model of obese type 2 diabetes. Diabetes. 2019;68:131–140. doi: 10.2337/db18-0304. [DOI] [PubMed] [Google Scholar]
- 3.Makena W, Hamman WO, Buraimoh AA, Dibal NI, Obaje SG. Therapeutic effects of balanitoside in streptozotocin-induced diabetic rats. J Taibah Univ Med Sci. 2018;13:402–406. doi: 10.1016/j.jtumed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Venkat Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 5.Gougeon R, Pencharz PB, Sigal RJ. Effect of glycemic control on the kinetics of whole body protein metabolism in obese subjects with non-insulin-dependent diabetes mellitus during iso and hypoenergetic feeding. Am J Clin Nutr. 1997;65:861–870. doi: 10.1093/ajcn/65.3.861. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and DiabetesRelated complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 8.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MJ, Lim Y. Protective effect of short-term Genistein supplementation on the early stage in diabetes-induced renal damage. Mediators of Inflammation Mediators Inflamm. 2013;2013:1–14. doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. J Clinic Endocrinol Metab. 2010;95:4206–4216. doi: 10.1210/jc.2010-0668. [DOI] [PubMed] [Google Scholar]
- 11.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. International diabetes federation diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clinic Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clinic Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 14.Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 15.Xie H, Huang S, Deng H, Wu Z, Ji A. Study on chemical components of Momordica charantia. Zhong Yao Cai. 1998;21:458–459. [PubMed] [Google Scholar]
- 16.Raman A, Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae) Phytomed. 1996;2:349–362. doi: 10.1016/S0944-7113(96)80080-8. [DOI] [PubMed] [Google Scholar]
- 17.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Wang R, Song X, Chibbar RC, Wang X, Wu L, Meng QH. Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutr. Res. 2008;28:464–471. doi: 10.1016/j.nutres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed I, Adeghate E, Sharma AK, Pallot DJ, Singh J. Effects of Momordica charantia L. fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes res. Clinic Pract. 1998;40:145–151. doi: 10.1016/s0168-8227(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZQ, Zhang XH, Yu Y, Poulev A, Ribnicky D, Cefalu WT. Bioactives from bitter melon enhance insulin signaling and modulate acyl carnitine content in skeletal muscle in high-fat diet fed mice. J Nutr Biochem. 2011;22:1064–1073. doi: 10.1016/j.jnutbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson TJ, Setchell KDR, Kendall C, Jenkins WC, Anderson DJA, Fanti JWP. Effect of soy protein-rich diet on renal function inyoung adults with insulin-dependent diabetes mellitus. Clin Nephrol. 2005;64:1–11. doi: 10.5414/cnp64001. [DOI] [PubMed] [Google Scholar]
- 22.Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med. 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- 23.Lorke D. A new approach to practical acute toxicity. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 24.El-Kordy EA, Alshahrani AM. Effect of genistein, a natural soy isoflavone on pancreatic β-cells of streptozotocin-induced diabetic rats: histological and immunohistochemical study. J Microsc Ultrastruct. 2015;3:108–119. doi: 10.1016/j.jmau.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Iliya IA, Mohammed B, Akuyam SA, Yaro JD, Bauchi ZM, Tanko M, Idoko J, Aghemunu IL, Yusuf B. Immunohistochemical evaluation of the antidiabetic potentials of S-allyl-cysteine (garlic) and mangiferin (mango) in type 2 diabetic rat model. Sub-Saharan Afr J Med. 2016;3:25–31. [Google Scholar]
- 27.Al Nahdi MT, John A, Raza H. Elucidation of molecular mechanisms of Streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxidative Med Cell Longevity. 2017;2017:1–15. doi: 10.1155/2017/7054272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Jokelainen J, Auvinen J, Puukka K, Kiukaanniemi SK, Järvelin M, Kettunen J, Mäkinen V, Ala-Korpela M. Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: an interventional study. BMC Med. 2017;17:217. doi: 10.1186/s12916-019-1440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucher J, Kleinridders A, Ronald Kahn CR. Insulin receptor signaling in Normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konsue A, Picheansoonthon C, Talubmook C. Fasting blood glucose levels and hematological values in Normal and Streptozotocin-induced diabetic rats of Mimosa pudica L. Extracts Pharmacogn J. 2017;9:315–322. [Google Scholar]
- 31.Joseph B, Jini D. Antidiabetic effects of Momordica charantia L. (bitter melon) and its medicinal potency. Asian Pac J Trop Dis. 2013;3:93–102. [Google Scholar]
- 32.Nkambo W, Anyama NG, Onegi B. In vivo hypoglycemic effect of methanolic fruit extract of Momordica charantia L. Afr Health Sci. 2013;13:933–939. doi: 10.4314/ahs.v13i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi B, Ata A, Kumar NVA, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Ayatollahi SA, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9:551. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan SP, Kha TC, Parks SE, Roach PD. Bitter melon (Mormodica charanchia L.) bioactive composition and health benefits: a review. Food Rev Inter. 2016;32:181–202. [Google Scholar]
- 35.Ummi R, Harijono ET, Edang S. Bioactive compound and nutritious characteristic of bitter melon fruit (Momordica charantia L.) RJOAS. 2018;7:308–316. [Google Scholar]
- 36.Babu PVA, Si H, Fu Z, Zhen W, Liu D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr. 2012;142:724–730. doi: 10.3945/jn.111.152322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55:165–166. doi: 10.2337/diabetes.55.04.06.db05-1089. [DOI] [PubMed] [Google Scholar]
- 38.Bakhtiari A, Hajian-Tilaki K, Omidvar S, Nasiri-Amiri F. Clinical and metabolic response to soy administration in older women with metabolic syndrome: a randomized controlled trial. Diabetol Metab Syndr. 2019;11:47. doi: 10.1186/s13098-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konya J, Sathyapalan T, Kilpatrick ES, Atkin SL. The Effects of Soy Protein and Cocoa with or without Isoflavones on Glycemic Control in Type 2 Diabetes. A Double-Blind, Randomized, Placebo-Controlled Study. Front Endocrinol. 2019;10:296. doi: 10.3389/fendo.2019.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the Atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65:1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 41.Antwi-Baffour S, Kyeremeh R, Boateng SO, Annison L, Seidu MA. Haematological parameters and lipid profile abnormalities among patients with Type-2 diabetes mellitus in Ghana. Lipids Health Dis. 2018;17:283. doi: 10.1186/s12944-018-0926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhowmik B, Siddiquee T, Mujumder A, Afsana F, Ahmed T, Mdala IA, Moreira NCV. Serum lipid profile and its association with diabetes and Prediabetes in a rural Bangladeshi population. Int J Environ Res Public Health. 2018;15:1944. doi: 10.3390/ijerph15091944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017;16:207. doi: 10.1186/s12944-017-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaisar T, Couzens E, Hwang A, Russell M, Barlow CE, DeFina LF, Hoofnagle AN, Kim F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS One. 2018;13:e0192616. doi: 10.1371/journal.pone.0192616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi Y, Lee C, Lee K, Jung S, Lee B. Increased hepatic fatty acid uptake and esterification contribute to tetracycline-induced Steatosis in mice. Toxicol Sci. 2015;145:273–282. doi: 10.1093/toxsci/kfv049. [DOI] [PubMed] [Google Scholar]
- 46.Temitope AG, Sheriff OL, Azeezat YF, Taofik A, Fatimah AI. Cardio-protective properties of Momordica charantia L.in albino rats. African J Sci Res. 2013;11:600–610. [Google Scholar]
- 47.Zhou L, Xiao X, Zhang Q, Zheng J, Deng M. Maternal Genistein intake mitigates the deleterious effects of high-fat diet on glucose and lipid metabolism and modulates gut microbiota in adult life of male mice. Front Physiol. 2019;10:985. doi: 10.3389/fphys.2019.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu Z, Gilbert ER, Pfeiffer L, Zhang Y, Fu Y, Liu D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl Physiol Nutr Metab. 2012;37:480–488. doi: 10.1139/h2012-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4:1–12. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soares JMD, Leal AEP, Silva JC, Almeida JR, de Oliveira HP. Influence of flavonoids on mechanism of modulation of insulin secretion. Phcog Mag. 2017;13:639–646. doi: 10.4103/pm.pm_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinol. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Mi J. Insulin resistance determined by homeostasis model assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5:71. doi: 10.1186/1758-5996-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morimoto A, Tatsumi Y, Soyano F, Miyamatsu N, Sonoda N, Godai K, Ohno Y, Noda M, Deura K. Increase in homeostasis model assessment of insulin resistance (HOMA-IR) had a strong impact on the development of type 2 diabetes in Japanese individuals with impaired insulin secretion: the Saku study. PLoS One. 2014;9:e105827. doi: 10.1371/journal.pone.0105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clinic Endocrinol Metab. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 55.Ha BG, Nagaoka M, Yonezawa T, Tanabe R, Woo JT, Kato H. Regulatory mechanism for the stimulatory action of genistein on glucose uptake in vitro and in vivo. J Nutr Biochem. 2012;23:501–509. doi: 10.1016/j.jnutbio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 56.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh N, Gupta M. Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J Exp Biol. 2007;45:1055–1062. [PubMed] [Google Scholar]



